Abstract
Background and purpose:
Hyperdynamic circulation and mesenteric hyperaemia are found in cirrhosis. To delineate the role of endocannabinoids in these changes, we examined the cardiovascular effects of anandamide, AM251 (CB1 antagonist), AM630 (CB2 antagonist) and capsazepine (VR1 antagonist), in a rat model of cirrhosis.
Experimental approach:
Cirrhosis was induced by bile duct ligation. Controls underwent sham operation. Four weeks later, diameters of mesenteric arteriole and venule (intravital microscopy), arterial pressure, cardiac output, systemic vascular resistance and superior mesenteric artery (SMA) flow were measured after anandamide, AM251 (with or without anandamide), AM630 and capsazepine administration. CB1, CB2 and VR1 receptor expression in SMA was assessed by western blot and RT-PCR.
Key results:
Anandamide increased mesenteric vessel diameter and flow, and cardiac output in cirrhotic rats, but did not affect controls. Anandamide induced a triphasic arterial pressure response in controls, but this pattern differed markedly in cirrhotic rats. Pre-administration of AM251 blocked the effects of anandamide. AM251 (without anandamide) increased arterial pressure and systemic vascular resistance, constricted mesenteric arterioles, decreased SMA flow and changed cardiac output in a time-dependent fashion in cirrhotic rats. Capsazepine decreased cardiac output and mesenteric arteriolar diameter and flow, and increased systemic vascular resistance in cirrhotic rats, but lacked effect in controls. Expression of CB1 and VR1 receptor proteins were increased in cirrhotic rats. AM630 did not affect any cardiovascular parameter in either group.
Conclusions and implications:
These data suggest that endocannabinoids contribute to hyperdynamic circulation and mesenteric hyperaemia in cirrhosis, via CB1- and VR1-mediated mechanisms.
Keywords: endocannabinoid, cirrhosis, anandamide, CB1, CB2, VR1, vanilloid, haemodynamics, hyperdynamic circulation
Introduction
Cirrhosis is associated with hyperdynamic circulation, defined as increased cardiac output and arterial vasodilatation, which is especially prominent in the splanchnic circulation. These abnormalities may play an important role in the development of several complications of cirrhosis including ascites, dilutional hyponatremia and hepatorenal syndrome (Chen et al., 2004; Garcia-Tsao, 2005). The pathogenesis of hyperdynamic circulation in cirrhosis remains unclear.
The biological effects of cannabis are mediated by specific receptors. To date, two cannabinoid (CB) receptors have been identified, the cannabinoid receptor subtype 1 (CB1) receptor, which is expressed in peripheral tissues including the heart and vasculature (Gebremedhin et al., 1999; Liu et al., 2000; Bonz et al., 2003) and cannabinoid receptor subtype 2 (CB2) receptors, expressed primarily by immune and hematopoietic cells (Munro et al., 1993). Endogenous ligands for these receptors also exist. These natural ligands are lipid-like substances called endocannabinoids, which include arachidonoyl ethanolamide or anandamide, and 2-arachidonoylglycerol (2-AG) (De Petrocellis et al., 2004).
In addition to their well-known neurobehavioral effects, endocannabinoids influence a number of other physiological functions, including cardiovascular variables (Jarai et al., 1999). In anaesthetized mice, anandamide and synthetic CBs given as intravenous (i.v.) bolus doses produce bradycardia and hypotension, which are absent in CB1 receptor gene knockout animals (Jarai et al., 1999; Ledent et al., 1999). In anaesthetized rats, anandamide induces a triphasic effect on blood pressure (Varga et al., 1995, 1996; Wagner et al., 2001).
However, recent work has highlighted the possibility that anandamide may exert markedly different cardiovascular effects in conscious animals compared to anaesthetized (Gardiner et al., 2002). Anandamide has been shown to be a vasorelaxant in different vascular beds, particularly in the resistance vasculature. This vasorelaxation has been reported to be both endothelium-independent and -dependent, depending on the vascular bed (Randall and Kendall, 1998; Mukhopadhyay et al., 2002). Therefore, although the cardiovascular effects are fairly well characterized in normal or noncirrhotic animals, the situation in cirrhosis remains incompletely clarified.
Recent studies have identified the endogenous CB system as a mediator of arterial hypotension in cirrhosis. Batkai et al. (2001) and Ros et al. (2002) showed that monocytes from cirrhotic rats have elevated anandamide levels, and CB1 receptor blockade by SR141716A increases arterial pressure in these animals. Batkai et al. (2001) also found a threefold increase in CB1 receptors on vascular endothelial cells isolated from cirrhotic patients compared to noncirrhotic controls. Although these studies examined arterial pressure and other systemic haemodynamic variables, an integrated in vivo systemic and splanchnic haemodynamic characterization of endocannabinoid effects has not previously been performed.
We therefore examined cardiovascular responses of cirrhotic rats to anandamide and the involvement of CB1 receptors in these effects. We also studied the effect of AM251, a CB1 receptor antagonist, AM630, a CB2 receptor antagonist and capsazepine, a vanilloid receptor 1 (VR1) antagonist, with particular emphasis on the splanchnic circulation.
Methods
Animal model
The study protocols were approved by the University of Calgary Faculty of Medicine Animal Care Committee, under the guidelines of the Canadian Council on Animal Care. Male Sprague–Dawley rats (Charles River, Canada) weighing 200–250 g were used in this study. Rats had free access to rat chow and water and were maintained on a 12-h light/dark cycle. Cirrhosis was produced by common bile duct ligation (BDL), as previously described (Ma et al., 1999). Briefly, the common bile duct in pentobarbital-anaesthetized (60 mg kg−1 i.p.) rats was doubly ligated and sectioned between the ligatures. Controls received sham operation. These rats were treated similarly to the BDL group except that the bile duct was visually inspected but not ligated. After operation, gentamicin aerosol was sprayed on the incision to prevent infection. Four weeks after surgery, BDL rats showed jaundice, muscle wasting, ascites and liver failure. This is a well-accepted rat model of biliary cirrhosis, and the liver histology and serum biochemistry typical of cirrhosis and liver failure have previously been documented in detail (Kountouras et al., 1984; Ma et al., 1999; Liu et al., 2000). All studies were performed 4 weeks after BDL or sham operation.
Intravital microscopy to measure vessel diameter
Cirrhotic and control rats were anaesthetized with ketamine (85 mg kg−1) and xylazine (15 mg kg−1) i.p., and placed on a heating pad to maintain body temperature at 37°C. The right jugular vein was cannulated with PE-10 for drug administration and the left carotid artery with PE-90 for measurement of mean arterial pressure (MAP). Then the abdomen was opened by a small incision for exteriorization of the ileal portion of the mesenteric bed. The method of intravital microscopy to observe vessel diameters has been previously described (Li et al., 2006). In brief, a small loop of mesentery was prepared for intravital microscopy by placing the loop on an adjustable stage and the area under the microscope light was covered by a glass slide. The mesenteric preparation was continuously superfused with normal saline to avoid temperature fluctuation and drying of the tissue by ambient air. All other exposed areas were covered with saline-soaked cotton gauze.
Diameter changes of mesenteric arterioles and venules (first order branch) in response to the stimuli were observed using a Mikron IV500L intravital microscope system (Mikron, San Marcos, CA, USA) with a 100 W mercury lamp (FluoArc for N HBO103, Carl Zeiss, Germany) attached to a fluorescence illuminator equipped with Blue filter blocks (excitation: 450–490 nm, emission: >520 nm, Carl Zeiss, Don Mills, ON, Canada). With the use of a × 20/0.50 W objective lens (Achroplan, water immersion, Zeiss) and a × 10 eyepiece, the image captured by a Pieper charge-coupled device video camera (FK 6990, Cohu, San Diego, CA, USA) was displayed on a video monitor and recorded on a videocassette for off-line evaluation. Contrast enhancement for visualization of the mesenteric vessels was achieved by i.v. injection of 5% fluorescein isothiocyanate (FITC)-labeled dextran (molecular weight, 150 000, 1 ml kg−1 body weight; Sigma, St Louis, MO, USA).
Anandamide (3 mg kg−1) was infused through a jugular vein over 4 min by a motorized infusion pump and data recorded for 20 min. In a separate experiment, AM251 (3 mg kg−1), a CB1 receptor antagonist, or saline were administered as an i.v. bolus 10 min before anandamide infusion and data recorded for 20 min. In another set of experiments, AM251, the CB2 receptor antagonist AM630 or capsazepine, 3 mg kg−1, were injected by a bolus through a jugular vein and data recorded for 60 min. A final protocol involved a combination of AM251 and capsazepine at the same doses in cirrhotic rats, injected at time 0 and 20 min with parameters followed for 60 min. In this protocol, half the animals were injected with AM251 first, then capsazepine, while the other half were administered these drugs in reverse order.
Cardiac output and superior mesenteric artery blood flow measurements
The animals were anaesthetized with ketamine and xylazine as previously described. The right femoral artery was cannulated to measure MAP using a polygraph recorder (Gould 2400, Oxnard, CA, USA), calibrated at each use with a pressure manometer. The left femoral vein was cannulated for drug administration. Cardiac output was measured by the thermodilution method. A microprobe adapter (Columbus Instruments, Columbus, OH, USA) was connected to a cardiac output computer (Columbus Instruments). A bolus of normal saline (200 μl) at 4±1°C was injected into a PE-50 catheter placed in the right atrium via a right jugular approach. The microprobe sensor was placed into the aortic arch through a left carotid approach. Cardiac index was calculated as cardiac output per 100 g body weight (ml min−1 100 g−1). Systemic vascular resistance (mm Hg ml−1 min−1 100 g−1; SVR) was calculated as MAP/cardiac index.
Superior mesenteric artery (SMA) blood flow was measured by transonic flowmetry. The SMA was gently dissected free from connective tissue, and a perivascular ultrasonic flowprobe (1-mm inside diameter) (Transonic Systems Inc., Columbus, OH, USA) was placed around this vessel close to its aortic origin. The flow probe was connected to an ultrasonic flowmeter (T206; Transonic Systems) to measure blood flow by the transonic transit time technique as described previously (Wen et al., 1996; Song et al., 2002). All drugs were injected via the left femoral vein.
Western blot for CB1, CB2 and VR1 receptor protein
Western blots were performed as previously described (Liu et al., 2001). Briefly, SMA from sham and BDL rats (2–3 rats for each sample) were immediately isolated after they had been killed and connective tissue was dissected out. An arterial segment of about 3–4 cm was then transferred to a Petri dish containing ice-cold normal saline. After removal of the blood within the lumen by gently squeezing, the segment was homogenized with a Kinamatica homogenizer (Brinkmann Instruments, Rexdale, ON, Canada) in a buffer containing 20 mM Tris HCl (pH 7.2), 0.2 mM phenylmethylsulphonyl fluoride, and 1 mM dithiothreitol. The supernatant from 4000 g centrifugation was collected and resuspended in Tris buffer containing proteinase inhibitor cocktail (Boehringer Mannheim, Mannheim, Germany). The protein concentration was determined with the Bio-Rad protein assay using bovine serum albumin (BSA) as a standard. Equal amounts (200 μg) of the denatured proteins per lane were loaded and separated on sodium dodecyl sulphate–10% polyacrylamide gels by electrophoresis. Proteins were then transferred to nitrocellulose by wet electroblotting at 4°C overnight. Blots were blocked for 2 h at room temperature with 10% BSA in 0.1% Tween Tris-buffered saline buffer (TBS-T) at pH 7.5 containing 20 mM Tris base, 137 mM NaCl, and 0.1% Tween-20. The membranes incubated with rabbit polyclonal anti-CB1 antibody (1:500, Affinity Bioreagents), goat polyclonal anti-CB2 antibody (1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA), or goat polyclonal anti-VR1 antibody (1:100, Santa Cruz Biotechnology) at 4°C overnight. The membranes were washed with TBS-T (three times, 15 min each) and then incubated at room temperature for 120 min with alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin (for CB1; 1:1000, Calbiochem, San Diego, CA, USA) or alkaline phosphatase-conjugated rabbit anti-goat immunoglobin (for CB2 and VR1; 1:1000, Sigma). The relative expression of CB1, CB2, and VR1 protein in the blots was quantified by computerized optical densitometric scanning using a Hewlett-Packard Scan Jet IIc scanner, DeskScan II software and the NIH Image program.
CB1 and CB2 mRNA expression by RT–PCR
SMA segments were prepared as described above. Total RNA from the tissue was extracted using the acid guanidinium isothiocyanate method (Wong et al., 1994). Total RNA was quantified using a GeneQuant spectrophotometer (Pharmacia, Piscataway, NJ, USA). The cDNA was obtained through RT–PCR by the method of Wong et al. (1994). Briefly, the reaction mixture containing 2 μg of the total RNA, first-strand buffer, 20 nM each of dATP, dGTP, dCTP and dTTP, 160 units of Superscript-RT, and 100 pmol of random hexamer oligodeoxynucleotides (Pharmacia) was incubated at 20°C for 10 min. The reverse transcription reaction was carried out in a thermal cycler (Barnstead/Thermolyne, Dubuque, IA, USA) at 42°C for 50 min, and the product was then heated to 95°C to stop the reaction.
CB1 (GenBank No. U40395), CB2 (GenBank No. AF17635), VR1 (GenBank No. AF327067), β-actin and RPL primers (β-actin as an internal control for CB1 and CB2 and RPL as an internal control for VR1) were synthesized by Gibco-BRL Life Technologies (Burlington, ON, Canada). Briefly, the initial reaction mixture contained 2 μl of RT product, 1 × PCR buffer, 4 nM of each deoxynucleotide, and 2 units of Taq DNA polymerase. Taq DNA polymerase was added to the PCR reaction mixture in the first denaturation step. A set of 36 cycles for CB1, CB2, VR1 and RPL and 30 cycles for β-actin were chosen to ensure that the amplification of PCR products was in the exponential range according to preliminary cycle test experiments. In each PCR cycle, heat denaturation was set at 94°C for 1 min, primer annealing at 60°C (for CB1, CB2 and β-actin) or 55°C (for VR1 and RPL) for 30 s, and polymerization at 72°C for 1 min. PCR product (10 μl) was electrophoresed in 1.5% agarose gels containing 0.2 μg ml−1 of ethidium bromide. The gels were scanned by computerized ultraviolet densitometric analysis in the same manner as the Western blots.
Chemical reagents
Water soluble forms of anandamide in 1:4 soya oil:water emulsion were purchased from Tocris Cookson Ltd (Ellisville, MO, USA). Stocks of AM251 and AM630 (both from Tocris Cookson) were dissolved in a mixture of DMSO: ethanol: Cremophor: normal saline, 1:1:1:17 by sonicating. Capsazepine (Tocris Cookson) was dissolved in DMSO 10%, Tween-80 10% and normal saline 80%. Other reagents were purchased from Sigma, Bio-Rad (Hercules, CA, USA), or Fisher Scientific and were the highest available grade.
Statistical analysis
The results are expressed as mean±s.e.m. Student's paired t-test was used to compare the differences between two groups and multiple comparisons were analysed by two-way ANOVA followed by Tukey's post hoc test. A P-value of less than 0.05 was considered to be significantly different.
Results
Effect of anandamide with or without pre-administration of AM251
Systemic haemodynamics
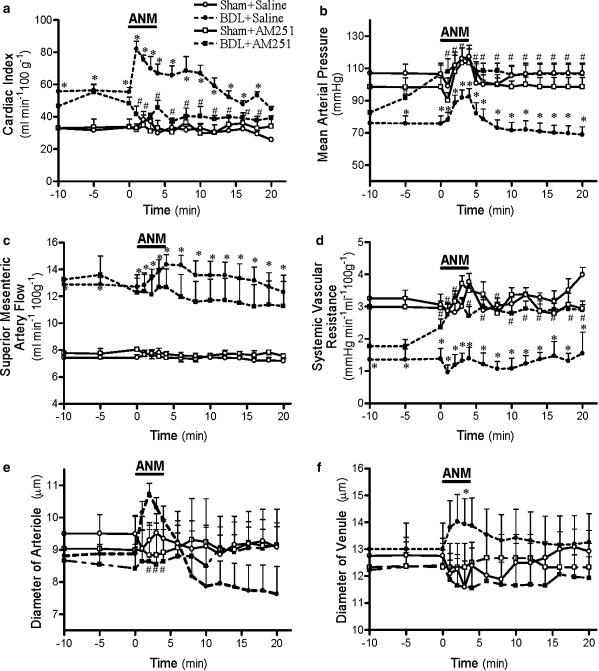
There was a significant increase in cardiac index of BDL rats during and after anandamide infusion (Figure 1a). AM251 significantly increased cardiac output of BDL rats 5 min after injection, but decreased cardiac output during and after anandamide infusion. Cardiac output of control animals remained unaffected by anandamide, AM251 or the combination of both drugs. Anandamide alone did not significantly affect SVR of control or BDL animals. Preadministration of AM251 before anandamide increased SVR in cirrhotic rats while it had no effect in controls (Figure 1d).
Figure 1.
Effect of anandamide (3 mg kg−1) (ANM) infusion with or without preadministration of AM251 (3 mg kg−1) on cardiac index (a), MAP (b), SMA flow (c), systemic vascular resistance (d), mesenteric arteriole diameter (e) and mesenteric venule diameter (f) in cirrhotic or sham-control rats. Values are the mean±s.e.m. of 6–8 rats per group. *P<0.05 for comparison of BDL-placebo group with the corresponding sham-placebo groups. #P<0.05 for comparison of the BDL-placebo group with the corresponding BDL+AM251 groups.
Anandamide induced a triphasic change in MAP of control rats (Figure 1b) consisting of (1) an initial short-lasting hypotension, (2) a pressor effect during the infusion period and (3) a delayed fall in blood pressure. In cirrhotic animals, the pattern was markedly different. The first phase was absent, the hypertensive second phase was accentuated and the hypotensive third phase even further delayed. Preadministration of AM251 blocked the third phase of anandamide-induced hypotension in both controls and cirrhotic animals.
Mesenteric haemodynamics
Anandamide increased SMA flow in BDL rats, an effect blocked by AM251 preadministration (Figure 1c). Anandamide had no significant effect in control animals, and AM251 did not change this.
Anandamide infusion significantly dilated mesenteric arterioles of cirrhotic rats but had no effect on controls (Figure 1e). Preadministration of AM251 completely blocked the vasodilator effect of anandamide on mesenteric arterioles of cirrhotic rats but had no effect on control animals. During anandamide infusion, BDL mesenteric venule diameter also significantly increased, but less prominently than in the arterioles (Figure 1f). Anandamide had no significant effect on mesenteric venule diameter of control animals with or without AM251 preadministration.
Effect of AM251, AM630 and capsazepine
Systemic haemodynamics
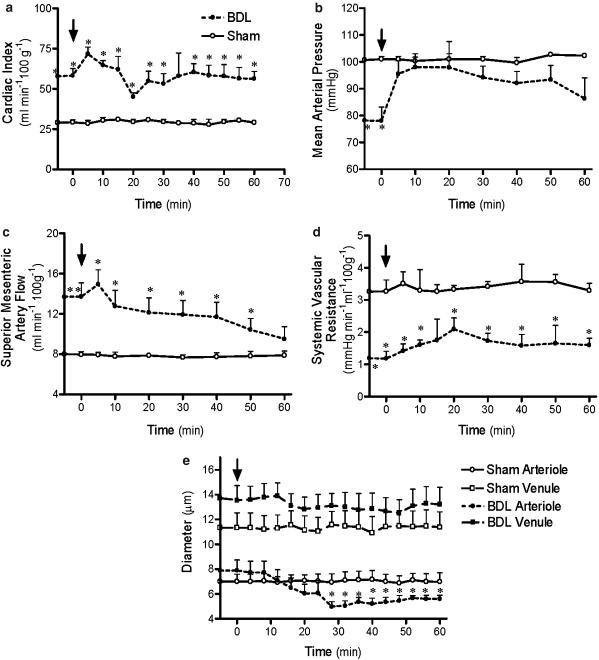
In BDL rats, cardiac output immediately rose after AM251 administration, peaking at 5 min, but then decreasing until a significant trough was attained at 20 min postinjection (Figure 2a). Cardiac output remained unaffected in control animals. MAP increased significantly within 5 min after AM251 in the cirrhotic rats but was unaffected in the control animals (Figure 2b). SVR increased after AM251 administration, peaking at 20 min (Figure 2d).
Figure 2.
Effect of AM251 (3 mg kg−1) administration on cardiac index (a), MAP (b), SMA flow (c), systemic vascular resistance (d) and mesenteric vessel diameter (e) in cirrhotic or sham-control rats. Values are the mean±s.e.m. of 6–8 rats per group. Arrows indicate time of administration of AM251. *P<0.05 for comparison of BDL group with the corresponding sham group.
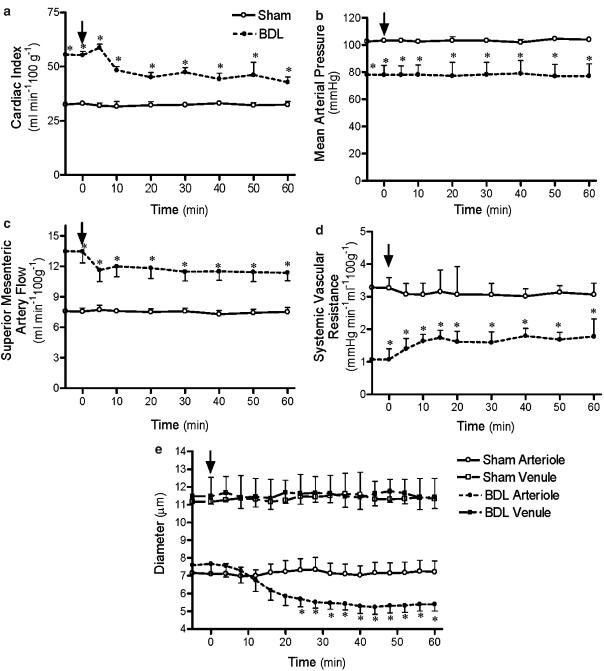
Capsazepine did not significantly affect MAP in cirrhotic or control rats (Figure 3b), or cardiac output and SVR in controls (Figure 3a, d). However, in cirrhotic rats, this drug reduced cardiac output (Figure 3a) and increased SVR (Figure 3d). AM630 did not affect any observed cardiovascular variable in this study in either control or BDL groups (data not shown).
Figure 3.
Effect of capsazepine (3 mg kg−1) administration on cardiac index (a), MAP (b), SMA flow (c), systemic vascular resistance (d) and mesenteric vessel diameter (e) in cirrhotic or sham-control rats. Values are the mean±s.e.m. of 6–8 rats per group. Arrows indicate time of administration of capsazepine. *P<0.05 for comparison of BDL group with the corresponding sham group.
Mesenteric haemodynamics
AM251 and capsazepine administered as single agents both significantly decreased SMA flow in cirrhotic rats, but neither affected control rats (Figures 2c and 3c).
AM251 administration significantly constricted mesenteric arterioles of BDL rats, while it had no effect on BDL venules or arterioles and venules of control rats (Figure 2e). Capsazepine similarly significantly constricted mesenteric arterioles of BDL rats, but did not affect venule diameters of BDL rats or any vessel diameters of the control rats (Figure 3e).
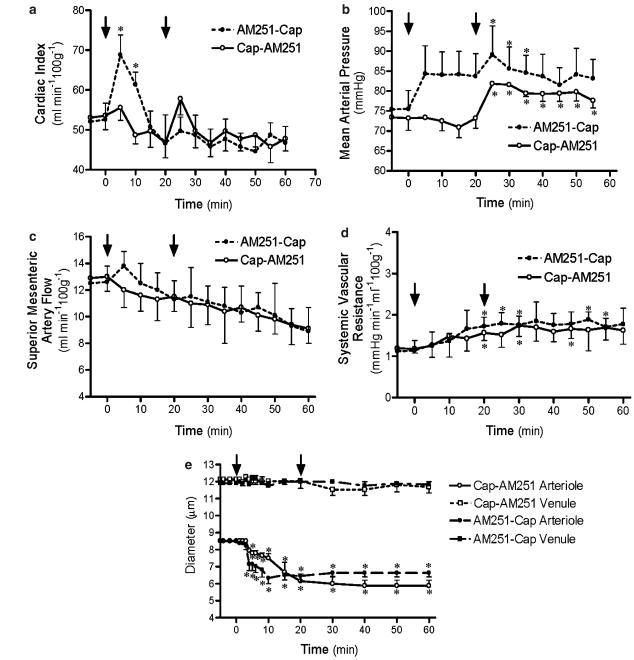
Sequential combination of both AM251 and capsazepine administration in the same cirrhotic animal generally did not produce additive or incremental effects beyond those observed with each drug alone, regardless of which drug was administered first (Figure 4b–e). The exception was the effect on cardiac output, which showed a differential response depending on which drug was administered first. When AM251 was administered first, the initial large increase in cardiac output was again observed and capsazepine administration 20 min later produced no noticeable effect on this variable (Figure 4a). When capsazepine was administered first, there was a slight decrease in cardiac output and the AM251-induced increase was markedly attenuated.
Figure 4.
Effects of combination sequential AM251 (3 mg kg−1) and capsazepine (3 mg kg−1) on cardiac index (a), MAP (b), SMA flow (c), systemic vascular resistance (d) and mesenteric vessel diameters (e) in cirrhotic rats. Values are the mean±s.e.m. of 4–6 rats per group. Arrows indicate time of administration of either AM251 or capsazepine. *P<0.05 significantly different from baseline value in each group.
CB1, CB2 and VR1 receptor mRNA and protein expression
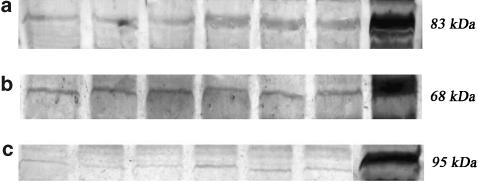
CB1 and VR1 protein expression were increased in cirrhotic rats compared to controls, while there was no significant difference in CB2 protein expression between BDL and control groups (Figure 5).
Figure 5.
Western blot protein expression of CB1 (a), CB2 (b) and VR1 (c) receptor protein in SMA of sham (lanes 1–3) and BDL (lanes 4–6) rats. Lane 7 is the positive control (CB1, cerebellum; CB2, spleen and VR1, cerebrum). BDL rats show significantly increased CB1 and VR1 protein expression compared to sham-controls. CB2 expression is not significantly different between the two groups.
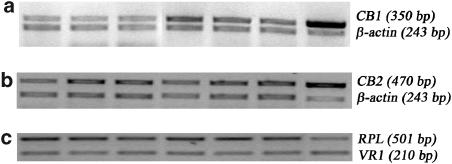
Representative RT–PCR photographs of CB1, CB2 and VR1 mRNA expression in SMA are shown in Figure 6. CB1 mRNA expression was elevated in cirrhotic rats compared to controls while there was no significant difference in CB2 or VR1 mRNA expression between the sham and BDL groups.
Figure 6.
RT–PCR of CB1 (a), CB2 (b) and VR1 (c) receptor mRNA in SMA of sham (lanes 1–3) and BDL (lanes 4–6) rats. Lane 7 is the positive control (CB1, cerebellum; CB2, spleen; and VR1, cerebrum). BDL rats show significantly increased CB1 mRNA expression, but unchanged CB2 and VR1 mRNA expression compared to sham-controls.
Discussion
This study showed an enhanced cardiovascular response to anandamide in cirrhotic rats, and CB1-mediated splanchnic vasodilation was especially prominent. Although recent studies have confirmed increased levels of endocannabinoids in cirrhosis (Batkai et al., 2001; Ros et al., 2002; Fernandez-Rodriguez et al., 2004), and they have been shown to have a role in arterial hypotension (Batkai et al., 2001; Ros et al., 2002), no previous study has assessed the cardiovascular reactivity of cirrhotic animals to anandamide in vivo.
Anandamide dilated mesenteric arterioles and to a lesser extent, venules, in cirrhotic rats. Preadministration of the CB1 antagonist AM251 blocked this effect. These results agree with the in vitro study of Domenicali et al. (2005) who showed that isolated mesenteric vessels of cirrhotic rats are more sensitive to anandamide than control vessels. This vasodilator response was reversed by the CB1 antagonist, SR141716A (Domenicali et al., 2005). However, the responses of mesenteric veins in cirrhosis have not been studied. Although mesenteric veins and venules share many of the same vasoactive systems as arteries, they have generally been ignored in cardiovascular studies in cirrhosis, as they are capacitance rather than resistance vessels. However, results from the present study, as well as those obtained from another investigation of intrahepatic veins (Li et al., 2006), demonstrate that these vessels may also play a role in cardiovascular regulation and merit further study in cirrhosis.
AM251 is a selective CB1 antagonist that is equipotent with SR141716A (Gatley et al., 1997). Several recent studies point to the existence of additional CB receptors distinct from CB1 and CB2. At least two of these, a putative Gi/Go-coupled endothelial receptor mediating vasodilatation (Jarai et al., 1999; Mukhopadhyay et al., 2002; Ho and Hiley, 2003) and a receptor postulated to be present on glutaminergic terminals in the hippocampus (Hajos and Freund, 2002), have been shown to be uniquely sensitive to inhibition by SR141716A but not by other CB1 antagonists such as AM251 (Batkai et al., 2004a). Therefore our use of AM251, which is more specific for the CB1 receptor especially in mesenteric beds, confirms a CB1-mediated effect of anandamide.
Although several in vitro studies show a direct vasodilator action of anandamide in normal rat and mouse mesenteric vascular beds (Randall et al., 1997; Jarai et al., 1999; Wagner et al., 1999; Garcia et al., 2001), to our knowledge, only one previous study (Gardiner et al., 2002) has examined mesenteric vessels in vivo. In that study, in conscious rats a decreased mesenteric vascular conductance was obtained in response to anandamide and this was unaffected by AM251 preadministration (Gardiner et al., 2002). This result accentuates the complex and divergent effect of anandamide in conscious compared to anaesthetized animals (Randall et al., 2004). Our result showing no significant vasoactivity of a 3 mg kg−1 dose in the mesenteric vessels of normal rats straddles the rift between the previous divergent in vitro and in vivo studies.
Previous studies in anaesthetized control rats showed that anandamide induces a triphasic blood pressure response, consisting of (1) an initial short-lasting hypotension related to VR1 receptors (Malinowska et al., 2001; Pacher et al., 2004), (2) a pressor response of unknown origin (Kunos et al., 2000) and (3) a delayed hypotension which is related to CB1 receptor activation (Varga et al., 1996). Recent work by Kwolek et al. (2005) suggests that the second pressor phase is due to a central effect of anandamide mediated via β2-adrenoceptors and/or NMDA receptors. In control animals, our data agree with previous results. AM251 preadministration blocked the third phase of anandamide-induced hypotension without affecting the first and second phases. Cirrhotic rats that received anandamide alone did not exhibit the first phase, had an enhanced second phase and a delayed third hypotensive phase. In contrast, Batkai et al. (2001), using an anandamide i.v. bolus of 4 mg kg−1 in BDL-cirrhotic rats, observed that the first phase was intact, the second phase enhanced and the third phase absent. Possible explanations for this discrepancy include differences in anandamide dose, mode of administration, anaesthetic and timing of observations. In our cirrhotic rats, preadministration of AM251 before anandamide eliminated the third phase, slightly blunted the enhanced second phase and exerted no effect on the absent first phase. These results are consistent with the known effects of AM251; it blocks CB1 receptors but does not affect VR1 receptors. As capsazepine administration did not affect arterial pressure in our cirrhotic rats, but instead decreased mesenteric arterial flow and calibre, we surmised that vanilloid receptors exert relatively little or no tonic influence on blood pressure regulation, but rather play a role in the splanchnic circulation of cirrhosis.
We used a pharmacological dose of anandamide, therefore extrapolation of these effects to the pathophysiological levels of endocannabinoids in cirrhosis is speculative. Moreover, several alternative possibilities to explain the data should be considered. For example, we cannot rule out the possibility that anandamide at the pathophysiological concentrations present in cirrhosis exerts at least part of its effects via other mechanisms, such as vanilloid receptors other than VR1 (Watanabe et al., 2003), interaction with other endocannabinoids such as 2-AG, or interaction with other vasoactive systems such as the sympathetic nervous system. In this respect, a recent study by Yang et al. (2006) demonstrated that the isolated perfused cirrhotic liver of the rat was more sensitive to anandamide-induced increase in intrahepatic resistance, mediated via eicosanoid generation.
Further delineation of the integrated cardiovascular response to CB and vanilloid receptor activation was provided by results obtained after examining the effects of AM251 and capsazepine alone. These results were generally similar in that neither drug affected cardiac responses in control animals, and in the cirrhotic rats, both induced systemic and mesenteric vasoconstriction. Important differences between the two drugs include the initial marked biphasic effect on cardiac output induced by AM251 compared to the persistent depression of cardiac output, for at least 60 min, in the capsazepine-treated animals. Somewhat surprisingly, the combined administration of these two drugs in the cirrhotic rats did not produce increased or additional effects compared to administration of either drug alone, irrespective of which drug was administered first. We propose that the degree of vasoconstriction induced by each drug may have reached a plateau beyond which the vessel would remain unresponsive to additional constrictor influences, and/or that reflex mechanisms might prevent further vasoconstriction from occurring, as it would harm the animal.
SMA responses to both anandamide (increased flow) and AM251 (decreased flow) were more pronounced in BDL cirrhotic rats compared to the controls. Moreover, BDL SMA showed increased CB1 receptor expression. These results all point towards an enhanced CB1-mediated mesenteric hyperaemia in cirrhotic rats.
AM251 also increased blood pressure of cirrhotic rats, in agreement with previous studies (Batkai et al., 2001; Ros et al., 2002). In cirrhotic rats, AM251 increased cardiac output at 5 min, but then progressively decreased it until a significant trough was reached at 20 min. Ros et al. (2002) showed that SR141716A did not significantly affect cardiac output of cirrhotic rats at 10 and 30 min after injection. We measured cardiac output every 5 min, which allowed a more precise determination of the time-dependent response to CB1 blockade. The overall effect on cardiac output presumably results from the combined effects induced by blockade of CB1-mediated cardiac negative inotropism and peripheral vasodilatation, complicated by the influence of cardiovascular reflexes. It is therefore not surprising that the overall changes in cardiac output were relatively modest and variable over time. The immediate positive-inotropic effect on cardiac output agrees with data from our previous study showing a direct cardiodepressant effect of endocannabinoids, mediated via ventricular CB1 receptors (Gaskari et al., 2005).
The lack of cardiovascular effect of AM630 confirms that the CB2 receptor is not involved in cardiovascular regulation in either normal or cirrhotic animals. Similarly, in control animals, AM251 alone had little haemodynamic effect, which suggests that the CB system is physiologically inactive under normal conditions; findings that agree with those from previous studies (Ledent et al., 1999; Batkai et al., 2004b).
The vanilloid VR1 receptor is part of a family of transient receptor potential channels (Benham et al., 2002) largely associated with small diameter primary afferent nerve fibres. This receptor is a nonselective cation channel that is activated by multiple noxious stimuli such as the naturally occurring vanilloid capsaicin, heat stress and acid (Szallasi, 2002). Anandamide and vanilloids share some structural similarities. In rat mesenteric arteries, the endothelium-independent vasodilator effect of anandamide is inhibited by the VR1 receptor antagonist capsazepine or by a calcitonin gene-related peptide (CGRP) receptor antagonist (Di Marzo et al., 1998). Anandamide binds to a cloned VR1 receptor with micromolar affinity (Di Marzo et al., 1998), and at nanomolar concentrations releases CGRP from sensory nerve terminals in the vascular adventitia (Zygmunt et al., 1999). Thus, it is likely that anandamide acts at VR1 receptors in sensory nerves to release the potent vasodilator peptide CGRP (Pacher et al., 2004). Accordingly, our study would have been incomplete without examination of the VR1-mediated effects of anandamide.
The VR1 receptor antagonist capsazepine induced mesenteric arteriolar constriction and decreased SMA blood flow in cirrhotic rats. Moreover, cirrhotic SMA showed increased VR1 receptor expression. These results suggest that the elevated level of anandamide in cirrhotic animals mediates mesenteric vasodilatation via both CB1 and VR1 receptors. The present results agree with those of Domenicali et al. (2005); they showed increased CB1 and VR1 receptor protein in 2nd- and 3rd-order mesenteric arterial branches from cirrhotic rats.
CGRP has positive chronotropic and inotropic effects (Wimalawansa, 1996). Shekhar et al. (1991) observed that CGRP infusion increased cardiac output by 72% while reducing right atrial and pulmonary wedge pressures in patients with congestive heart failure. In cirrhosis, circulating CGRP levels are elevated, and correlate with indices of hyperdynamic circulation (Henriksen et al., 2001). In our cirrhotic rats, capsazepine decreased cardiac output and increased systemic vascular resistance. We therefore believe that the blockade of VR1 abrogated the CGRP-releasing effect of elevated anandamide levels in the cirrhotic rats. On the other hand, capsazepine did not affect any haemodynamic variable in our control rats, suggesting that VR1 receptors are not tonically active in the normal circulation.
The depressant effect of capsazepine on cardiac output was highlighted in the sequential combination protocol by the different patterns of response depending on whether AM251 or capsazepine was administered first. Whether or not the capsazepine-induced cardiodepression is due to a peripheral vascular or direct cardiac effect of vanilloid receptor blockade remains unclear. Although the in vivo haemodynamic effects of acute vanilloid receptor blockade have not previously been studied in cirrhosis, these results agree with our previous studies in which neonatal capsaicin administration was used to ablate the primary afferent nerves. Using such a protocol, we demonstrated that such treatment prevents the development of hyperdynamic circulation and other cardiovascular abnormalities when cirrhosis or portal hypertension is induced in these animals in adult life (Lee and Sharkey, 1993; Li et al., 2003).
In conclusion, these results indicate that endogenous CB compounds such as anandamide play a role in the pathogenesis of hyperdynamic circulation in cirrhosis, particularly in the mesenteric circulation. These effects are primarily mediated via stimulation of CB1 and VR1 receptor pathways.
Acknowledgments
This study was funded by research operating grants from the Canadian Institutes of Health Research (CIHR). SAG was supported by a Canadian Heart and Stroke Foundation Doctoral Research Award, and SKB by a sabbatical leave award from the Yonsei University Wonju College of Medicine and by Visiting Fellowship awards from the Korean Association for Study of Liver and GlaxoSmithKline Korea. SSL was supported by an Alberta Heritage Foundation for Medical Research (AHFMR) Senior Scholarship award.
Abbreviations
- 2-AG
2-arachidonoylglycerol
- BDL
bile duct ligated
- CB1
cannabinoid receptor subtype 1
- CB2
cannabinoid receptor subtype 2
- CGRP
calcitonin gene-related peptide
- FITC
fluorescein isothiocyanate
- MAP
mean arterial pressure
- SMA
superior mesenteric artery
- SVR
systemic vascular resistance
- VR1
vanilloid receptor 1
Conflict of interest
The authors state no conflict of interest.
References
- Batkai S, Jarai Z, Wagner JA, Goparaju SK, Varga K, Liu J, et al. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat Med. 2001;7:827–832. doi: 10.1038/89953. [DOI] [PubMed] [Google Scholar]
- Batkai S, Pacher P, Jarai Z, Wagner JA, Kunos G. Cannabinoid antagonist SR-141716 inhibits endotoxic hypotension by a cardiac mechanism not involving CB1 or CB2 receptors. Am J Physiol Heart Circ Physiol. 2004a;287:H595–H600. doi: 10.1152/ajpheart.00184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batkai S, Pacher P, Osei-Hyiaman D, Radaeva S, Liu J, Harvey-White J, et al. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation. 2004b;110:1996–2002. doi: 10.1161/01.CIR.0000143230.23252.D2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham CD, Davis JB, Randall AD. Vanilloid and TRP channels: a family of lipid-gated cation channels. Neuropharmacology. 2002;42:873–888. doi: 10.1016/s0028-3908(02)00047-3. [DOI] [PubMed] [Google Scholar]
- Bonz A, Laser M, Kullmer S, Kniesch S, Babin-Ebell J, Popp V, et al. Cannabinoids acting on CB1 receptors decrease contractile performance in human atrial muscle. J Cardiovac Pharmacol. 2003;41:657–664. doi: 10.1097/00005344-200304000-00020. [DOI] [PubMed] [Google Scholar]
- Chen YC, Gines P, Yang J, Summer SN, Falk S, Russell NS, et al. Increased vascular heme oxygenase-1 expression contributes to arterial vasodilation in experimental cirrhosis in rats. Hepatology. 2004;39:1075–1087. doi: 10.1002/hep.20151. [DOI] [PubMed] [Google Scholar]
- Garcia-Tsao G. Portal hypertension. Curr Opin Gasroenterol. 2005;21:313–322. doi: 10.1097/01.mog.0000158110.13722.e0. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Cascio MG, Di Marzo V. The endocannabinoid system: a general view and latest additions. Br J Pharmacol. 2004;141:765–774. doi: 10.1038/sj.bjp.0705666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Bisogno T, Melck D, Ross R, Brockie H, Stevenson L, et al. Interactions between synthetic vanilloids and the endogenous cannabinoid system. FEBS Lett. 1998;436:449–454. doi: 10.1016/s0014-5793(98)01175-2. [DOI] [PubMed] [Google Scholar]
- Domenicali M, Ros J, Fernandez-Varo G, Cejudo-Martin P, Crespo M, Morales-Ruiz M, et al. Increased anandamide induced relaxation in mesenteric arteries of cirrhotic rats: role of cannabinoid and vanilloid receptors. Gut. 2005;54:522–527. doi: 10.1136/gut.2004.051599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Rodriguez CM, Romero J, Petros TJ, Bradshaw H, Gasalla JM, Gutierrez ML, et al. Circulating endogenous cannabinoid anandamide and portal, systemic and renal hemodynamics in cirrhosis. Liver Int. 2004;24:477–483. doi: 10.1111/j.1478-3231.2004.0945.x. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, March JE, Kemp PA, Bennett T. Complex regional haemodynamic effects of anandamide in conscious rats. Br J Pharmacol. 2002;135:1889–1896. doi: 10.1038/sj.bjp.0704649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia N, Jr, Jarai Z, Mirshahi F, Kunos G, Sanyal AJ. Systemic and portal hemodynamic effects of anandamide. Am J Physiol Gastrointest Liver Physiol. 2001;280:G14–G20. doi: 10.1152/ajpgi.2001.280.1.G14. [DOI] [PubMed] [Google Scholar]
- Gaskari SA, Liu H, Moezi L, Li Y, Baik SK, Lee SS. Role of endocannabinoids in the pathogenesis of cirrhotic cardiomyopathy in bile duct-ligated rats. Br J Pharmacol. 2005;146:315–323. doi: 10.1038/sj.bjp.0706331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatley SJ, Lan R, Pyatt B, Gifford AN, Volkow Nd, Makriyannis A. Binding of the non-classical cannabinoid CP 55,940, and the diarylpyrazole AM251 to rodent brain cannabinoid receptors. Life Sci. 1997;61:191–197. doi: 10.1016/s0024-3205(97)00690-5. [DOI] [PubMed] [Google Scholar]
- Gebremedhin D, Lange AR, Campbell WB, Hillard CJ, Harder DR. Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. Am J Physiol. 1999;276:H2085–H2093. doi: 10.1152/ajpheart.1999.276.6.H2085. [DOI] [PubMed] [Google Scholar]
- Hajos N, Freund TF. Pharmacological separation of cannabinoid sensitive receptors on hippocampal excitatory and inhibitory fibers. Neuropharmacol. 2002;43:503–510. doi: 10.1016/s0028-3908(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Henriksen JH, Moller S, Schifter S, Abrahamsen J, Becker U. High arterial compliance in cirrhosis is related to low adrenaline and elevated circulating calcitonin gene related peptide but not to activated vasoconstrictor systems. Gut. 2001;49:112–118. doi: 10.1136/gut.49.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WS, Hiley CR. Vasodilator actions of abnormal-cannabidiol in rat isolated small mesenteric artery. Br J Pharmacol. 2003;138:1320–1332. doi: 10.1038/sj.bjp.0705160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci USA. 1999;23:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kountouras J, Billing BH, Scheuer PJ. Prolonged bile duct obstruction: a new experimental model for cirrhosis in the rat. Br J Exp Pathol. 1984;65:305–311. [PMC free article] [PubMed] [Google Scholar]
- Kunos G, Jarai Z, Batkai S, Goparaju SK, Ishac EJ, Liu J, et al. Endocannabinoids as cardiovascular modulators. Chem Phys Lipids. 2000;108:159–168. doi: 10.1016/s0009-3084(00)00194-8. [DOI] [PubMed] [Google Scholar]
- Kwolek G, Zakrzeska A, Schlicker E, Gothert M, Godlewski G, Malinowska B. Central and peripheral components of the pressor effect of anandamide in urethane-anaesthetized rats. Br J Pharmacol. 2005;145:567–575. doi: 10.1038/sj.bjp.0706195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Lee SS, Sharkey KA. Capsaicin treatment blocks the development of hyperkinetic circulation in portal hypertensive and cirrhotic rats. Am J Physiol Gastrointest Liver Physiol. 1993;264:G868–G873. doi: 10.1152/ajpgi.1993.264.5.G868. [DOI] [PubMed] [Google Scholar]
- Li Y, Song D, Zhang Y, Lee SS. Effect of neonatal capsaicin treatment on haemodynamics and renal function in cirrhotic rats. Gut. 2003;52:293–299. doi: 10.1136/gut.52.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu H, Gaskari SA, Mccafferty DM, Lee SS. Hepatic venous dysregulation contributes to blood volume pooling in cirrhotic rats. Gut. 2006;55:1030–1035. doi: 10.1136/gut.2005.082446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Song D, Lee SS. Role of heme oxygenase-carbon monoxide pathway in pathogenesis of cirrhotic cardiomyopathy in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;280:G68–G74. doi: 10.1152/ajpgi.2001.280.1.G68. [DOI] [PubMed] [Google Scholar]
- Liu J, Gao B, Mirshahi F, Sanyal AJ, Khanolkar AD, Makriyannis A, et al. Functional CB1 cannabinoid receptors in human vascular endothelial cells. Biochem J. 2000;346:835–840. [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Zhang Y, Huet PM, Lee SS. Differential effects of jaundice and cirrhosis on beta-adrenoceptor signaling in three rat models of cirrhotic cardiomyopathy. J Hepatol. 1999;30:485–491. doi: 10.1016/s0168-8278(99)80109-3. [DOI] [PubMed] [Google Scholar]
- Malinowska B, Kwolek G, Gothert M. Anandamide and methanandamide induce both vanilloid VR1- and cannabinoid CB1 receptor-mediated changes in heart rate and blood pressure in anaesthetized rats. Naunyn Schmiedeberg Arch Pharmacol. 2001;364:562–569. doi: 10.1007/s00210-001-0498-6. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Chapnick BM, Howlett AC. Anandamide-induced vasorelaxation in rabbit aortic rings has two components: G protein dependent and independent. Am J Physiol Heart Circ Physiol. 2002;282:H2046–H2054. doi: 10.1152/ajpheart.00497.2001. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G.Haemodynamic profile and responsiveness to anandamide of TRPV1 receptor knock-out mice J Physiol 2004558647–657.(E-pub April 30, 2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall MD, Mcculloch AI, Kendall DA. Comparative pharmacology of endothelium-derived hyperpolarizing factor and anandamide in rat isolated mesentery. Eur J Pharmacol. 1997;333:191–197. doi: 10.1016/s0014-2999(97)01137-0. [DOI] [PubMed] [Google Scholar]
- Randall MD, Kendall DA. Anandamide and endothelium-derived hyperpolarizing factor act via a common vasorelaxant mechanism in rat mesentery. Eur J Pharmacol. 1998;346:51–53. doi: 10.1016/s0014-2999(98)00003-x. [DOI] [PubMed] [Google Scholar]
- Randall MD, Kendall DA, O'sullivan S. The complexities of the cardiovascular actions of cannabinoids. Br J Pharmacol. 2004;142:20–26. doi: 10.1038/sj.bjp.0705725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros J, Claria J, To-Figueras J, Planaguma A, Cejudo-Martin P, Fernandez-Varo G, et al. Endogenous cannabinoids: a new system involved in the homeostasis of arterial pressure in experimental cirrhosis in the rat. Gastroenterology. 2002;122:85–93. doi: 10.1053/gast.2002.30305. [DOI] [PubMed] [Google Scholar]
- Shekhar YC, Anand IS, Sarma R, Ferrari R, Wahi PL, Poole-Wilson PA. Effects of prolonged infusion of human alpha calcitonin gene-related peptide on hemodynamics, renal blood flow and hormone levels in congestive heart failure. Am J Cardiol. 1991;67:732–736. doi: 10.1016/0002-9149(91)90531-o. [DOI] [PubMed] [Google Scholar]
- Song D, Liu H, Sharkey KA, Lee SS. Hyperdynamic circulation in portal hypertensive rats is dependent on central c-fos gene expression. Hepatology. 2002;35:159–166. doi: 10.1053/jhep.2002.30417. [DOI] [PubMed] [Google Scholar]
- Szallasi A. Vanilloid (capsaicin) receptors in health and disease. Am J Clin Pathol. 2002;118:110–121. doi: 10.1309/7AYY-VVH1-GQT5-J4R2. [DOI] [PubMed] [Google Scholar]
- Varga K, Lake KD, Huangfu D, Guyenet PG, Kunos G. Mechanism of the hypotensive action of anandamide in anesthetized rats. Hypertension. 1996;28:682–686. doi: 10.1161/01.hyp.28.4.682. [DOI] [PubMed] [Google Scholar]
- Varga K, Lake K, Martin BR, Kunos G. Novel antagonist implicates the CB1 cannabinoid receptor in the hypotensive action of anandamide. Eur J Pharmacol. 1995;278:279–283. doi: 10.1016/0014-2999(95)00181-j. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Jarai Z, Batkai S, Kunos G. Hemodynamic effects of cannabinoids: coronary and cerebral vasodilation mediated by cannabinoid CB(1) receptors. Eur J Pharmacol. 2001;423:203–210. doi: 10.1016/s0014-2999(01)01112-8. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Varga K, Jarai Z, Kunos G. Mesenteric vasodilation mediated by endothelial anandamide receptors. Hypertension. 1999;33:429–434. doi: 10.1161/01.hyp.33.1.429. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- Wen C, Li M, Whitworth JA. Validation of transonic small animal flowmeter for measurement of cardiac output and regional blood flow in the rat. J Cardiovasc Pharmacol. 1996;27:482–486. doi: 10.1097/00005344-199604000-00005. [DOI] [PubMed] [Google Scholar]
- Wimalawansa SJ. Calcitonin gene-related peptide and its receptors: molecular genetics, physiology, pathophysiology, and therapeutic potentials. Endocr Rev. 1996;17:533–585. doi: 10.1210/edrv-17-5-533. [DOI] [PubMed] [Google Scholar]
- Wong H, Anderson WD, Cheng T, Riabowol KT. Monitoring mRNA expression by polymerase chain reaction: the ‘primer-dropping' method. Anal Biochem. 1994;223:251–258. doi: 10.1006/abio.1994.1581. [DOI] [PubMed] [Google Scholar]
- Yang YY, Lin HC, Huang YT, Lee TY, Hou MC, Wang WY, et al. Roles of anandamide in the hepatic microcirculation in cirrhotic rats. Am J Physiol Gastrointest Liver Physiol. 2006;290:G328–G334. doi: 10.1152/ajpgi.00367.2005. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]