Abstract
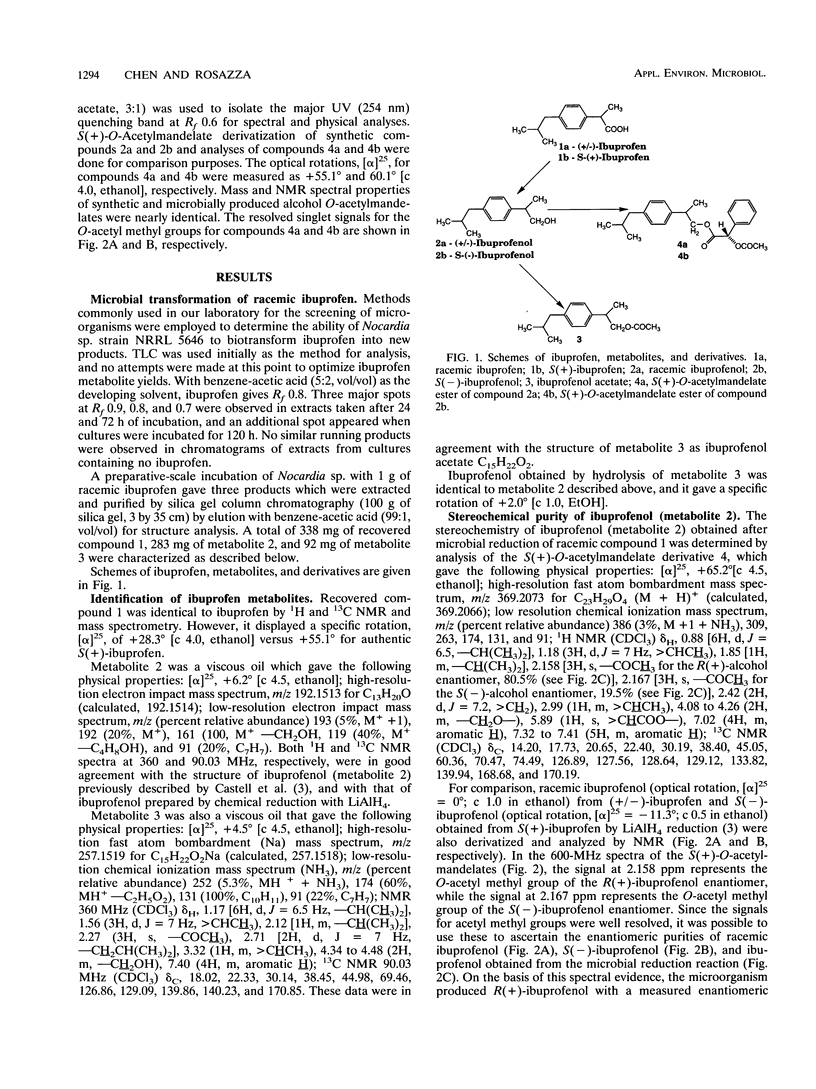
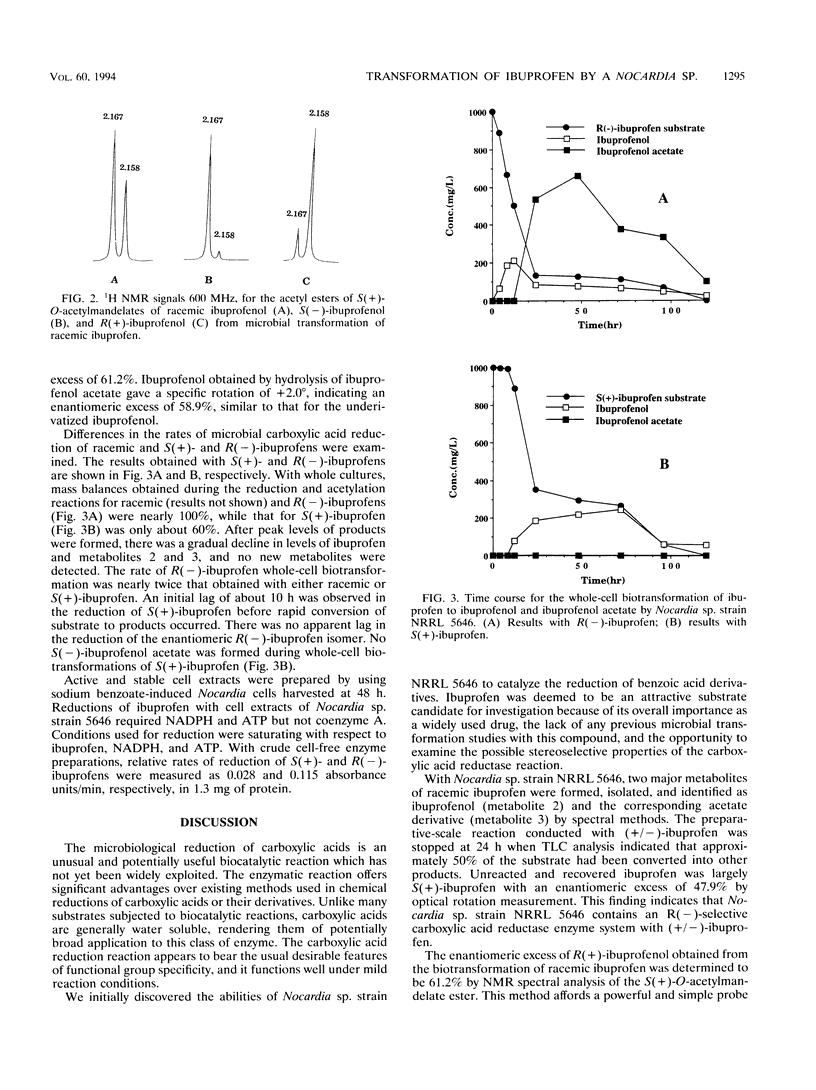
The carboxylic acid functional group of ibuprofen [α-methyl-4-(2-methylpropyl) benzene acetic acid] is reduced to the corresponding alcohol and subsequently esterified to the acetate derivative by cultures of Nocardia species strain NRRL 5646. The alcohol and ester microbial transformation products were isolated, and their structures were determined by 1H and 13C nuclear magnetic resonance spectroscopy and mass spectrometry. By derivatization of synthetic and microbiologically produced ibuprofen alcohols with S(+)-O-acetylmandelic acid, nuclear magnetic resonance analysis indicated that the carboxylic acid reductase of Nocardia sp. is R enantioselective, giving alcohol products with an enantiomeric excess of 61.2%. The R enantioselectivity of the carboxylic acid reductase enzyme system was confirmed by using cell extracts together with ATP and NADPH in the reduction of isomeric ibuprofens.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arfmann H. A., Abraham W. R. Microbial reduction of aromatic carboxylic acids. Z Naturforsch C. 1993 Jan-Feb;48(1-2):52–57. doi: 10.1515/znc-1993-1-210. [DOI] [PubMed] [Google Scholar]
- Betts R. E., Walters D. E., Rosazza J. P. Microbial transformations of antitumor compounds. 1. Conversion of acronycine to 9-hydroxyacronycine by Cunninghamella echinulata. J Med Chem. 1974 Jun;17(6):599–602. doi: 10.1021/jm00252a006. [DOI] [PubMed] [Google Scholar]
- Castell J. V., Gomez M. J., Miranda M. A., Morera I. M. Photolytic degradation of ibuprofen. Toxicity of the isolated photoproducts on fibroblasts and erythrocytes. Photochem Photobiol. 1987 Dec;46(6):991–996. doi: 10.1111/j.1751-1097.1987.tb04882.x. [DOI] [PubMed] [Google Scholar]
- Chen C. S., Chen T., Shieh W. R. Metabolic stereoisomeric inversion of 2-arylpropionic acids. On the mechanism of ibuprofen epimerization in rats. Biochim Biophys Acta. 1990 Jan 29;1033(1):1–6. doi: 10.1016/0304-4165(90)90185-y. [DOI] [PubMed] [Google Scholar]
- Geisslinger G., Stock K. P., Bach G. L., Loew D., Brune K. Pharmacological differences between R(-)- and S(+)-ibuprofen. Agents Actions. 1989 Jun;27(3-4):455–457. doi: 10.1007/BF01972851. [DOI] [PubMed] [Google Scholar]
- Kantor T. G. Ibuprofen. Ann Intern Med. 1979 Dec;91(6):877–882. doi: 10.7326/0003-4819-91-6-877. [DOI] [PubMed] [Google Scholar]
- Simon L. S., Mills J. A. Drug therapy: nonsteroidal antiinflammatory drugs (first of two parts). N Engl J Med. 1980 May 22;302(21):1179–1185. doi: 10.1056/NEJM198005223022105. [DOI] [PubMed] [Google Scholar]
- Ward O. P., Young C. S. Reductive biotransformations of organic compounds by cells or enzymes of yeast. Enzyme Microb Technol. 1990 Jul;12(7):482–493. doi: 10.1016/0141-0229(90)90063-v. [DOI] [PubMed] [Google Scholar]
- el-Sharkawy S. H., Yang W., Dostal L., Rosazza J. P. Microbial oxidation of oleic acid. Appl Environ Microbiol. 1992 Jul;58(7):2116–2122. doi: 10.1128/aem.58.7.2116-2122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


