Abstract
We report here the clinical, genetic and molecular characterization of three Han Chinese pedigrees with maternally transmitted aminoglycoside-induced and nonsyndromic bilateral hearing loss. Clinical evaluation revealed the wide range of severity, age-at-onset and audiometric configuration of hearing impairment in matrilineal relatives in these families. The penetrances of hearing loss in these pedigrees were 28%, 20%, and 15%, with an average of 21%, when aminoglycoside-induced deafness was included. When the effect of aminoglycosides was excluded, the penetrances of hearing loss in these seven pedigrees were 21%, 13% and 8%, with an average of 14%. Sequence analysis of the complete mitochondrial genomes in these pedigrees showed the presence of the deafness-associated 12S rRNA C1494T mutation, in addition to distinct sets of mtDNA polymorphism belonging to Eastern Asian haplogroups F1a1, F1a1 and D5a2, respectively. This suggested that the C1494T mutation occurred sporadically and multiplied through evolution of the mtDNA. The absence of functionally significant mutations in tRNA and rRNAs or secondary LHON mutations in their mtDNA suggest that these mtDNA haplogroup-specific variants may not play an important role in the phenotypic expression of the C1494T mutation in those Chinese families. In addition, the lack of significant mutation in the GJB2 gene ruled out the possible involvement of GJB2 in the phenotypic expression of the C1494T mutation in those affected subjects. However, aminoglycosides and other nuclear modifier genes play a modifying role in the phenotypic manifestation of the C1494T mutation in these Chinese families.
Keywords: hearing loss, 12S rRNA, mitochodnrial DNA, penetrance, mutation, Chinese, aminoglycoside ototoxicity
1. Introduction
Mutations in mitochondrial DNA (mtDNA), especially in the 12S rRNA gene, are one of the important causes of both aminoglycoside induced and nonsyndromic hearing loss (Fischel-Ghodsian 2005; Guan 2005). Of these, the A1555G mutation in the highly conserved A-site of the 12S rRNA has been associated with both aminoglycoside-induced and nonsyndromic hearing loss in many families worldwide (Prezant et al., 1993; Matthijis et al., 1996; Pandya et al., 1997; Usami et al., 1997; Estivill et al., 1998; del Castillo et al., 2003; Li et al. 2004a; 2004b; 2005b;; Young et al., 2005; Yuan et al., 2005; Zhao et al., 2005b; Jacobs et al., 2005). However, the homoplasmic C1494T mutation in the highly conserved decoding site of this rRNA has been associated with both aminoglycoside-induced and non-syndromic hearing loss in only three Chinese families and three Spanish pedigrees (Zhao et al., 2004; Wang et al., 2006; Han et al., 2007; Rodriguez-Ballesteros et al., 2006). Matrilineal relatives within and among families carrying the A1555G or C1494T mutation exhibited a wide range of penetrance, severity and age-of-onset in hearing loss (Estivill et al., 1998; del Castillo et al., 2003; Li et al. 2004b; Young et al., 2005; Yuan et al., 2005; Zhao et al., 2004). Functional characterization of cell lines derived from matrilineal relatives of a large Arab-Israeli family or one large Chinese family demonstrated that the A1555G or C1494T mutation led to mild mitochondrial dysfunction and sensitivity to aminoglycosides (Guan et al., 1996; 2000; 2001; Zhao et al. 2004; 2005a). These findings strongly indicated that the A1555G and C1494T mutations by themselves are insufficient to produce the deafness phenotype. Therefore, other modifier factors including aminoglycosides, nuclear modifier genes and mitochondrial variants/haplotypes modulate the expressivity and penetrance of hearing loss associated with the A1555G or C1494T mutation (Bykhovskaya et al., 1998; Estivill et al., 1998; Fischel-Ghodsian 2005; Guan et al., 1996; 2000; 2001; 2006; Young et al., 2006; Zhao et al., 2004).
To further investigate the molecular mechanism of maternally transmitted hearing loss, we have initiated a systematic and extended mutational screening of the 12S rRNA gene in several cohorts of hearing-impaired subjects (Li et al., 2004a; Li et al. 2005b; Dai et al., 2006; Young et al., 2005; 2006; Zhao et al., 2005b; Tang et al., 2007). In the previous investigation, we showed the highly variable penetrance and expressivity of hearing loss in 36 Han Chinese families carrying the A1555G mutation (Li et al., 2004b; 2005b; Young et al., 2005; 2006; Zhao et al., 2005b; Dai et al., 2005; Yuan et al., 2005; Tang et al. 2007) and three Han Chinese pedigrees carrying the C1494T mutation (Zhao et al. 2004; Wang et al., 2005, Han et al. 2007). Sequence analysis of complete mitochondrial genomes as well as clinical and genetic valuations in these Chinese pedigrees suggested that five mitochondrial tRNA variants: tRNAGlu A14693G, tRNAThr T15908C, tRNAArg T10454C, tRNASer(UCN) G7444A and tRNACys G5821A, may influence the phenotypic manifestation of the A1555G mutation (Yuan et al., 2005; Young et al., 2006; Zhao et al., 2005b), while the tRNAAla T5628C variant may have a modifying role in the phenotypic manifestation of the 12S rRNA C1494T mutation in a large Chinese family with hearing loss (Han et al., 2007).
In the present study, we performed the clinical, molecular and genetic characterization of another three Han Chinese pedigrees carrying the C1494T mutation. A wide range of severity, age-at-onset and penetrances of hearing loss was observed in the matrilineal relatives within and among these three families. To assess the contribution that mtDNA variants make toward the phenotypic expression of the C1494T mutation, we performed PCR-amplification of fragments spanning entire mitochondrial genome and subsequent DNA sequence analysis in the matrilineal relatives of those families. Furthermore, it has been implied that GJB2 mutations modulate the severity of hearing loss associated with the 12S rRNA A1555G mutation (Abe et al. 2001). In fact, the Connexin 26 (GJB2) gene is a potential candidate modifier gene as mutations in this gene are the most common cause of hereditary hearing loss (Friedman and Griffith 2003). To examine the role of the GJB2 gene in the phenotypic expression of the C1494T mutation, we performed a mutational screening of the GJB2 gene in the hearing-impaired and normal hearing subjects of these three families.
2. Subjects and methods
2.1. Subjects and audiological examinations
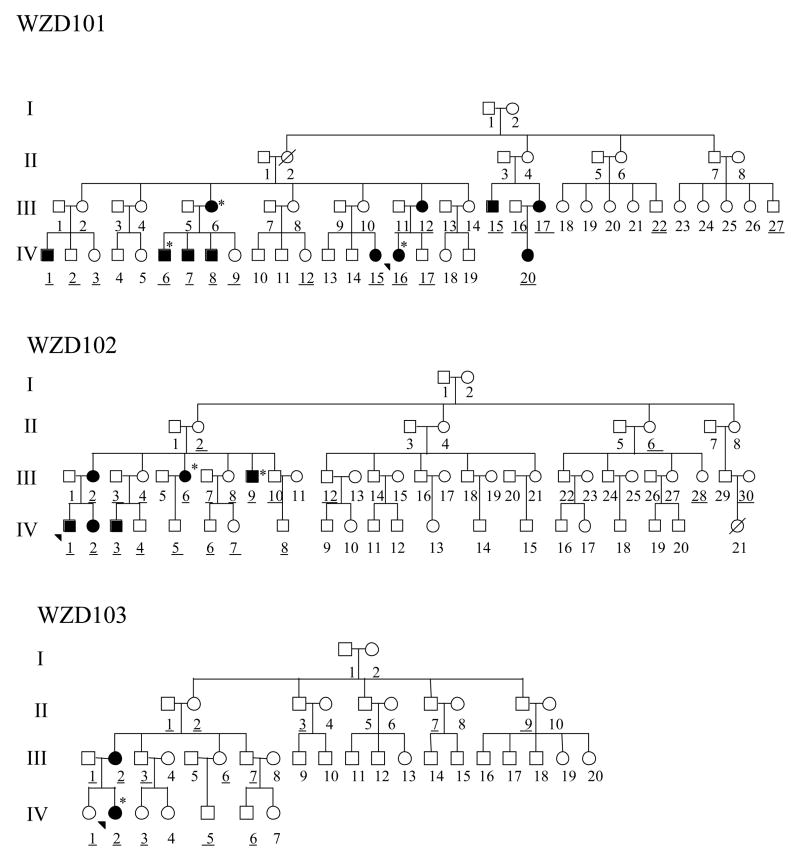
As the part of genetic screening program for hearing impairment, three Han Chinese families, as shown in Figure 1, were ascertained through the Otology Clinic of the First Affiliated Hospital, Wenzhou Medical College, China. A comprehensive history and physical examination were performed to identify any syndromic findings, the history of the use of aminoglycosides, genetic factors related to the hearing impairment in members of this pedigree. An age-appropriate audiological examination was performed and this examination included pure-tone audiometry (PTA) and/or auditory brainstem response (ABR), immittance testing and Distortion product otoacoustic emissions (DPOAE). The PTA was calculated from the sum of the audiometric thresholds at 500, 1000, 2000, 4000 and 8000 Hz. The severity of hearing impairment was classified into five grades: normal <26 Decibel (dB); mild =26–40 dB; moderate=41–70 dB; severe=71–90 dB; and profound >90 dB. Informed consent was obtained from participants prior to their participation in the study, in accordance with the Cincinnati Children’s Hospital Medical Center Institutional Review Board and Ethics Committee of Wenzhou Medical College.
Figure 1.
Three Han Chinese pedigrees with aminoglycoside-induced and nonsyndromic hearing loss. Hearing impaired individuals are indicated by filled symbols. Arrow denotes probands. Asterisks denote individuals who had a history of exposure to aminoglycosides. Subjects used for the genotyping analysis were underlined.
2.2. Mutational analysis of mitochondrial genome
Genomic DNA was isolated from whole blood of participants using Puregene DNA Isolation Kits (Gentra Systems, Minneapolis, MN). Subject’s DNA fragments spanning the entire mitochondrial 12S rRNA gene were amplified by PCR using oligodeoxynucleotides corresponding to positions 618–635 and 1988–2007 (Li et al., 2004b). The entire mitochondrial genomes of three probands and their mothers carrying the C1494T mutation were PCR amplified in 24 overlapping fragments by use of sets of the light-strand and the heavy strand oligonucleotide primers, as described elsewhere (Rieder et al., 1998). Each fragment was purified and subsequently analyzed by direct sequencing in an ABI 3700 automated DNA sequencer using the Big Dye Terminator Cycle sequencing reaction kit. The resultant sequence data were compared with the updated consensus Cambridge sequence (GenBank accession number: NC_001807) (Andrews et al., 1999).
2.3. Mutational analysis of GJB2 gene
The DNA fragments spanning the entire coding region of GJB2 gene were amplified by PCR using the following oligodeoxynucleotides: forward-5′TATGACACTCCCCAGCACAG3′ and reverse-5′GGGCAATGCTTAAACTGGC3′. PCR amplification and subsequent sequencing analysis were performed as detailed elsewhere (Li et al., 2004a). The results were compared with the wild type GJB2 sequence (GenBank accession number: M86849) to identify the mutations.
3. Results
3.1. Clinical and genetic evaluation of three Chinese pedigrees
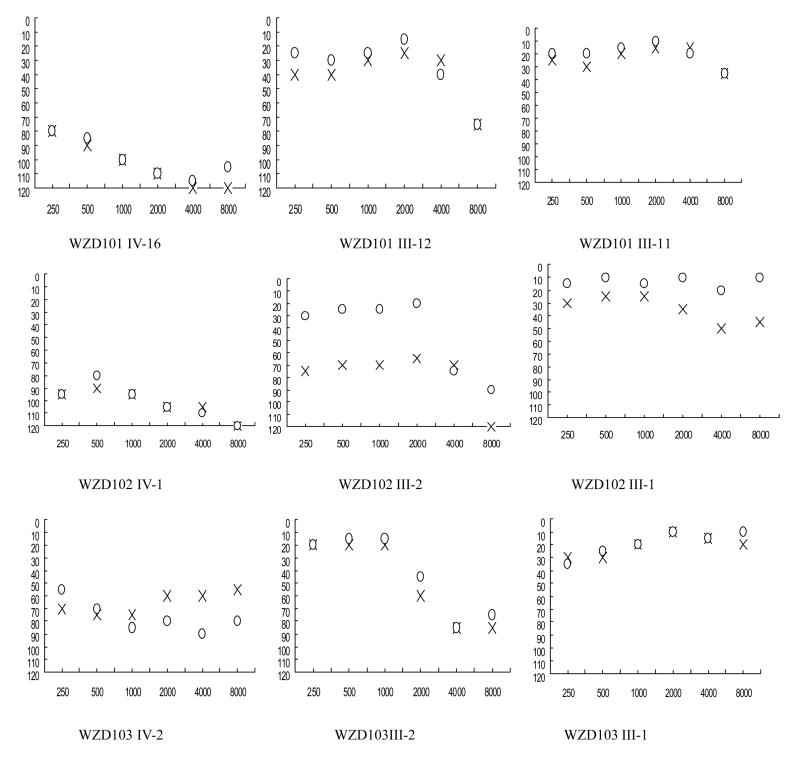
In the family WZD101 from Jiangxi Province in Southeastern China, the proband (IV-16) came to the otology clinic of Wenzhou Medical College at the age of 14 year old. She was treated with gentamycin (3–5 mg/kg/dose every 8 h) for high fever at the age of one year old. She exhibited bilateral hearing impairment one week later after the drug administration. As illustrated in Figure 2, audiological evaluation showed that she had profound hearing loss (103 dB at right ear, 109 dB at left ear, respectively) with a sloped-shaped pattern, her mother (III-12) exhibited a mild hearing loss (36 dB and 40 dB at right and left ears, respectively) and her father (III-11) had normal hearing. Further family history and clinical evaluations showed that one male (IV-6) and his mother (III-6), who received a treatment of gentamycin for illness, exhibited profound bilateral hearing loss after a treatment of this antibiotic. In the absence of aminoglycosides, seven of other 36 matrilineal relatives had a variable severity of hearing impairment in this maternal kindred, ranging from profound hearing impairment (IV-1, IV-7, IV-8, IV-20), severe hearing impairment (III-15, III-17), to moderate hearing impairment (IV-15). In addition, parental relative (III-5) exhibited a mild hearing loss (24 dB at right ear, 45 dB at left ear). All affected individuals displayed the loss of the high frequencies and their hearing impairments were symmetric. However, the other members of this family had normal hearing.
Figure 2.
Air conduction audiogram of six affected subjects with the C1494T mutation and three married-in Chinese controls. Symbols: X-left, O-right ear.
In the family WZD102, the proband IV-1, was a 19 year old man. He suffered bilateral hearing impairment after his birth. As shown in Figure 2, audiological evaluation showed that he had profound hearing loss (100 dB at both right and left ears; a flatted-shaped pattern). This family originated from Jiangxi Province. Of other family members, five (three females and two males) of other 29 matrilineal relatives exhibited hearing deficit, whereas other family members have normal hearing. Of these, two affected subjects (III-6 and III-9), exhibiting profound hearing loss, had a history of exposure to aminoglycosides. In the absence of these drugs, their mother (III-2) had moderate hearing loss, his sister (IV-2) exhibited severe hearing loss, and his cousin (IV-3) suffered from moderate hearing loss, while other members of this family showed normal hearing.
The proband IV-2 of WZD103 family from Zhejiang Province, Eastern China, as shown in Figure 2, was diagnosed as severe hearing loss (81dB and 65 dB at right and left ears, respectively; a flat-shaped pattern) by the Otology Clinic of Wenzhou Medical College at the age of 12 year old. She was treated with gentamycin (3–5 mg/kg/dose every 8 h) for high fever at the age of 2 and half year old. She experienced bilateral hearing impairment one month after the drug administration. Only her mother (III-2) had moderate hearing loss, while of other 11 matrilineal relatives and other members in this family exhibited normal hearing.
There is no evidence that any member of these families had any other known cause to account for hearing impairment. A comprehensive history and physical examination as well as audiological examination were performed to identify any syndromic findings, the history of the use of aminoglycosides, genetic factors related to the hearing impairment in all available members of three Han Chinese pedigrees. In fact, comprehensive family medical histories of those probands and other members of these Chinese families showed no other clinical abnormalities, including diabetes, muscular diseases, visual loss, and neurological disorders.
3.2. Mutational analysis of mitochondrial genome
The maternal transmission of aminoglycoside-induced and nonsyndromic hearing loss in these families suggested the mitochondrial involvement and led us to analyze the mitochondrial genome of matrilineal relatives. First, DNA fragments spanning mitochondrial 12S rRNA gene were PCR amplified from each proband. Each fragment was purified and subsequently analyzed by DNA sequencing. We failed to detect mutations at positions 961, 1095 and 1555 in the 12S rRNA gene. However, the C1494T mutation in the same gene was found in these subjects. The subsequent DNA sequence analysis of these available members of these pedigrees indicated that the C1494T mutation was indeed present in homoplasmy in matrilineal relatives but not other members of these families (data not shown).
To assess the role of mtDNA variants in the phenotypic expression of the C1494T mutation, we performed a PCR-amplification of fragments spanning entire mitochondrial genome and subsequent DNA sequence analysis in three probands and their mothers. In addition to the identical C1494T mutation, as shown in Table 1, these subjects exhibited distinct sets of mtDNA polymorphism. Of other nucleotide changes in these mitochondrial genomes, there are 24 (1 novel and 23 known) variants in the D-loop, three known variants in 12S rRNA gene, one known variant in the 16S rRNA gene, one known variant in the tRNAThr gene, 24 known and 1 novel silent variants in the protein encoding genes as well as 13 known missense mutations in the protein encoding genes (Brandon et al., 2005). These missense mutations are the C5178A (L237M) and A5301G (I278V) in the ND2 gene, the A8701G (T59A), A8860G (T112A) and G9053A (S176D) in A6 gene, the A10398G (T114A) in the ND3 gene, the T10609C (M47T) in the ND4L gene, the A12026G (I503V) in the ND4 gene, the G12406A (V24A), G13759A (A475T) and G13928C (S531T) in the ND5 gene, the A15326G (T194A) and G15884A (A380T) in the cyto b gene. These variants in RNAs and polypeptides were further evaluated by phylogenetic analysis of these variants and sequences from other organisms including mouse (Bibb et al., 1981), bovine (Gadaleta et al., 1989), and Xenopus laevis (Roe et al., 1985). However, none of these variants showed evolutionary conservation and the implication of functional significance.
Table 1.
mtDNA mutations in three Chinese families with hearing impairment
| Gene | position | Replacement | Conservation (H/B/M/X)a | CRSb | WZD101 | WZD102 | WZD103 | Previously reportedc,d |
|---|---|---|---|---|---|---|---|---|
| D-Loop | 73 | A to G | A | G | G | G | Yes | |
| 150 | C to T | C | T | Yes | ||||
| 249 | del A | A | del A | del A | Yes | |||
| 263 | A to G | A | G | G | G | Yes | ||
| 310 | T-CT, CTC or TC | T | TC | TC | CTC | Yes | ||
| 489 | T to C | T | C | Yes | ||||
| 514 | del C | C | del C | Yes | ||||
| 515 | del A | A | del A | Yes | ||||
| 523 | del A | A | del A | del A | Yes | |||
| 524 | del C | C | del C | del C | No | |||
| 16092 | T to C | T | C | Yes | ||||
| 16129 | G to A | G | A | A | Yes | |||
| 16161 | T to C | T | C | Yes | ||||
| 16162 | A to G | A | G | G | Yes | |||
| 16164 | A to G | A | G | Yes | ||||
| 16172 | T to C | T | C | C | Yes | |||
| 16182 | A to C | A | C | Yes | ||||
| 16183 | A to C | A | C | Yes | ||||
| 16189 | T to C | T | C | Yes | ||||
| 16223 | C to T | C | T | Yes | ||||
| 16266 | C to T | C | T | Yes | ||||
| 16304 | T to C | T | C | C | Yes | |||
| 16362 | T to C | T | C | Yes | ||||
| 16519 | T to C | T | C | C | Yes | |||
| 12S rRNA | 752 | C to T | C/C/A/- | C | T | Yes | ||
| 1107 | T to C | T | C | Yes | ||||
| 1438 | A to G | A/A/A/G | A | G | G | G | Yes | |
| 1494 | C to T | C/C/C/C | C | T | T | T | Yes | |
| 16S rRNA | 2706 | A to G | A/G/A/A | A | G | G | G | Yes |
| ND1 | 3970 | C to T | C | T | T | Yes | ||
| 4086 | C to T | C | T | T | Yes | |||
| ND2 | 4769 | A to G | A | G | G | G | Yes | |
| 4883 | C to T | C | T | Yes | ||||
| 4985 | G to A | G | A | A | A | Yes | ||
| 5178 | C to T (Leu to Met) | L/T/T/T | C | T | Yes | |||
| 5301 | A to G (Ile to Val) | I/I/M/L | A | G | Yes | |||
| CO1 | 6392 | T to C | T | C | C | Yes | ||
| 6962 | G to A | G | A | A | Yes | |||
| 7028 | C to T | C | T | T | T | Yes | ||
| ATPase6 | 8701 | A to G (Thr to Ala) | T/S/L/Q | A | G | Yes | ||
| 8860 | A to G (Thr to Ala) | T/A/A/T | A | G | G | G | Yes | |
| 9053 | G to A (Ser to Asp) | S/G/G/T | G | A | A | Yes | ||
| 9180 | A to G | A | G | Yes | ||||
| CO3 | 9540 | T to C | T | C | Yes | |||
| 9548 | G to A | G | A | A | Yes | |||
| ND3 | 10310 | G to A | G | A | A | Yes | ||
| 10397 | A to G | A | G | Yes | ||||
| 10398 | A to G (Thr to Ala) | T/T/T/A | A | G | Yes | |||
| 10400 | C to T | C | T | Yes | ||||
| ND4L | 10609 | T to C (Met to Thr) | M/T/T/T | T | C | C | Yes | |
| ND4 | 10873 | T to C | T | C | Yes | |||
| 11176 | G to A | G | A | A | yes | |||
| 11719 | G to A | G | A | A | A | Yes | ||
| 11944 | T to C | T | C | Yes | ||||
| 12026 | A to G (Ile to Val) | I/I/M/L | A | G | Yes | |||
| ND5 | 12406 | G to A (Val to Ile) | V/F/S/F | G | A | A | Yes | |
| 12561 | G to A | G | A | A | No | |||
| 12705 | C to T | C | T | Yes | ||||
| 12882 | C to T | C | T | Yes | ||||
| 13759 | G to A (Ala to Thr) | A/T/T/T | G | A | A | Yes | ||
| 13928 | G to C (Ser to Thr) | S/T/S/T | G | C | C | Yes | ||
| Cyt b | 14783 | T to C | T | C | Yes | |||
| 15043 | G to A | G | A | Yes | ||||
| 15301 | G to A | G | A | Yes | ||||
| 15326 | A to G (Thr to Ala) | T/M/I/I | A | G | G | G | Yes | |
| 15749 | C to A | C | A | No | ||||
| 15884 | G to A (Aa to Thr) | A/W/Y/W | G | A | A | Yes | ||
| tRNAThr | 15891 | C to G | C/T/T/C | C | G | Yes |
Conservation of amino acid for polypepides or nucleotide for RNAs in human (H), bovine (B), mouse (M), and Xenopus laevis (X);
CRS: Cambridge reference sequence (29);
See the online mitochondrial genome database http://www.mitomap.org.
3.4. Mutational analysis of GJB2
To examine the role of GJB2 gene in phenotypic expression of the C1494T mutation, we performed the mutational screening of GJB2 gene in 16 symptomatic and 10 asymptomatic C1494T carriers of these three Chinese pedigrees. None of variants in GJB2 gene was found in these affected matrilineal relatives of these Chinese pedigrees. Indeed, the absence of variant in the GJB2 gene in those subjects with hearing impairment indicates that the GJB2 gene may not be a modifier of the phenotypic effects of the C1494T mutation in those subjects.
4. Discussion
In the present study, we have performed the clinical, genetic and molecular characterization of three Chinese pedigree with aminoglycoside-induced and nonsyndromic hearing impairment. Hearing impairment as a sole clinical phenotype was mostly present in the maternal lineage of those pedigrees, suggesting that the mtDNA mutation is the molecular basis for this disorder. In fact, the C1494T mutation was identified to be homoplasmy in matrilineal relatives of these Chinese families. This mutation was first identified in a large Chinese family with maternally inherited aminoglycoside-induced and nonsyndromic hearing impairment (Zhao et al., 2004) and subsequently in other two Chinese pedigrees (Wang et al., 2006; Han et al., 2007) and three Spanish families (Rodriguez-Ballesteros et al., 2006). As shown in Table 2, the penetrance of hearing loss (affected matrilineal relatives/total matrilineal relatives) in WZD101, WZD102 and WZD103 pedigrees exhibited 28%, 20%, and 15% respectively, while the penetrances of hearing loss in BJ1, BJ2 and BJ3 were 51%, 78%, and 23% (Zhao et al., 2004; Wang et al., 2006; Han et al., 2007), when aminoglycoside-induced deafness was included. When the effect of aminoglycosides was excluded, the penetrances of hearing loss in WZD101, WZD102 and WZD103 pedigrees were 21%, 13% and 8%, respectively, whereas the penetrances of hearing loss in BJ1, BJ2 and BJ3 pedigrees were 31%, 22%, and 20% (Zhao et al., 2004; Wang et al., 2006; Han et al., 2007), respectively. This suggested that the average penetrance of hearing loss in the absence of aminoglycosides is approximately 19% in these Chinese pedigrees carrying the C1494T mutation. However, three Spanish pedigrees exhibited higher penetrance of hearing loss than these Chinese pedigrees (Rodriguez-Ballesteros et al., 2006). Furthermore, all affected matrilineal relatives in these Chinese families exhibited the variable severity, age-at-onset and audiometric configuration of hearing impairment, although these subjects shared some common features: being bilateral and sensorineural hearing loss of high frequencies.
Table 2.
Summary of clinical and molecular data for six Chinese families carrying the C1494T mutation
| Pedigree | Number of matrilineal relatives | aPenetrance (including the use of drugs) (%) | Penetrance (excluding the use of drugs) (%) | mtDNA haplogroup |
|---|---|---|---|---|
| WZD101 | 39 | 28.2 | 20.5 | F1a1 |
| WZD102 | 30 | 20 | 13.3 | F1a1 |
| WZD103 | 13 | 15.4 | 7.7 | D5a2a |
| bBJ1 | 40 | 51 | 31 | D |
| cBJ2 | 9 | 77.8 | 22.2 | R |
| dBJ3 | 65 | 23.1 | 20 | F1 |
affected matrilineal relatives/total affected matrilineal relatives
Our previous investigations showed that the C1494T mutation led to mild mitochondrial dysfunction and sensitivity to aminoglycosides (Zhao et al. 2004; 2005a), indicating that the C1494T mutation itself is insufficient to produce the deafness phenotype. Thus, other modifier factors including nuclear modifier genes, aminoglycosides, and mitochondrial variants/haplotypes contribute to various penetrances and expressivities of hearing loss among families carrying the C1494T mutation. The observation that six matrilineal relatives of these pedigrees suffered from aminoglycoside-induced hearing loss indicated that the aminoglycosides indeed contributed to the deafness expression of the C1494T mutation. The phenotypic variability of matrilineal relatives from within and among families including the variable severity and audiometric configuration of hearing impairment suggests a role of nuclear modifier genes in the phenotypic manifestation of the C1494T mutation as described other pedigrees carrying the A1555G mutation (Guan et al., 1996; 2001; Guan et al., 2006). The lack of significant mutation in the GJB2 gene ruled out the possible involvement of GJB2 in the phenotypic expression of the C1494T mutation in those affected subjects, as in the cases of mtDNA T7511C mutation in a Japanese pedigree (Li et al., 2005a) and other Chinese pedigrees carrying the A1555G mutation (Young et al., 2006; Tang et al. 2007). However, other nuclear modifier genes may modulate the phenotypic variability of the C1494T mutation in these families.
In addition, the mtDNA variants have been shown to modulate the phenotypic manifestation of the deafness-associated mtDNA mutations. The secondary LHON-associated T4216C and G13708A mutations may increase the penetrance of vision loss associated with ND4 G11778A mutation (Torroni et al., 1997) or hearing loss associated with the tRNASer(UCN) A7445G mutation (Guan et al., 1998), while the ND1 T3308C and tRNAAla T5655C mutations likely caused higher penetrance of hearing loss in an African pedigree than Japanese and French families carrying the T7511C mutation (Li et al., 2004c; Li et al., 2005a). Most recently, mtDNA variants tRNAGlu A14693G, tRNAThr T15908C, tRNAArg T10454C, tRNASer(UCN) G7444A and tRNACys G5821A may contribute to higher penetrance of hearing loss in five Han Chinese pedigrees carrying the A1555G mutation (Young et al., 2006; Zhao et al., 2005b; Yuan et al., 2005), while the tRNAAla T5628C variant may have a modifying role in the phenotypic manifestation of the C1494T mutation in a large Chinese family (Han et al., 2007). Here, there were the distinct sets of sequence variations in their mitochondrial genomes of these Chinese pedigrees. As shown in Table 2, their mtDNAs belong to Eastern Asian haplogroups F1a1, F1a1 and D5a2 (Tanaka et al., 2004), respectively, while other three Chinese mitochondrial genomes harboring the C1494T mutation belong to haplogroups D, R, and F1 (Zhao et al., 2004; Wang et al., 2006; Han et al., 2007) and mtDNAs from three Spanish families carrying the same C1494T mutation belong to haplogroups H, U5b and U6a (Rodriguez-Ballesteros et al., 2006), respectively. This suggests that the C1494T mutation, similar to the A1555G mutation (Torroni et al., 1999; Abe et al., 1998; Tang et al., 2007), occurred sporadically and multiplied through evolution of the mtDNA. Despite the presence of distinct sets of mtDNA variants, there was the absence of functionally significant mutations in tRNA and rRNAs or secondary LHON mutations in these three Chinese families, similar to the previously identified 11 Chinese pedigrees carrying the A1555G mutation (Young et al., 2005; Tang et al., 2007). These data suggest that these mtDNA haplogroup-specific variants may not play an important role in the deafness expression of the C1494T mutation in those Chinese families.
Acknowledgments
This work was supported by Public Health Service grants RO1DC05230 and RO1DC07696 from the National Institute on Deafness and Other Communication Disorders, and RO1NS44015 from the National Institute of Neurological Disorders and Stroke and grants from National Basic Research Priorities Program of China 2004CCA02200, Ministry of Public Heath of Zhejiang Province 2006A100 and Ministry of Science and Technology of Zhejiang Province 2007G50G2090026 to M.X.G and a grant from Science and Technology bureau of Wenzhou City Y2006A031 Z. L.
Abbreviations
- mtDNA
mitochondrial DNA
- dB
decibel
- LHON
Leber’s hereditary optic neuropathy
- PCR
Polymerase Chain Reaction
Footnotes
The first five authors had equally contributed to this work
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe S, Usami S, Shinkawa H, Weston MD, Overbeck LD, Hoover DM, Kenyon JB, Horai S, Kimberling WJ. Phylogenetic analysis of mitochondrial DNA in Japanese pedigrees of sensorineural hearing loss associated with the A1555G mutation. Eur J Hum Genet. 1998;6:563–569. doi: 10.1038/sj.ejhg.5200239. [DOI] [PubMed] [Google Scholar]
- Abe S, Kelley PM, Kimberling WJ, Usami SI. Connexin 26 gene (GJB2) mutation modulates the severity of hearing loss associated with the 1555A-->G mitochondrial mutation. Am J Med Genet. 2001;103:334–338. [PubMed] [Google Scholar]
- Andrews RM, Kubacka I, Chinnery PF, Lightowlers RN, Turnbull DM, Howell N. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat Genet. 1999;23:147. doi: 10.1038/13779. [DOI] [PubMed] [Google Scholar]
- Bibb MJ, Van Etten RA, Wright CT, Walberg MW, Clayton DA. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981;26:167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Brandon MC, Lott MT, Nguyen KC, Spolim S, Navathe SB, Baldi P, Wallace DC. MITOMAP: a human mitochondrial genome database--2004 update. Nucleic Acids Res. 2005;33:D611–613. doi: 10.1093/nar/gki079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bykhovskaya Y, Shohat M, Ehrenman K, Johnson D, Hamon M, Cantor RM, Aouizerat B, Bu X, Rotter JI, Jaber L, Fischel-Ghodsian N. Evidence for complex nuclear inheritance in a pedigree with nonsyndromic deafness due to a homoplasmic mitochondrial mutation. Am J Med Genet. 1998;77:421–426. doi: 10.1002/(sici)1096-8628(19980605)77:5<421::aid-ajmg13>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- del Castillo FJ, Rodriguez-Ballesteros M, Martin Y, Arellano B, Gallo-Teran J, Morales-Angulo C, Ramirez-Camacho R, Cruz Tapia M, Solanellas J, Martinez-Conde A, Villamar M, Moreno-Pelayo MA, Moreno F, del Castillo I. Heteroplasmy for the 1555A>G mutation in the mitochondrial 12S rRNA gene in six Spanish families with non-syndromic hearing loss. J Med Genet. 2003;40:632–636. doi: 10.1136/jmg.40.8.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai P, et al. Extremely low penetrance of deafness associated with the mitochondrial 12S rRNA mutation in 16 Chinese families: implication for early detection and prevention of deafness. Biochem Biophys Res Commun. 2006;340:194–199. doi: 10.1016/j.bbrc.2005.11.156. [DOI] [PubMed] [Google Scholar]
- Estivill X, Govea N, Barcelo E, Badenas C, Romero E, Moral L, Scozzari R, D’Urbano L, Zeviani M, Torroni A. Familial progressive sensorineural deafness is mainly due to the mtDNA A1555G mutation and is enhanced by treatment with aminoglycosides. Am J Hum Genet. 1998;62:27–35. doi: 10.1086/301676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischel-Ghodsian N. Genetic factors in aminoglycoside toxicity. Pharmacogenomics. 2005;6:27–36. doi: 10.1517/14622416.6.1.27. [DOI] [PubMed] [Google Scholar]
- Friedman TB, Griffith AJ. Human nonsyndromic sensorineural deafness. Annu Rev Genomics Hum Genet. 2003;4:341–402. doi: 10.1146/annurev.genom.4.070802.110347. [DOI] [PubMed] [Google Scholar]
- Gadaleta G, Pepe G, De Candia G, Quagliariello C, Sbisa E, Saccone C. The complete nucleotide sequence of the Rattus norvegicus mitochondrial genome: cryptic signals revealed by comparative analysis between vertebrates. J Mol Evol. 1989;28:497–516. doi: 10.1007/BF02602930. [DOI] [PubMed] [Google Scholar]
- Guan MX. Prevalence of mitochondrial 12S rRNA mutations associated with aminoglycoside ototoxicity. The Voltra Review. 2005;105:211–237. doi: 10.1016/j.mito.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Guan MX, Fischel-Ghodsian N, Attardi G. Biochemical evidence for nuclear gene involvement in phenotype of non-syndromic deafness associated with mitochondrial 12S rRNA mutation. Hum Mol Genet. 1996;5:963–997. doi: 10.1093/hmg/5.7.963. [DOI] [PubMed] [Google Scholar]
- Guan MX, Enriquez JA, Fischel-Ghodsian N, Puranam R, Lin CP, Marion MA, Attardi G. The Deafness-associated mtDNA 7445 mutation, which affects tRNASer(UCN) precursor processing, has long-range effects on NADH dehydrogenase ND6 subunit gene expression. Mol Cell Biol. 1998;18:5868–5879. doi: 10.1128/mcb.18.10.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan MX, Fischel-Ghodsian N, Attardi G. A biochemical basis for the inherited susceptibility to aminoglycoside ototoxicity. Hum Mol Genet. 2000;9:1787–1793. doi: 10.1093/hmg/9.12.1787. [DOI] [PubMed] [Google Scholar]
- Guan MX, Fischel-Ghodsian N, Attardi G. Nuclear background determines biochemical phenotype in the deafness-associated mitochondrial 12S rRNA mutation. Hum Mol Genet. 2001;10:573–580. doi: 10.1093/hmg/10.6.573. [DOI] [PubMed] [Google Scholar]
- Guan MX, et al. Mutation in TRMU related to transfer RNA modification modulates the phenotypic expression of the deafness-associated mitochondrial 12S ribosomal RNA mutations. Am J Hum Genet. 2006;79:291–302. doi: 10.1086/506389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, et al. The mitochondrial tRNAAla T5628C variant may have a modifying role in the phenotypic manifestation of the 12S rRNA C1494T mutation in a large Chinese family with hearing loss. Biochem Biophys Res Commun. 2007;357:554–560. doi: 10.1016/j.bbrc.2007.03.199. [DOI] [PubMed] [Google Scholar]
- Jacobs HT, et al. Mitochondrial DNA mutations in patients with postlingual, nonsyndromic hearing impairment. Eur J Hum Genet. 2005;13:26–33. doi: 10.1038/sj.ejhg.5201250. [DOI] [PubMed] [Google Scholar]
- Li R, Greinwald JH, Yang L, Choo DI, Wenstrup RJ, Guan MX. Molecular analysis of mitochondrial 12S rRNA and tRNASer(UCN) genes in paediatric subjects with nonsyndromic hearing loss. J Med Genet 2004. 2004a;41:615–620. doi: 10.1136/jmg.2004.020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Xing G, Yan M, Cao X, Liu XZ, Bu X, Guan MX. Cosegregation of C-insertion at position 961 with A1555G mutation of mitochondrial 12S rRNA gene in a large Chinese family with maternally inherited hearing loss. Am J Med Genet. 2004b;124A:113–117. doi: 10.1002/ajmg.a.20305. [DOI] [PubMed] [Google Scholar]
- Li X, Fischel-Ghodsian N, Schwartz F, Yan Q, Friedman RA, Guan MX. Biochemical characterization of the mitochondrial tRNASer(UCN) T7511C mutation associated with nonsyndromic deafness. Nucleic Acids Res. 2004c;32:867–877. doi: 10.1093/nar/gkh226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Ishikawa K, Deng JH, Yang L, Tamagawa Y, Bai Y, Ichimura K, Guan MX. Maternally inherited nonsyndromic hearing loss is associated with the mitochondrial tRNASer(UCN) 7511C mutation in a Japanese family. Biochem Biophys Res Commun. 2005a;328:32–37. doi: 10.1016/j.bbrc.2004.12.140. [DOI] [PubMed] [Google Scholar]
- Li Z, Li R, Chen J, Liao Z, Zhu Y, Qian Y, Xiong S, Heman-Ackah S, Wu J, Choo DI, Guan MX. Mutational analysis of the mitochondrial 12S rRNA gene in Chinese pediatric subjects with aminoglycoside induced and non-syndromic hearing loss. Hum Genet. 2005b;117:9–15. doi: 10.1007/s00439-005-1276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijs G, Claes S, Longo-Bbenza B, Cassiman J-J. Non-syndromic deafness associated with a mutation and a polymorphism in the mitochondrial 12S ribosomal RNA gene in a large Zairean pedigree. Eur J Hum Genet. 1996;4:46–51. doi: 10.1159/000472169. [DOI] [PubMed] [Google Scholar]
- Pandya A, Xia X, Radnaabazar J, Batsuuri J, Dangaansuren B, Fischel-Ghodsian N, Nance WE. Mutation in the mitochondrial 12S ribosomal-RNA gene in 2 families from Mongolia with matrilineal aminoglycoside ototoxicity. J Med Genet. 1997;34:169–172. doi: 10.1136/jmg.34.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prezant TR, Agapian JV, Bohlman MC, Bu X, Oztas S, Qiu WQ, Arnos KS, Cortopassi GA, Jaber L, Rotter JI, Shohat M, Fischel-Ghodsian N. Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nat Genet. 1993;4:289–294. doi: 10.1038/ng0793-289. [DOI] [PubMed] [Google Scholar]
- Rieder MJ, Taylor SL, Tobe VO, Nickerson DA. Automating the identification of DNA variations using quality-based fluorescence re-sequencing: analysis of the human mitochondrial genome. Nucleic Acids Res. 1998;26:967–973. doi: 10.1093/nar/26.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe A, Ma DP, Wilson RK, Wong JF. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J Biol Chem. 1985;260:9759–9774. [PubMed] [Google Scholar]
- Rodriguez-Ballesteros M, Olarte M, Aguirre LA, Galan F, Galan R, Vallejo LA, Navas C, Villamar M, Moreno-Pelayo MA, Moreno F, del Castillo I. Molecular and clinical characterisation of three Spanish families with maternally inherited non-syndromic hearing loss caused by the 1494C->T mutation in the mitochondrial 12S rRNA gene. J Med Genet. 2006;43:e54. doi: 10.1136/jmg.2006.042440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, et al. Mitochondrial genome variation in eastern Asia and the peopling of Japan. Genome Res. 2004;14:1832–1850. doi: 10.1101/gr.2286304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, et al. Very low penetrance of hearing loss in seven Han Chinese pedigrees carrying the deafness-associated 12S rRNA A1555G mutation. Gene. 2007;293:11–19. doi: 10.1016/j.gene.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Torroni A, Petrozzi M, D’Urbano L, Sellitto D, Zeviani M, Carrara F, Carducci C, Leuzzi V, Carelli V, Barboni P, De Negri A, Scozzari R. Haplotype and phylogenetic analyses suggest that one European-specific mtDNA background plays a role in the expression of Leber hereditary optic neuropathy by increasing the penetrance of the primary mutations 11778 and 14484. Am J Hum Genet. 1997;60:1107–1121. [PMC free article] [PubMed] [Google Scholar]
- Torroni A, et al. The A1555G mutation in the 12S rRNA gene of human mtDNA: recurrent origins and founder events in families affected by sensorineural deafness. Am J Hum Genet. 1999;65:1349–1358. doi: 10.1086/302642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usami SI, Abe S, Kasai M, Shinkawa H, Moeller B, Kenyon JB, Kimberling WJ. Genetic and clinical features of sensorineural hearing loss associated with the 1555 mitochondrial mutation. Laryngoscope. 1997;107:483–490. doi: 10.1097/00005537-199704000-00011. [DOI] [PubMed] [Google Scholar]
- Wang Q, et al. Clinical and molecular analysis of a four-generation Chinese family with aminoglycoside-induced and nonsyndromic hearing loss associated with the mitochondrial 12S rRNA C1494T mutation. Biochem Biophys Res Commun. 2006;340:583–588. doi: 10.1016/j.bbrc.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Young WY, Zhao L, Qian Y, Wang Q, Li N, Greinwald JH, Jr, Guan MX. Extremely low penetrance of hearing loss in four Chinese families with the mitochondrial 12S rRNA A1555G mutation. Biochem Biophys Res Commun. 2005;328:1244–1251. doi: 10.1016/j.bbrc.2005.01.085. [DOI] [PubMed] [Google Scholar]
- Young WY, Zhao L, Qian Y, Li R, Chen J, Yuan H, Dai P, Zhai S, Han D, Guan MX. Variants in mitochondrial tRNAGlu, tRNAArg and tRNAThr may influence the phenotypic manifestation of deafness-associated 12S rRNA A1555G mutation in three Chinese families with hearing loss. Am J Med Genet. 2006;140:2188–2197. doi: 10.1002/ajmg.a.31434. [DOI] [PubMed] [Google Scholar]
- Yuan H, et al. Cosegregation of the G7444A mutation in the mitochondrial COI/tRNASer(UCN) genes with the 12S rRNA A1555G mutation in a Chinese family with aminoglycoside-induced and non-syndromic hearing loss. Am J Med Genet. 2005;138A:133–140. doi: 10.1002/ajmg.a.30952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Li R, Wang Q, Yan Q, Deng JH, Han D, Bai Y, Young WY, Guan MX. Maternally inherited aminoglycoside-induced and non-syndromic deafness is associated with the novel C1494T mutation in the mitochondrial 12S rRNA gene in a large Chinese family. Am J Hum Genet. 2004;74:139–152. doi: 10.1086/381133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Young WY, Yan Q, Li R, Cao J, Wang Q, Li X, Peters JL, Han D, Guan MX. Functional characterization of the mitochondrial 12S rRNA C1494T mutation associated with aminoglycoside-induced and nonsyndromic hearing loss. Nucleic Acid Res. 2005a;33:1132–1139. doi: 10.1093/nar/gki262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Wang Q, Qian Y, Li R, Cao J, Hart LC, Zhai S, Han D, Young WY, Guan MX. Clinical evaluation and mitochondrial genome sequence analysis of two Chinese families with aminoglycoside-induced and nonsyndromic hearing loss. Biochem Biophys Res Commun. 2005b;336:967–973. doi: 10.1016/j.bbrc.2005.08.199. [DOI] [PubMed] [Google Scholar]