Abstract
It has been postulated that infections, inflammatory processes and resulting cytokines may be causative factors in emotional disorders, including depression and anxiety. Support for this possibility has been sought in studies of animal behavior following administration of interleukin-1 (IL-1) and lipopolysaccharide (LPS). However, such treatments induce a variety of behavioral responses, collectively known as sickness behavior, some of which could affect the performance in tests used to assess anxiety and depression. Thus the effects of peripheral administration of IL-1β and LPS on the behavior of mice were studied in the elevated plus-maze (EPM) and the open field (OF). Mouse IL-1β (30, 100, 300, and 1000 ng) was injected intraperitoneally 30 or 60 min, and LPS (0.5, 1 and 5 μg) 120 minutes before the tests.
IL-1β and LPS induced dose-dependent decreases in open arm entries and the time spent on the open arms in the EPM, effects considered to reflect anxiety-like behavior. However, entries to all arms were also reduced in a dose-dependent manner, indicating a decrease in general activity. In the OF, IL-1β and LPS decreased the number of line-crossings in the center of the field, that can also be considered to reflect anxiety-like behavior. However, this effect was accompanied by a similar decrease in line-crossings in the periphery, as well as in rears and climbs. Thus the doses of IL-1β and LPS necessary to induce these effects also decreased locomotor activity in the EPM and OF. Therefore, the behavioral responses induced by IL-1β and LPS in the EPM and the OF considered to reflect anxiety must be interpreted in the light of this reduction in overall activity. Thus the results do not provide unequivocal support for the suggestion that LPS or IL-1 mediate anxiety. Nevertheless, because infections, endotoxins, and the ensuing cytokines cause alterations in CNS norepinephrine and serotonin, they may contribute to emotionality, and perhaps to anxiety.
Introduction
Administration of interleukin-1 (IL-1) and lipopolysaccharide (LPS) induces physiological changes like those associated with physical or psychogenic stressors in rats and mice (Connor et al., 1998; Dunn et al., 1999; Anisman et al., 2002). Both IL-1 and LPS activate brain norepinephrine and serotonin metabolism (Dunn, 1988; Kabiersch et al., 1988; Dunn, 2006), and activate the hypothalamic-pituitary-adrenal (HPA) axis so that plasma concentrations of ACTH and glucocorticoids are increased (Besedovsky et al., 1986; Dunn, 1988, 2000). Furthermore, the peripheral sympathetic system is stimulated. Similar physiological responses occur during anxiety. Moreover, the brain noradrenergic systems have long been implicated in mediating anxiety (Charney et al., 1998; Clement and Chapouthier, 1998).
Systemic administration of IL-1 or LPS has also been demonstrated to produce a spectrum of behavioral responses known as “sickness behavior” (Hart, 1988; Kent et al., 1992; Dantzer et al., 2001). These include decreased locomotor activity (Otterness et al., 1988), exploration (Spadaro and Dunn, 1990; Dunn et al., 1991), and feeding (McCarthy et al., 1985), behaviors that may be displayed by fearful or anxious animals. These well-documented findings raised the question of whether IL-1 may mediate fear or anxiety-like behavior in animals. If indeed IL-1 and LPS elicit or enhance anxiety-like responses in animal models, it would suggest that anxiety in humans could be precipitated or exaggerated by immune system disturbances. Because cytokines and LPS activate the HPA axis and central noradrenergic systems, some researchers have suggested that immune activation could influence affective state, including anxiety-related behavior (Leonard and Song, 1996; Lacosta et al., 1999). However, review of the published animal studies indicates that the results are equivocal, because changes in locomotor present an experimental confound (see also Weiss et al., 1998; Wall and Messier, 2001).
The present study aimed to determine whether peripherally administered IL-1 and LPS specifically affect anxiety-like responses in the elevated plus-maze (EPM) and the open field (OF), the tests most often used to assess anxiogenic and anxiolytic properties of various treatments. A spectrum of behaviors was assessed to distinguish anxiety-like behavior from other symptoms of sickness behavior.
Materials and Methods
Animals
Six separate batches of CD-1 albino, VAF+ male mice were obtained from Charles River (R16 colony Raleigh-Durham facility) and upon arrival housed in an animal colony room maintained at 23 ± 1°C and with 12 h light-dark cycle with lights on at 7 AM. The animals were kept in plastic cages with wood shaving bedding. They were kept in groups of 4-5 to avoid behavioral changes that may result from single housing - usually an increase in aggressive and fear-like behavior. Mice were given free access to water and Purina chow pellets and changes in body weight resulting from the experimental treatments were recorded. The animals were 9-14 weeks old at the beginning of the experiments. All experiments were conducted in accordance with the NIH Guide on the care and use of animals for research, and a protocol approved by the LSUHSC-Shreveport Institutional Animal Care and Use Committee.
Materials
Recombinant mouse IL-1β (R&D Systems, Minneapolis) and E. coli lipopolysaccharide (LPS; 026:B6, Sigma L3755) dissolved in sterile saline (0.9% NaCl) were injected intraperitoneally (ip) in a volume of 0.1 ml. Control animals were injected with saline.
Behavioral Tests
Mice were subjected to the elevated plus maze test (EPM) and the open field test (OF) following treatment with IL-1 or LPS. The animals were tested only once as repeated exposure to the EPM has been shown to affect behavior independent of the experimental factors (Lister, 1987; Lapin, 1995).
Elevated Plus-Maze Test (EPM)
The elevated plus-maze was made of a black Plexiglas cross (arms 30 cm long × 5 cm wide) elevated 40 cm above the floor (Gorman and Dunn, 1993; Weninger et al., 1999). Two opposite arms were enclosed by the transparent walls (30 cm long × 15 cm high) and the two other arms were open. A mouse was placed in the center of the apparatus facing an enclosed arm and observed for 5 min. The number of entries into the open and enclosed arms, and the time spent on the open arms was scored. The total number of entries into the arms (enclosed plus open), the ratio of open to total arm entries, and the time in the closed arms were calculated. This test was performed first because of the common observation that the EPM is very sensitive to environmental factors.
Open Field Test (OF)
Locomotor activity was quantified for 5 minutes in an open field, a white Plexiglas box 59 × 59 cm with its floor divided into 16 squares. Out previous studies have indicated that this period is sufficient to indicate differences between treatment groups, and that if the test is longer than that, the mice habituate to the apparatus, decreasing differences between the groups. Four squares were defined as the center and the 12 squares along the walls as the periphery. Each mouse was gently placed in the very center of the box and activity was scored as a line crossing when a mouse removed all four paws from one square and entered another. Line crossings in the central four squares and in the peripheral 12 squares of the OF were counted separately. Rears were scored when a mouse raised both front paws from the floor, and climbs when an animal leaned its front paws against a wall.
Experimental Procedure
In the animal room, each mouse was removed from its cage, gently weighed, injected intraperitoneally, and returned to its home cage. Saline, IL-1β (30, 100, 300, or 1000 ng/mouse) was injected 30 or 60 min, and saline or LPS (0.5, 1 or 5 μg) 120 min before the test in the EPM. These time points were chosen on the basis of our previous behavioral, endocrine and neurochemical studies (Dunn, 1988, 1992; Swiergiel et al., 1997; Dunn, 2000). When mice were presented with sweetened milk, the response to IL-1 appeared around 15-20 min, and persisted for approximately 2 h, with the maximum response between 30 and 90 minutes (Swiergiel et al., 1997). The increase in plasma corticosterone occurred over a similar time period with a peak around 2 h, closely paralleling the brain noradrenergic response, while the increases in tryptophan and in serotonin metabolism occurred between 90 min and 4 hours (Dunn, 1988, 1992, 2000). For LPS, a delay of 120 min was chosen because this was typically the time of the most profound response in sweetened milk drinking, elevation of plasma corticosterone, and brain noradrenergic activity (Dunn, 1988, 1992; Swiergiel et al., 1997).
All observations were conducted in the animal colony room to avoid exposing the mice to unfamiliar and, potentially aversive and stressful surroundings. The tests were performed between 10 AM and 2 PM. White room illumination was turned off and the apparati were illuminated with red light. An infrared-sensitive video camera was placed above the apparati and the animals were observed on a TV monitor placed in a corridor adjacent to the colony room. All tests were videotaped. After completion of the EPM test, an observer entered the room, removed the mouse from the EPM, placed it in the open field and left the room. There was approximately a 2 min interval between the EPM and the OF tests. Observers who scored behaviors were unaware of the experimental treatments.
Data Analysis
To determine main effects of IL-1β or LPS, one-way analysis of variance (ANOVA) was performed. ANOVA was followed by Fisher’s Least Significant Difference (LSD) test for a priori designed pair-wise comparisons (saline control versus a single dose of IL-1β or LPS). Data are reported as the mean ± standard error of the mean.
RESULTS
Behavioral effects of IL-1β
IL-1β administered 60 minutes before testing
Elevated Plus-Maze
mIL-1β injected ip 60 min before testing decreased activity in the EPM (Figure 1). ANOVA indicated significant reductions in entries into the open arms (F4,51 = 3.29, p < 0.05), the total number of arm entries (F4,51 = 3.01, p < 0.05), the percentage of entries into the open arms (F4,51 = 2.99, p < 0.05), the ratio of open to closed arm entries (F4,51 = 2.84, p < 0.05), and the time spent on the open arms (F4,51 = 3.05, p < 0.05), whereas the reduction in closed arm entries was not statistically significant (F4,51 = 1.33). Post hoc Fisher’s LSD tests indicated that each of these decreases was confined to the three higher doses of IL-1β(100, 300 and 1000 ng); the lowest dose of mIL-1β (30 ng) had no statistically significant effects. Thus, arm entries were decreased at doses of 100 ng or more of IL-1β, but this effect was somewhat selective for the open arms as indicated by the significant reduction in the percentage of entries into open arms.
Figure 1.
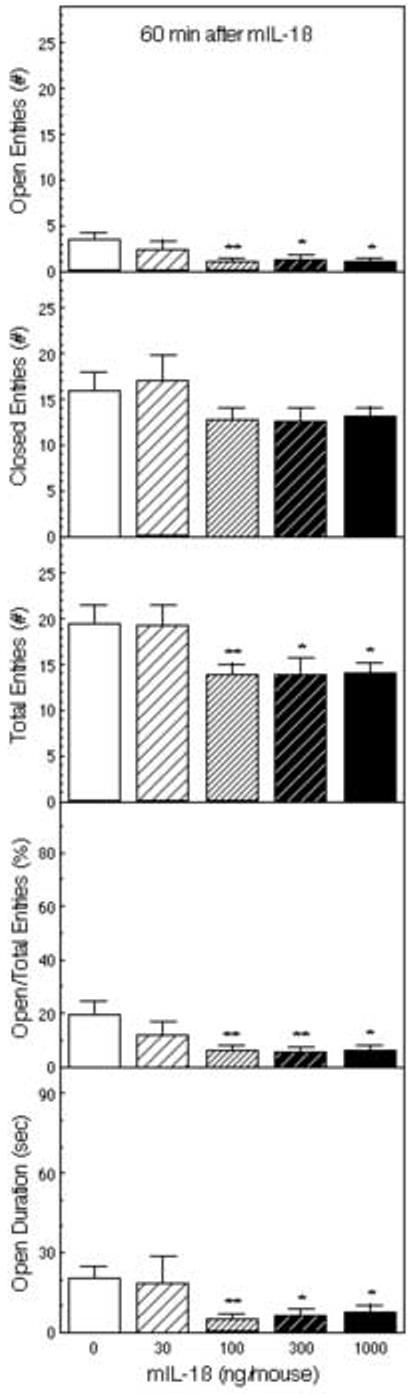
The effect of IL-1β on behavior of mice in the elevated plus-maze (EPM). Mice (n = 11) were injected ip with saline or 30, 100, 300, or 1000 ng of IL-1β 60 min before testing for 5 minutes in the EPM. The number of entries into the open and closed arms, the ratio of the open arm entries to the total entries, and the time spent on the open arms are depicted *Significantly different from saline-treated mice (*p < 0.05; **p < 0.01).
Open Field
The same mice were subsequently tested in the open field approximately two minutes after testing in the EPM. IL-1β significantly decreased the number of line crossings in the center (F4,51 = 4.59, p < 0.01), in the periphery (F4,51 = 5.41, p < 0.001), and the total number of line crossings (F4,51 = 5.79, p < 0.001; Figure 2). Post hoc Fisher’s LSD tests indicated that 100, 300 and 1000 ng of mIL-1β significantly decreased line crossings, whereas the lowest dose (30 ng) tended to increase activity, although this effect was not statistically significant. The percentage of central line-crossings was decreased, but the overall effect of IL-1β was not statistically significant (F4,50 = 2.06). IL-1β also decreased the number of rears (F3,9 = 4.04, p < 0.05), climbs (F3,9 = 3.67, p < 0.06), and the sum of rears and climbs (F3,9 = 7.27, p < 0.01) (Figure 2).
Figure 2.
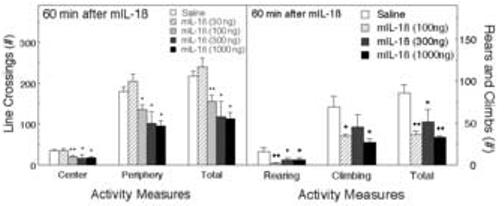
The effect of IL-1β on activity in the open field (OF). Mice (n = 11) were tested in the OF within 2 min of testing in the EPM (67 min after the injections; the same animals as in Fig. 1). Activity was scored for 5 min. The numbers of line crossings in the central and peripheral areas of the field and the total are depicted (Left panel), along with the numbers of rears and climbs (Right panel). *Significantly different from saline-treated mice (*p < 0.05; **p < 0.01).
IL-1β administered 30 minutes before testing
Elevated Plus-Maze
In a separate batch of mice, mIL-1β was administered 30 min before testing in the EPM using doses of 0, 30, 100 or 300 ng. As indicated in Figure 3, IL-1β decreased the numbers of open arm entries (F3,52 = 4.58, p < 0.01), closed arm entries (F3,52 = 7.40, p < 0.001), and the total number of entries into the open and closed arms (F3,52 = 8.54, p < 0.001). Post hoc Fisher’s LSD tests indicated that the 100 and 300 ng doses of mIL-1β significantly reduced the number of arm entries (open, closed and the total). The percentage of entries into the open arms (F3,52 = 1.80), the ratio of open to closed arm entries (F3,52 = 1.63), and the time spent on the open arms (F3,52 = 1.80) were also decreased by IL-1β, but these effects were not statistically significant.
Figure 3.
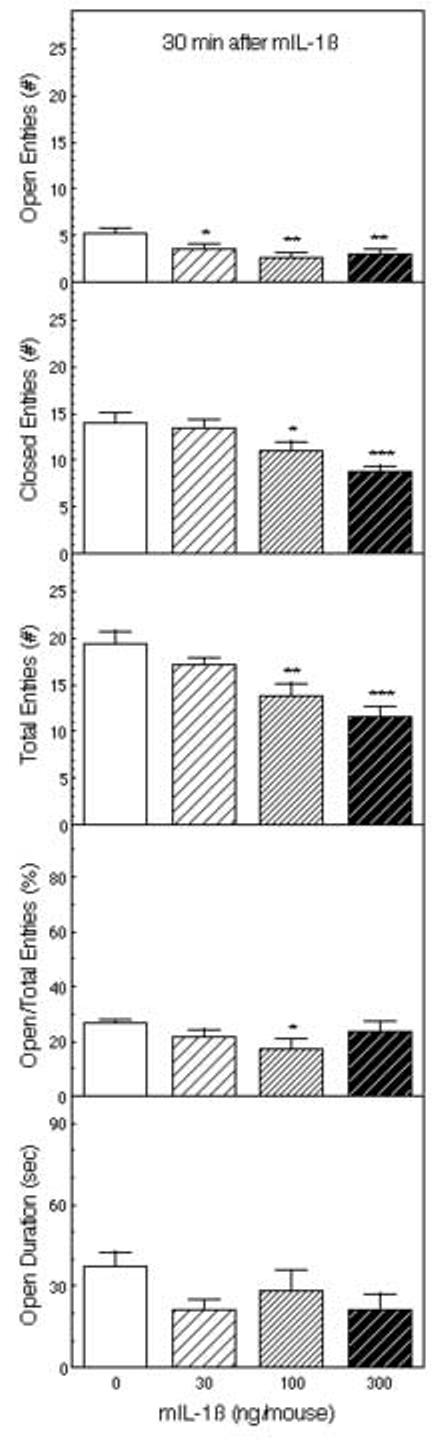
The effect of IL-1β on behavior in the EPM. Mice were injected ip with saline or 30, 100 or 300 ng of IL-1β (n = 14) 30 min before testing for 5 minutes in the EPM. The number of entries into the open and closed arms, the ratio of the open arm entries to the total entries, and the time spent on the open arms are depicted *Significantly different from saline-treated mice (*p < 0.05; **p < 0.01; ***p < 0.001).
Open Field Test
The same mice were then tested in the open field. IL-1β decreased line crossings in the center, in the periphery, and the total number of line crossings, but none of these effects reached statistical significance (Figure 4). However, as in the first experiment, in comparison to the control group, mice treated with 100 and 300 ng of mIL-1β displayed fewer line-crossings, whereas the lowest dose of 30 ng slightly increased line-crossings, although once again this effect was not statistically significant. The percentage of central crossings was not significantly altered. IL-1β also decreased the number of climbs (F3,53 = 5.17, p < 0.01), and the sum of rears and climbs (F3,53 = 4.95, p < 0.01), but the decrease in the number of rears was not statistically significant (F3,53 = 0.76).
Figure 4.
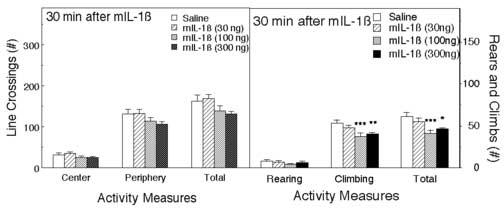
The effect of IL-1β on activity in the OF. The same mice as in Fig. 3, were observed in the OF after testing in the EPM and 37 minutes after the injections. Activity was scored for 5 minutes. The number of line crossings in the central and peripheral areas of the field and the total are depicted (left panel), and the rears and climbs (right panel). *Significantly different from saline-treated mice (*p < 0.05; **p < 0.01).
Consistent with the results of our preliminary studies, these data indicate that IL-1β administered ip at doses of 100 ng or more 30 or 60 minutes before testing consistently decreased entries onto the open arms of the EPM and line crossings in the center of the OF, both of which are normally considered to indicate anxiogenic effects. However, the same treatments also decreased the overall activity in the maze as indicated by reductions in entries into all arms of the maze. Thus the potential “tanxiogenic” effects are to some extent confounded by changes in overall activity.
Behavioral effects of LPS
Elevated Plus-Maze
LPS (1 and 5 μg) administered ip 120 minutes before testing decreased activity in the EPM (Figure 5). The number of open arm entries (F2,36 = 11.9, p < 0.001), closed arm entries (F2,36 = 10.3, p < 0.001), the total number of arm entries (F2,36 = 15.1, p < 0.001), and the time spent on the open arms (F2,36 = 8.49, p < 0.001) were all significantly decreased. These behavioral activities were depressed to a similar extent by 1 and 5 μg of LPS (p < 0.001). Less affected were the ratio of open to total arm entries (F2,36 = 2.26) and the percentage of open to closed arm entries (F2,36 = 2.24). The post hoc tests indicated that the percentage of entries into the open arms was significantly decreased only by the 5 μg dose of LPS.
Figure 5.
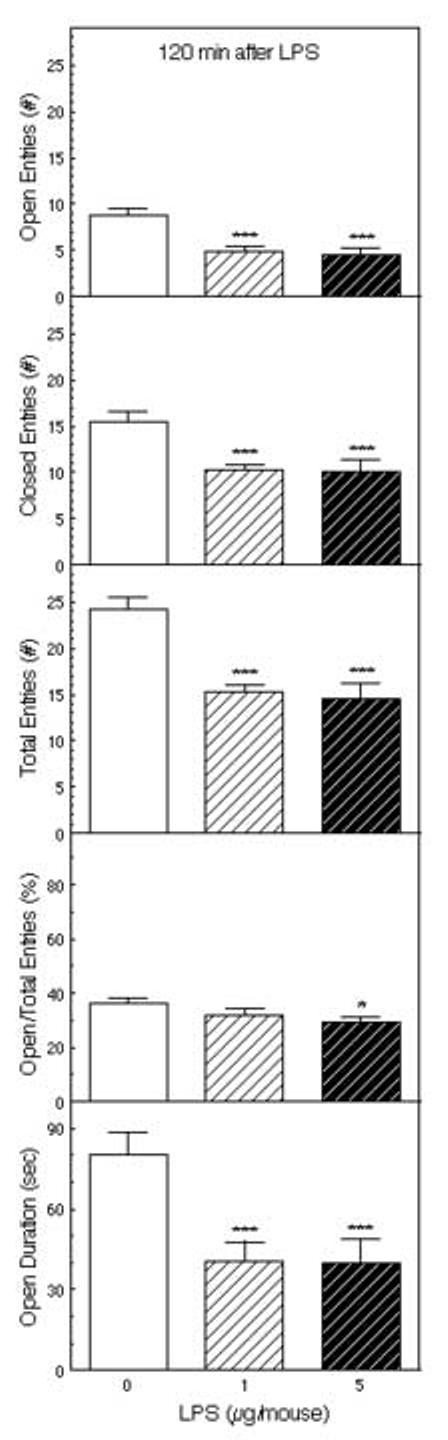
The effect of LPS on behavior in the EPM. Mice (n = 13) were injected ip with saline or 1 or 5 μg of LPS 120 min before testing for 5 minutes in the EPM. The number of entries into the open and closed arms, the ratio of the open arm entries to the total entries, and the time spent on the open arms are depicted *Significantly different from saline-treated mice (**p < 0.01; ***p < 0.001).
Open Field Test
In the subsequent open field test, LPS significantly decreased peripheral (F2,36 = 3.86, p < 0.05) and total line crossings (F2,36 = 4.15, p < 0.05), but the decrease in line-crossings in the central part of the OF fell short of statistical significance (F2,36 = 2.30, p < 0.12, Figure 6). Rears and climbs were not scored in this test.
Figure 6.
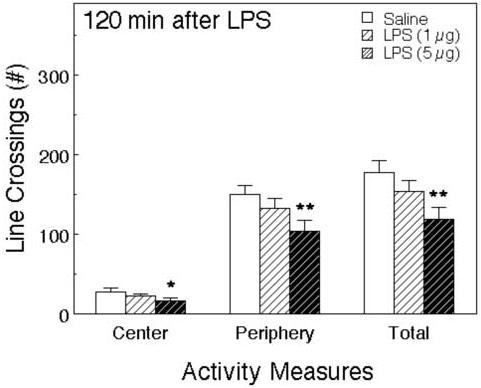
The effect of LPS on activity in the OF. The same mice as Fig. 5 were observed in the OF after testing in the EPM 127 minutes after the injections. Activity was scored for 5 minutes. The number of line crossings in the central and peripheral areas of the field and the total are depicted. *Significantly different from saline-treated mice (*p < 0.05; **p < 0.01; ***p < 0.001).
In a separate experiment in which 0.5, 1 and 5 μg doses of LPS were tested, very similar results were obtained. In this experiment, LPS significantly decreased open arm entries, closed arm entries, total entries, and the time spent on the open arms of the EPM. The percentage of open arm entries and the ratio of open to closed arm entries were also decreased by LPS. The total number of rears and climbs was significantly decreased by LPS.
Thus these results confirm the results of our preliminary experiments indicating that LPS, like IL-1, reduced open arm entries and the time spent on the open arms in the EPM, as well as reducing line crossings in the center of an OF. However, once again, there were overall reductions in locomotor activity indicated by decreases in the total number of arm entries in the EPM, and the total number of line crossings and the number of line crossings in the periphery of the OF.
DISCUSSION
In the elevated plus-maze, ip IL-1β decreased entries into the open arms at doses of 100 ng/mouse or more, and decreased the time spent on the open arms (Figure 1). The number of closed arm entries and the total number of arm entries were also decreased (Figure 3). There was a trend indicating selective effects on open arm entries, indicated by reductions in the percentage of open arm entries and the ratio of open to central arm entries, but this effect was not robust. Ip administration of LPS had very similar effects: reductions in the number of open arm entries, accompanied by reductions in the number of closed arm entries and the total number of arm entries (Figure 5).
In the open field, IL-1β decreased line-crossings in the center of the field, but also depressed line-crossings in the periphery, and the total number of line-crossings (Figures 2 and 4). As in the EPM, these effects were observed only at doses of 100 ng of IL-1β or more. The numbers of rears and climbs were also reduced. LPS induced similar effects; reductions in central line-crossings, as well as peripheral line-crossings (Figure 5), and rears and climbs (Figure 6). Decreases in line-crossings in the center of the OF are regarded as indicating increases in anxiety. However, the fact that all activities were decreased by IL-1β and LPS presents a significant experimental confound.
Purported anxiogenic effects of IL-1 and LPS have been reported in several previous studies. Montkowski et al. (1997) reported that intracerebroventricular (icv) administration of hIL-1β induced anxiety-like behavior in the EPM 20 min later. The effects were dose-dependent, such that no significant effects were observed after 0.001 or 0.01 ng, whereas an increase in the percentage of time spent on the open arms was observed at the 0.1 ng dose. Higher doses elicited no statistically significant effects, except that a 100 ng dose significantly decreased the percentage of time spent on the open arms. This same dose also decreased locomotor activity. Both the 0.1 and 100 ng doses decreased locomotor activity and food intake in the home cages over the next 24 h. The results in this study may have been affected because the EPM test was conducted in each rat both immediately before and 20 min and after IL-1 injection, even though it is well established that prior exposure to the apparatus markedly alters performance in this test (Lister, 1987; Lapin, 1995. Rather similar dose-dependent results were obtained in a study by Zubareva et al. (2001) who reported that low doses of IL-1β administered to rats reduced anxiety-like behavior in the EPM, whereas high doses increased anxiety, but both doses depressed social and exploratory behaviors.
Connor et al. (1998) reported that hIL-1β (20 ng icv) decreased the number of open arm entries and the time spent on the open arms 45 min after injection in rats, while increasing the time spent on the closed arms and decreasing the percentage of time spent on the open arms and the percentage of open arm entries. Subsequently, Song et al. (2003) reported that icv rat IL-1β (rIL-1β, 15 ng) decreased the ratio of open to closed arm entries and the time spent on the open arms compared to that on the closed arms in the EPM. It also decreased line-crossings and rears in the open field, especially line crossings in the center of the field. In another report, Song et al. (2004) injected rats subchronically icv with rIL-1β (5 or 50 ng for 5 consecutive days). These treatments decreased the number of rears and the number of entries into the central area of an open field. They also decreased the ratio of the number of entries into the open and closed arms of the EPM, as well as the ratio of the times spent on the open and closed arms. In a more recent study, Song et al. (2006) reported that rIL-1β (1 μg/rat, ip) increased locomotor activity, grooming and defecation in an open field, whereas a lower dose (10 ng) increased rearing and grooming. Curiously, icv injection of IL-1 (5, but not 50 ng) decreased rearing but increased grooming. Both ip (1 μg) and icv rIL-1β (5 and 50 ng) decreased entries into the central area. In this report, ip rIL-1β (0.01, 0.1 and 1 μg) did not alter the number of entries into the open and closed arms of the EPM or the times spent on either open or closed arms, but the ratio of open to closed entries was decreased by the 0.01 and 0.1 μg doses. Icv IL-1β (5 but not 50 ng) decreased the time spent on the open arms, and increased that on the closed arms. However, after subchronic icv administration both 5 and 50 ng doses of rIL-1β decreased open arm entries and time, but the 50 ng dose also decreased closed arm entries, and increased the time spent on the closed arms, thus decreasing the proportion of time spent on the open arms.
In another recent study, Cragnolini et al. (2006) infused rIL-1β icv (30 or 50 ng) and tested the rats in the EPM 60 min later. The number of open arm entries, the time spent on the open arms, and the percentage of time spent on the open arms was significantly decreased by both doses of IL-1β. However, although not statistically significant, the number of entries into the closed arms was also reduced. The total number of arm entries was also reduced, although this effect was only statistically significant at the 50 ng dose. The percentage of entries into the open arms was also reduced, but this effect was not statistically significant at either dose. These results closely resemble the current data, even though the study was performed in rats, and employed icv administration of IL-1.
Suggestive evidence for a role of IL-1 in the EPM was obtained from studies of transgenic mice over-expressing the gene for the IL-1-receptor antagonist (IL-1ra) in which the transgenic animals exhibited increased locomotor activity in the open field, and increased time spent on the closed relative to the open arms of the EPM, both indicative of decreased anxiety (Oprica et al., 2005).
Lacosta et al. (1999) reported that ip administration of LPS to mice dose-dependently decreased exploration in the illuminated part of the light-dark box at doses of 1 μg/mouse or higher. At a dose of 5 μg it also decreased the number of open arm entries and the time spent on the open arms in the EPM, but only induced a small decrease (not statistically significant) in the number of closed arm entries. In an open field, doses of 0.5, 1 and 5 μg reduced locomotor activity, rearing and contact with a novel object. Once again these results closely resemble those of the present study, using the same preparation of LPS in mice. Lacosta et al. (1999) suggested that LPS and “ensuing cytokines” may contribute to emotionality, and “perhaps even anxiety-related disturbances.” In rats, injection of LPS (0.25, 0.5 and 1 mg/kg ip) decreased the percentages of open arm entries, and of the time spent on the open arms, but increased the percentage of closed arm entries (Nava and Caputi, 1999; Nava and Carta, 2001). The numbers of entries were not reported.
Although there is clearly some variation in the results of the various studies, there appears to be general agreement that IL-1 and LPS administration both reduce open arm entries and the time spent on the open arms in the EPM, as well as avoidance of the central area of the OF. Moreover, most of the studies found reductions in overall activity in both tests. There is also remarkable similarity in the effects of IL-1 and LPS in both rats and mice, and between intracerebral and peripheral administration of IL-1 and LPS.
It has been suggested that infections, inflammatory processes may be causative factors in emotional disorders, including anxiety (Leonard and Song, 1996; Anisman and Merali, 1999). Cytokines, mainly IL-1, have been implicated in mediating such disorders and, at the experimental level, administration of IL-1 would be expected to augment or evoke anxiety-related behaviors.
The elevated plus-maze is a commonly used test of emotionality and anxiety in mice and rats (Handley and Mithani, 1984; Treit, 1985; Lister, 1987; Espejo, 1997; Wall and Messier, 2001). The test was based on the conflict between the drive to explore, and the innate avoidance of a novel and an open space (Montgomery, 1955; Treit et al., 1993). The number of entries into the open arms and the time spent on the open arms are used to assess a state of fear or anxiety (Lister, 1987; Cruz et al., 1994). The ratio of the open to closed or total arm entries can be treated as a measure of the relative aversiveness of the open arms, that has been regarded as an index of anxiety (Pellow et al., 1985; Lister, 1987; Handley and McBlane, 1993). A decrease in this ratio may suggest an anxiogenic effect of a treatment; it means that the treatment increased avoidance, expressed as decreased entries into the aversive, open areas of the apparatus while having little effect on activity in the closed arms. An unchanged ratio suggests that a decrease in the open arm entries was accompanied by a proportional decrease in the closed arm entries. The number of entries into the closed arms has been suggested as a pure index of locomotor activity (Cruz et al., 1994; Rodgers and Johnson, 1995) and the total number of entries into the arms (enclosed plus open) is considered to reflect general locomotor activity (Lister, 1987; Rodgers and Johnson, 1995).
The open field test has also been used widely to assess anxiety and locomotor performance (Ramos and Mormede, 1997; Choleris et al., 2001; Prut and Belzung, 2003). Some classical anxiolytics increase entries into aversive parts in both apparati: open arms in the EPM and the center of the OF (Prut and Belzung, 2003). Activity in the central part of the open field is thought to correlate with a degree of fear, while the behavior in the peripheral zone and along the walls of the field is thought to reflect general activity (Belzung and Griebel, 2001; Prut and Belzung, 2003). In general, to indicate anxiogenic or anxiolytic effects of a treatment, the control animals should display a reasonably high number of entries into the aversive parts of the apparatus and be quite active on the closed arms of the EPM or in the peripheral areas of the OF. Anxiety-like behavior is revealed by diminished exploration of the aversive space with relatively unaffected ambulatory activity in the safe areas.
The potential for changes in locomotor activity to confound the interpretation of the results has long been recognized (e.g., (Lister, 1987; Weiss et al., 1998). Thus it was suggested that changes in the number of open arm entries or the time spent on the open arms can only be interpreted in terms of fear or anxiety provided that there were no changes in the total number of entries (see Weiss et al., 1998). However, as pointed out by Weiss et al. (1998), there may be problems when the treatment tested alters the preference for the open arms, and the total number of entries in the same direction. In their own study, they observed that d-amphetamine, a classic stimulant, increased open arm entries in the elevated zero-maze without altering the total number of entries (Weiss et al., 1998). The results with amphetamine closely resembled those obtained with the classic anxiolytic benzodiazepine, chlordiazepoxide. They concluded that “under certain test conditions, psychostimulants can induce false positives in elevated maze models.” We suggest that the present results indicate that treatments that decrease locomotor activity similarly confound the interpretation of anxiogenic effects in the OF and the EPM, and that other behavioral tests should be considered and developed.
It is concluded that LPS and IL-1β produce responses that could be interpreted as anxiety-like behavior in the EPM and the OF. However, the anxiety-like behavioral patterns induced cannot clearly be dissociated from changes in locomotor activity. In the present experiments the substantial decrease induced by IL-1β and LPS in the number of total entries or line crossings suggests that the decrease in open arm entries and duration may not reflect anxiety, but rather the effects of LPS and IL-1 on locomotion. This confounding factor should be taken into consideration in the experiments using the EPM or OF in attempt to unmask any anxiogenic properties of LPS or IL-1.
Acknowledgements
This research was supported by a grant from the National Institutes of Health (NS 35370). A.H.S. also has an appointment in the Department of Animal Behavior, Institute of Genetics and Animal Breeding, Polish Academy of Sciences, Jastrzebiec, Poland. We are grateful for the excellent technical assistance of Daniel F. King of Louisiana Tech University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anisman H, Hayley S, Turrin N, Merali Z. Cytokines as a stressor: Implications for depressive illness. Intl J Neuropsychopharmacol. 2002;5:357–373. doi: 10.1017/S1461145702003097. [DOI] [PubMed] [Google Scholar]
- Anisman H, Merali Z. Anhedonic and anxiogenic effects of cytokine exposure. Adv. Exptl Med. Biol. 1999;461:199–233. doi: 10.1007/978-0-585-37970-8_12. [DOI] [PubMed] [Google Scholar]
- Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- Besedovsky HO, del Rey A, Sorkin E, Dinarello CA. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986;233:652–654. doi: 10.1126/science.3014662. [DOI] [PubMed] [Google Scholar]
- Charney DS, Grillon C, Bremner JD. The neurobiological basis of anxiety and fear: circuits, mechanisms, and neurochemical interactions (Part I) Neuroscientist. 1998;4:35–44. [Google Scholar]
- Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev. 2001;25:235–260. doi: 10.1016/s0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- Clement Y, Chapouthier G. Biological bases of anxiety. Neurosci Biobehav Rev. 1998;22:623–633. doi: 10.1016/s0149-7634(97)00058-4. [DOI] [PubMed] [Google Scholar]
- Connor TJ, Song C, Leonard BE, Merali Z, Anisman H. An assessment of the effects of central interleukin-1β, -2, -6, and tumor necrosis factor-α administration on some behavioural, neurochemical, endocrine and immune parameters in the rat. Neuroscience. 1998;84:923–933. doi: 10.1016/s0306-4522(97)00533-2. [DOI] [PubMed] [Google Scholar]
- Cragnolini AB, Schiöth HB, Scimonelli TN. Anxiety-like behavior induced by IL-1β is modulated by a-MSH through central melanocortin-4 receptors. Peptides. 2006;27:1451–1456. doi: 10.1016/j.peptides.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Cruz APM, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav. 1994;49:171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Bluthé R-M, Castanon N, Chauvet N, Capuron L, Goodall G, Kelley KW, Konsman J-P, Layé S, Parnet P, Pousset F. Cytokine effects on behavior. In: Ader R, Felten D, Cohen N, Ader R, Felten D, Cohens N, editors. Psychoneuroimmunology. Academic Press; San Diego, CA: 2001. pp. 703–727. [Google Scholar]
- Dunn AJ. Systemic interleukin-1 administration stimulates hypothalamic norepinephrine metabolism parallelling the increased plasma corticosterone. Life Sci. 1988;43:429–435. doi: 10.1016/0024-3205(88)90522-x. [DOI] [PubMed] [Google Scholar]
- Dunn AJ. Endotoxin-induced activation of cerebral catecholamine and serotonin metabolism: comparison with interleukin-1. J Pharmacol Exptl Therap. 1992;261:964–969. [PubMed] [Google Scholar]
- Dunn AJ. Cytokine activation of the HPA axis. Ann NY Acad Sci. 2000;917:608–617. doi: 10.1111/j.1749-6632.2000.tb05426.x. [DOI] [PubMed] [Google Scholar]
- Dunn AJ. Effects of cytokines and infections on brain neurochemistry. Clin Neurosci Res. 2006;6:52–68. doi: 10.1016/j.cnr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Antoon M, Chapman Y. Reduction of exploratory behavior by intraperitoneal injection of interleukin-1 involves brain corticotropin-releasing factor. Brain Res. Bull. 1991;26:539–542. doi: 10.1016/0361-9230(91)90092-x. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Wang J-P, Ando T. Effects of cytokines on cerebral neurotransmission: Comparison with the effects of stress. Adv Exptl Med Biol. 1999;461:117–127. doi: 10.1007/978-0-585-37970-8_8. [DOI] [PubMed] [Google Scholar]
- Espejo EF. Effects of weekly or daily exposure to the elevated plus-maze in male mice. Behav. Brain Res. 1997;87:233–238. doi: 10.1016/s0166-4328(97)02286-9. [DOI] [PubMed] [Google Scholar]
- Gorman AL, Dunn AJ. β-Adrenergic receptors are involved in stress-related behavioral changes. Pharmacol Biochem Behav. 1993;45:1–7. doi: 10.1016/0091-3057(93)90078-8. [DOI] [PubMed] [Google Scholar]
- Handley SL, McBlane JW. 5HT drugs in animal models of anxiety. Psychopharmacol. 1993;112:13–20. doi: 10.1007/BF02247358. [DOI] [PubMed] [Google Scholar]
- Handley SL, Mithani S. Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of ‘fear’-motivated behaviour. N-S Archs Pharmacol. 1984;327:1–5. doi: 10.1007/BF00504983. [DOI] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Kabiersch A, del Rey A, Honegger CG, Besedovsky HO. Interleukin-1 induces changes in norepinephrine metabolism in the rat brain. Brain Beha Immun. 1988;2:267–274. doi: 10.1016/0889-1591(88)90028-1. [DOI] [PubMed] [Google Scholar]
- Kent S, Bluthé R-M, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992;13:24–28. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- Lacosta S, Merali Z, Anisman H. Behavioral and neurochemical consequences of lipopolysaccharide in mice: anxiogenic-like effects. Brain Res. 1999;818:291–303. doi: 10.1016/s0006-8993(98)01288-8. [DOI] [PubMed] [Google Scholar]
- Lapin IP. Only controls: Effect of handling, sham injection, and intraperitoneal injection of saline on behavior of mice in an elevated plus-maze. J Pharmacol Tox Methods. 1995;34:73–77. doi: 10.1016/1056-8719(95)00025-d. [DOI] [PubMed] [Google Scholar]
- Leonard BE, Song C. Stress and the immune system in the etiology of anxiety and depression. Pharmacol Biochem Behav. 1996;54:299–303. doi: 10.1016/0091-3057(95)02158-2. [DOI] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacol. 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- McCarthy DO, Kluger MJ, Vander AJ. Suppression of food intake during infections: is interleukin-1 involved? Amer. J. Clin. Nutr. 1985;42:1179–1182. doi: 10.1093/ajcn/42.6.1179. [DOI] [PubMed] [Google Scholar]
- Montgomery KC. The relation between fear induced by novel stimulation and exploratory behavior. J Comp Physiol Psychol. 1955;48:254–260. doi: 10.1037/h0043788. [DOI] [PubMed] [Google Scholar]
- Montkowski A, Landgraf R, Yassouridis A, Holsboer F, Schöbitz B. Central administration of IL-1 reduces anxiety and induces sickness behavior in rats. Pharmacol Biochem Behav. 1997;58:329–336. doi: 10.1016/s0091-3057(97)00244-x. [DOI] [PubMed] [Google Scholar]
- Nava F, Caputi AP. Central effects of cromoglycate sodium salt in rats treated with lipopolysaccharide. Europ J Pharmacol. 1999;367:351–359. doi: 10.1016/s0014-2999(98)00986-8. [DOI] [PubMed] [Google Scholar]
- Nava F, Carta G. Melatonin reduces anxiety induced by lipopolysaccharide in the rat. Neurosci. Letts. 2001;307:57–60. doi: 10.1016/s0304-3940(01)01930-9. [DOI] [PubMed] [Google Scholar]
- Oprica M, Zhu S, Goiny M, Pham TM, Mohammed AH, Winblada B, Bartfai T, Schultzberg M. Transgenic overexpression of interleukin-1 receptor antagonist in the CNS influences behaviour, serum corticosterone and brain monoamines. Brain Behav Immun. 2005;19:223–234. doi: 10.1016/j.bbi.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Otterness IG, Seymour PA, Golden HW, Reynolds JA, Daumy GO. The effects of continuous administration of murine interleukin-1α in the rat. Physiol Behav. 1988;43:797–804. doi: 10.1016/0031-9384(88)90379-4. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Europ J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Ramos A, Mormede P. Stress and emotionality: a multidimensional and genetic approach. Neurosci Biobehav Rev. 1997;22:33–57. doi: 10.1016/s0149-7634(97)00001-8. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Johnson NJT. Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol Biochem Behav. 1995;52:297–303. doi: 10.1016/0091-3057(95)00138-m. [DOI] [PubMed] [Google Scholar]
- Song C, Horrobin DF, Leonard BE. The comparison of changes in behavior, neurochemistry, endocrine, and immune functions after different routes, doses and durations of administrations of IL-1β in rats. Pharmacopsychiat. 2006;39:88–99. doi: 10.1055/s-2006-941557. [DOI] [PubMed] [Google Scholar]
- Song C, Leonard BE, Horrobin DF. Dietary ethyl-eicosapentaenoic acid but not soybean oil reverses central interleukin-1-induced changes in behavior, corticosterone and immune response in rats. Stress. 2004;7:43–54. doi: 10.1080/10253890410001667188. [DOI] [PubMed] [Google Scholar]
- Song C, Li X, Leonard BE, Horrobin DF. Effects of dietary n-3 or n-6 fatty acids on interleukin-1β-induced anxiety, stress, and inflammatory responses in rats. J Lipid Res. 2003;44:1984–1991. doi: 10.1194/jlr.M300217-JLR200. [DOI] [PubMed] [Google Scholar]
- Spadaro F, Dunn AJ. Intracerebroventricular administration of interleukin-1 to mice alters investigation of stimuli in a novel environment. Brain Behav Immun. 1990;4:308–322. doi: 10.1016/0889-1591(90)90034-n. [DOI] [PubMed] [Google Scholar]
- Swiergiel AH, Smagin GN, Dunn AJ. Influenza virus infection of mice induces anorexia: comparison with endotoxin and interleukin-1 and the effects of indomethacin. Pharmacol Biochem Behav. 1997;57:389–396. doi: 10.1016/s0091-3057(96)00335-8. [DOI] [PubMed] [Google Scholar]
- Treit D. Animal models for the study of anti-anxiety agents: a review. Neurosci Biobehav Rev. 1985;9:203–22. doi: 10.1016/0149-7634(85)90046-6. [DOI] [PubMed] [Google Scholar]
- Treit D, Menard J, Royan C. Anxiogenic stimuli in the elevated plus-maze. Pharmacol. Biochem. Behav. 1993;44:463–470. doi: 10.1016/0091-3057(93)90492-c. [DOI] [PubMed] [Google Scholar]
- Wall PM, Messier C. Methodological and conceptual issues in the use of the elevated plus-maze as a psychological measurement instrument of animal anxiety-like behavior. Neurosci Biobehav Rev. 2001;25:275–286. doi: 10.1016/s0149-7634(01)00013-6. [DOI] [PubMed] [Google Scholar]
- Weiss SM, Wadsworth G, Fletcher A, Dourish CT. Utility of ethological analysis to overcome locomotor confounds in elevated maze models of anxiety. Neurosci Biobehav Rev. 1998;23:265–2671. doi: 10.1016/s0149-7634(98)00027-x. [DOI] [PubMed] [Google Scholar]
- Weninger SC, Dunn AJ, Muglia LJ, Dikkes P, Miczek KA, Swiergiel AH, Berridge CW, Majzoub JA. Stress-induced behaviors require the CRH receptor, but not CRH. Proc. Natl. Acad. Sci. USA. 1999;96:8283–8288. doi: 10.1073/pnas.96.14.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubareva OE, Efremov OM, Simbirtsev AS, Klimenko VM. Interleukin-1 beta and depressive states. Ross. Fiziol. Zh. Im. I. M. Sechenova. 2001;87:1450–1456. [PubMed] [Google Scholar]


