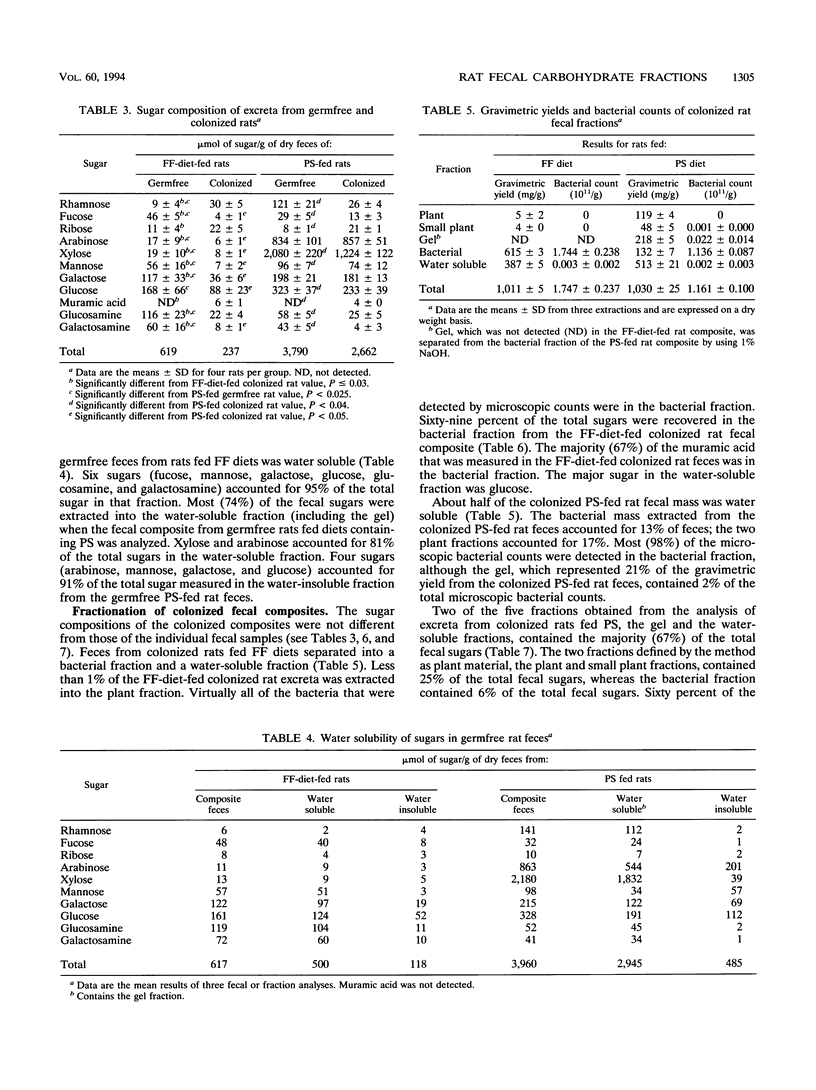
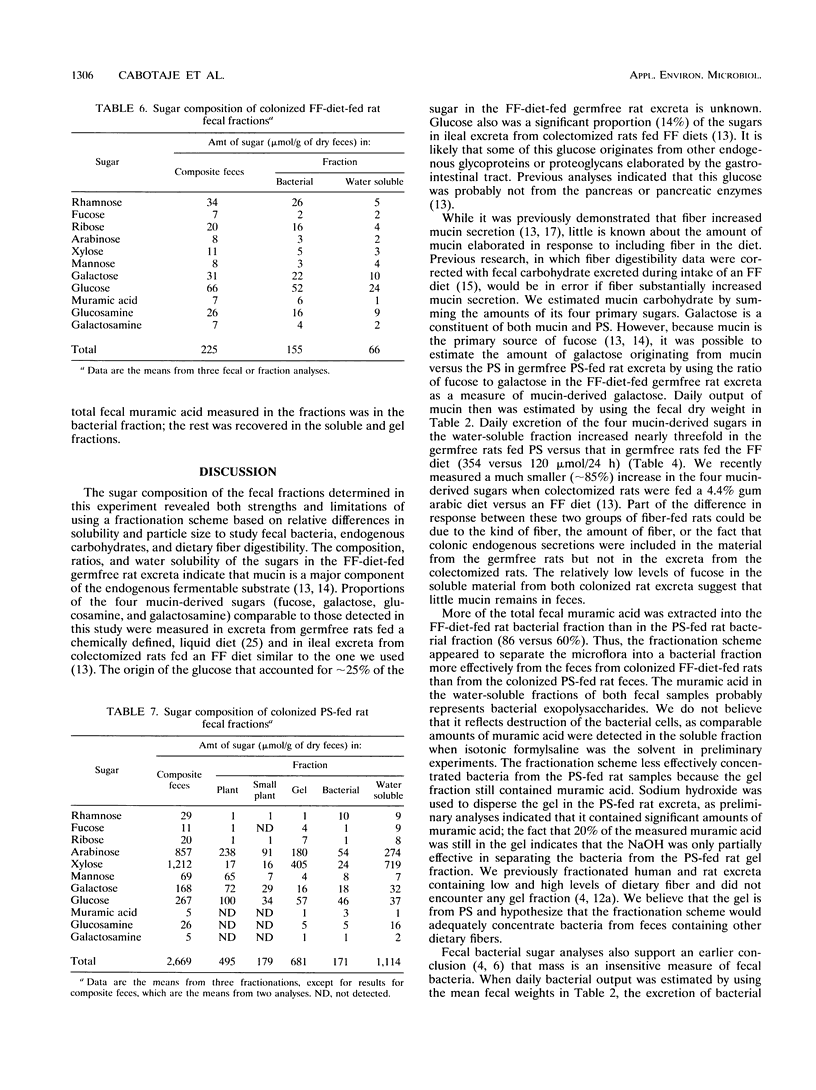
Abstract
The effect of psyllium on mucin secretion was determined by comparing water-soluble and -insoluble fractions of excreta from germfree rats fed a fiber-free (FF) diet or a diet containing psyllium seed husk (PS). Excreta from the same rats after colonization with a rat mixed cecal culture were separated into water-soluble, plant, and bacterial fractions to compare the remaining carbohydrate and the mass of bacteria. The sugar composition and water solubility of carbohydrate in excreta from germfree rats fed FF diets indicated that a primary fermentable substrate was mucin. PS increased fecal excretion of mucin-derived sugars almost threefold in germfree rats. Fecal carbohydrate was reduced from 619 to 237 mumol/g of dry feces and mostly in the bacterial fraction when rats fed an FF diet were colonized. The total sugar content and the amount of muramic acid, but not bacterial counts and mass, indicated that PS increased fecal bacteria. Fractionation of excreta from PS-fed rats was complicated by a gel which, based on sugar composition, was PS. Sugar composition of the water-soluble fraction from excreta from PS-fed rats suggested that it contained some bacterial component, possibly exopolysaccharides and some of the PS, but not mucin. PS digestibility ranged from 60 to 80%, depending on what fecal fraction was used for output. Because of the presence of PS-derived sugars in the gel and soluble fraction, it was not possible to determine which, if any, of the PS digestibilities was correct.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Cabotaje L. M., López-Guisa J. M., Shinnick F. L., Marlett J. A. Neutral sugar composition and gravimetric yield of plant and bacterial fractions of feces. Appl Environ Microbiol. 1990 Jun;56(6):1786–1792. doi: 10.1128/aem.56.6.1786-1792.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsum E., Eriksson C., Göranzon H., Sohlström A. Composition of faeces from human subjects consuming diets based on conventional foods containing different kinds and amounts of dietary fibre. Br J Nutr. 1990 Jul;64(1):171–186. doi: 10.1079/bjn19900019. [DOI] [PubMed] [Google Scholar]
- Kraus R. J., Shinnick F. L., Marlett J. A. Simultaneous determination of neutral and amino sugars in biological materials. J Chromatogr. 1990 Jul 27;513:71–81. doi: 10.1016/s0021-9673(01)89426-7. [DOI] [PubMed] [Google Scholar]
- Livesey G. Energy values of unavailable carbohydrate and diets: an inquiry and analysis. Am J Clin Nutr. 1990 Apr;51(4):617–637. doi: 10.1093/ajcn/51.4.617. [DOI] [PubMed] [Google Scholar]
- Monsma D. J., Vollendorf N. W., Marlett J. A. Determination of fermentable carbohydrate from the upper gastrointestinal tract by using colectomized rats. Appl Environ Microbiol. 1992 Oct;58(10):3330–3336. doi: 10.1128/aem.58.10.3330-3336.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyman M., Asp N. G. Fermentation of dietary fibre components in the rat intestinal tract. Br J Nutr. 1982 May;47(3):357–366. doi: 10.1079/bjn19820047. [DOI] [PubMed] [Google Scholar]
- Satchithanandam S., Vargofcak-Apker M., Calvert R. J., Leeds A. R., Cassidy M. M. Alteration of gastrointestinal mucin by fiber feeding in rats. J Nutr. 1990 Oct;120(10):1179–1184. doi: 10.1093/jn/120.10.1179. [DOI] [PubMed] [Google Scholar]
- Sharon N. The bacterial cell wall. Sci Am. 1969 May;220(5):92–98. doi: 10.1038/scientificamerican0569-92. [DOI] [PubMed] [Google Scholar]
- Shinnick F. L., Longacre M. J., Ink S. L., Marlett J. A. Oat fiber: composition versus physiological function in rats. J Nutr. 1988 Feb;118(2):144–151. doi: 10.1093/jn/118.2.144. [DOI] [PubMed] [Google Scholar]
- Stephen A. M., Cummings J. H. Mechanism of action of dietary fibre in the human colon. Nature. 1980 Mar 20;284(5753):283–284. doi: 10.1038/284283a0. [DOI] [PubMed] [Google Scholar]
- Stephen A. M., Cummings J. H. The microbial contribution to human faecal mass. J Med Microbiol. 1980 Feb;13(1):45–56. doi: 10.1099/00222615-13-1-45. [DOI] [PubMed] [Google Scholar]
- Stevens J., Levitsky D. A., VanSoest P. J., Robertson J. B., Kalkwarf H. J., Roe D. A. Effect of psyllium gum and wheat bran on spontaneous energy intake. Am J Clin Nutr. 1987 Nov;46(5):812–817. doi: 10.1093/ajcn/46.5.812. [DOI] [PubMed] [Google Scholar]
- Vahouny G. V., Le T., Ifrim I., Satchithanandam S., Cassidy M. M. Stimulation of intestinal cytokinetics and mucin turnover in rats fed wheat bran or cellulose. Am J Clin Nutr. 1985 May;41(5):895–900. doi: 10.1093/ajcn/41.5.895. [DOI] [PubMed] [Google Scholar]
- Wold J. K., Khan R., Midtvedt T. Intestinal glycoproteins of germfree rats. Chemical composition of intestinal and fecal mucus from germfree rats fed a chemically defined diet. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(4):525–530. [PubMed] [Google Scholar]



