Abstract
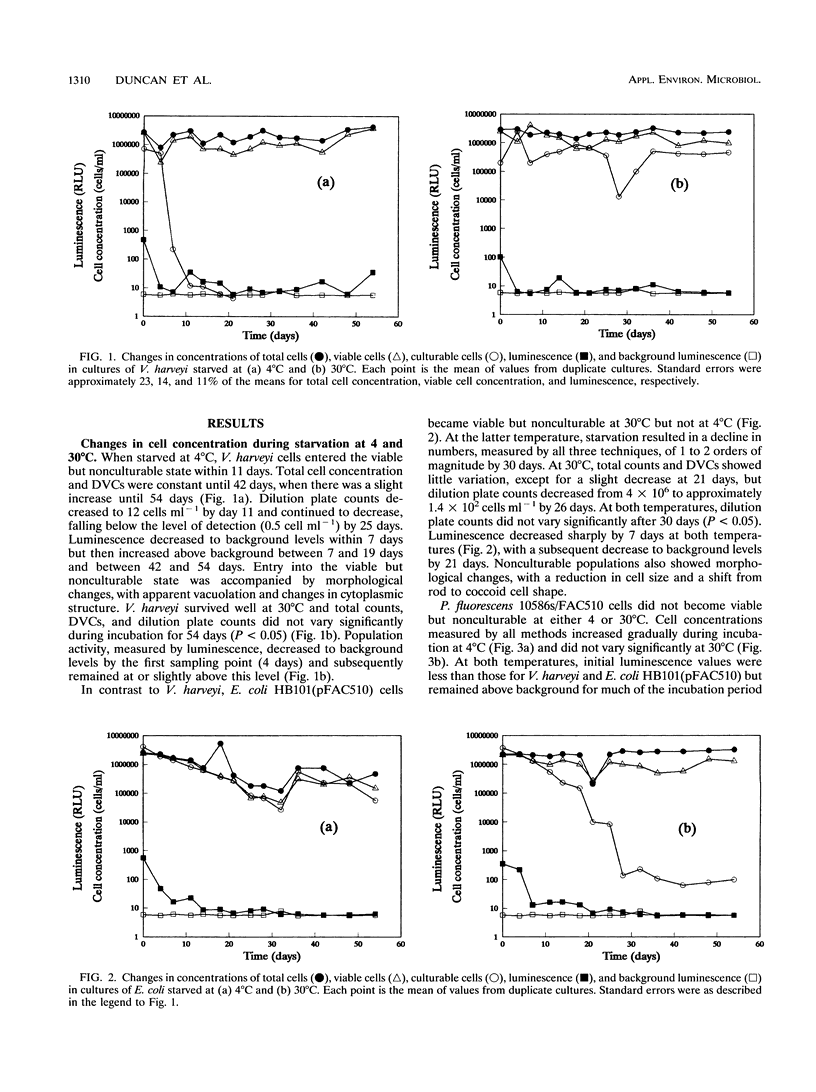
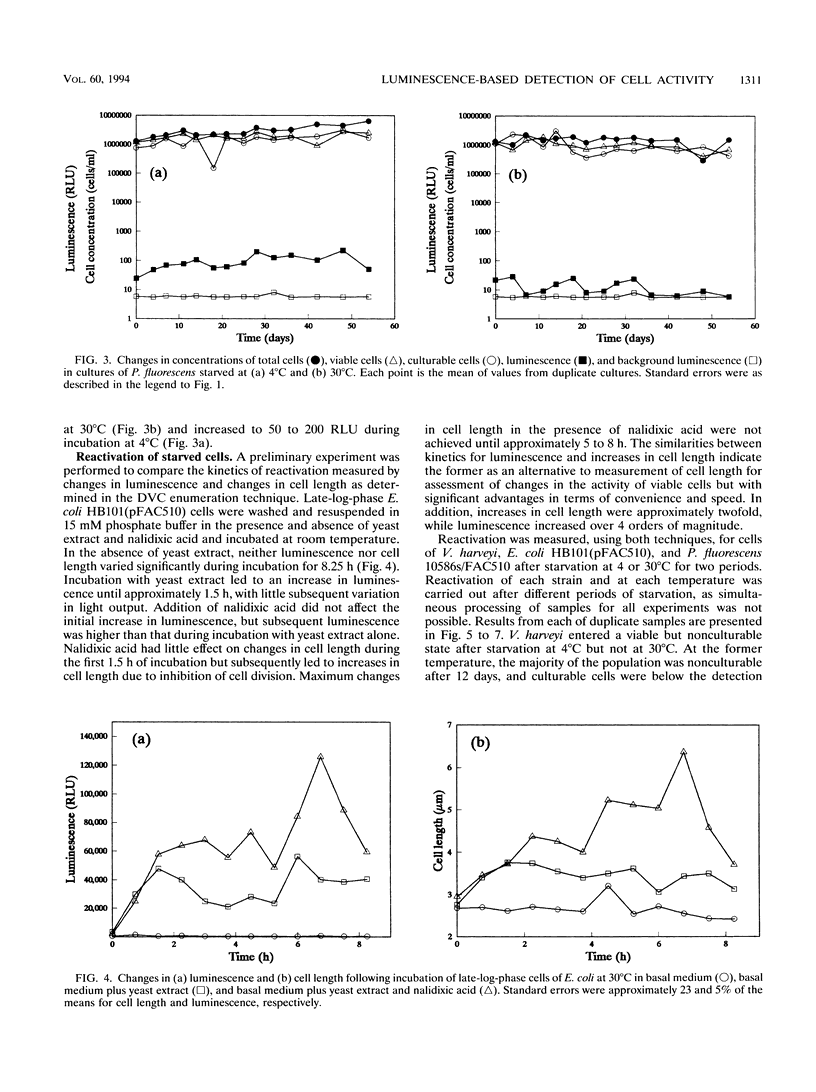
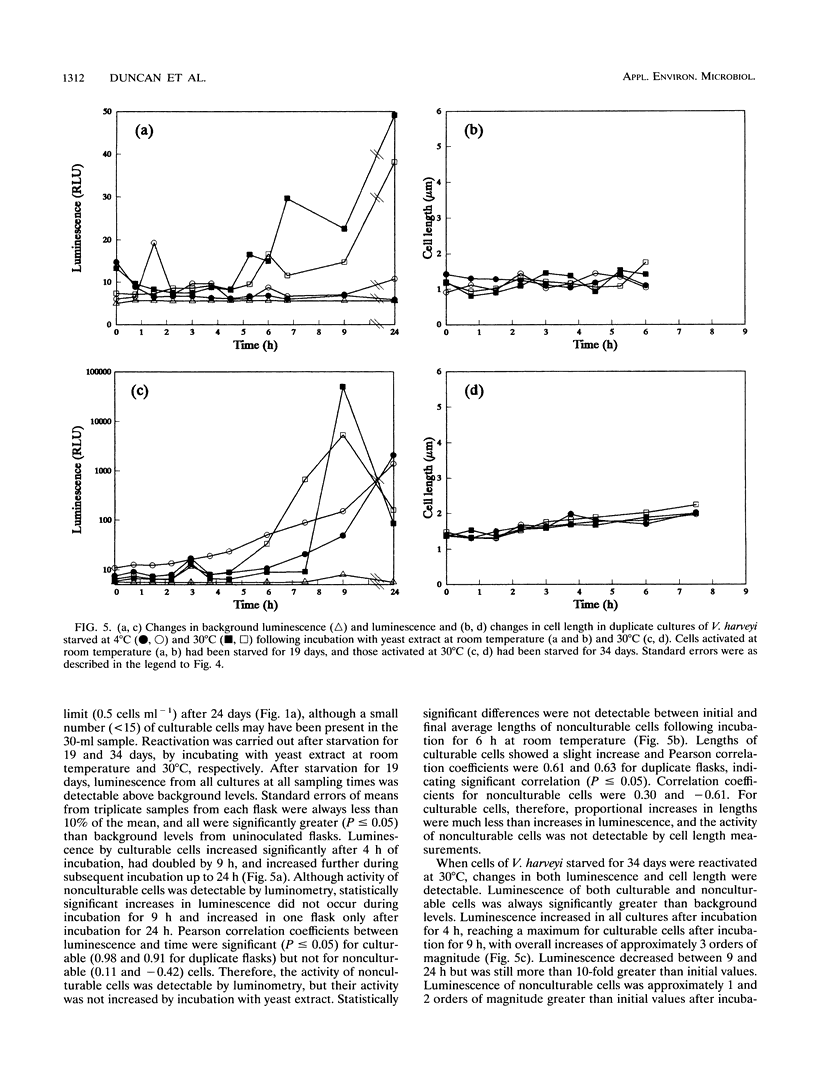
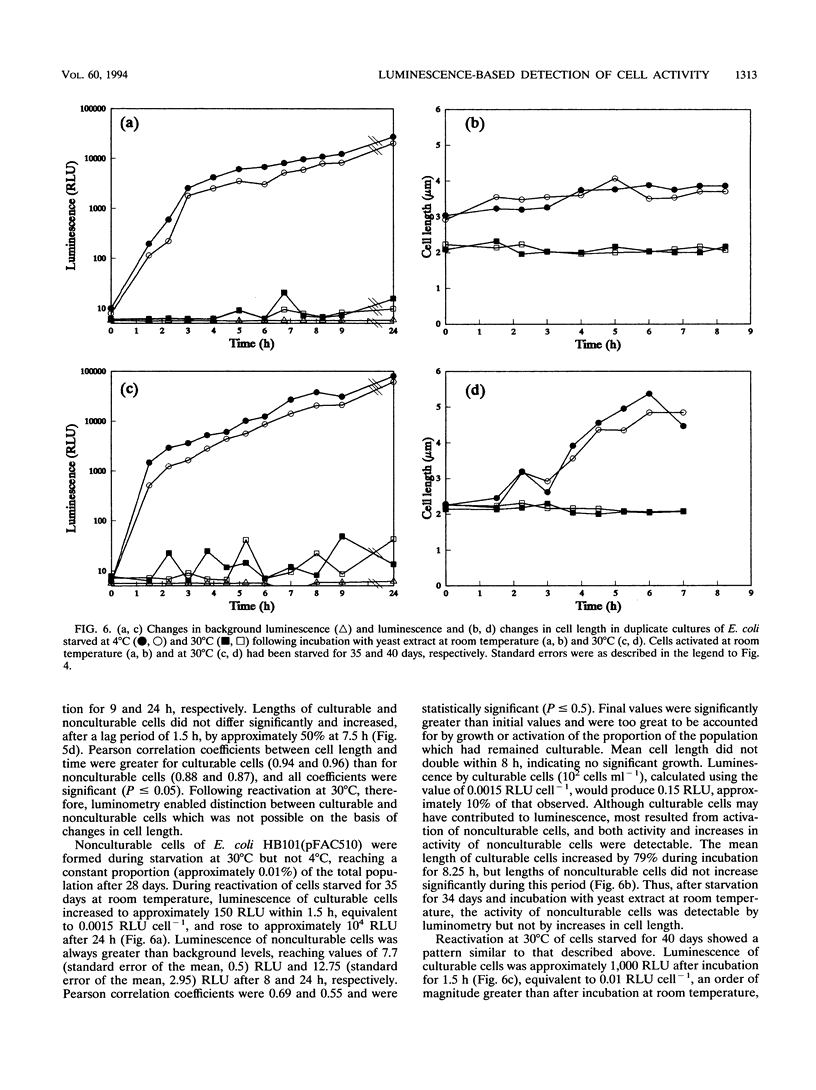
A naturally luminescent bacterium, Vibrio harveyi, and two bacteria, Escherichia coli and Pseudomonas fluorescens, which had been genetically marked with luminescence were starved in liquid medium at 4 and 30 degrees C for 54 days. Total cell concentrations and concentrations of culturable and viable cells were determined by acridine orange staining, dilution plate counting, and direct viable counting, respectively, and population activity was measured by luminometry. V. harveyi became nonculturable but maintained viability during starvation at 4 degrees C and maintained both culturability and viability at 30 degrees C. In contrast, E. coli became viable but nonculturable during starvation at 30 degrees C but not at 4 degrees C. Luminescence of nonculturable cells of both strains, and culturable cells of V. harveyi, decreased to background levels during starvation. Luminescence of starved culturable cells of E. coli also fell below background levels but occasionally increased to detectable values. Viable, nonculturable forms of P. fluorescens were not detected at either temperature, and cells starved at 4 degrees C showed no decrease in luminescence measured during incubation of samples at 25 degrees C. Following incubation of late-log-phase cells with yeast extract and nalidixic acid, changes in light output directly paralleled changes in cell length, as observed during direct viable counting. Quantification of changes in luminescence following incubation of starved cells with yeast extract enabled measurement of the activity of both culturable and viable but nonculturable cells. Measurement of luminescence was significantly more sensitive, rapid, and convenient in quantifying activity following nutrient amendment than measurement of changes in cell length.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson I. C., Rhodes M. W., Kator H. I. Seasonal variation in survival of Escherichia coli exposed in situ in membrane diffusion chambers containing filtered and nonfiltered estuarine water. Appl Environ Microbiol. 1983 Jun;45(6):1877–1883. doi: 10.1128/aem.45.6.1877-1883.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale M. J., Fry J. C., Day M. J. Plasmid transfer between strains of Pseudomonas aeruginosa on membrane filters attached to river stones. J Gen Microbiol. 1987 Nov;133(11):3099–3107. doi: 10.1099/00221287-133-11-3099. [DOI] [PubMed] [Google Scholar]
- Brauns L. A., Hudson M. C., Oliver J. D. Use of the polymerase chain reaction in detection of culturable and nonculturable Vibrio vulnificus cells. Appl Environ Microbiol. 1991 Sep;57(9):2651–2655. doi: 10.1128/aem.57.9.2651-2655.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd J. J., Xu H. S., Colwell R. R. Viable but nonculturable bacteria in drinking water. Appl Environ Microbiol. 1991 Mar;57(3):875–878. doi: 10.1128/aem.57.3.875-878.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure K., Simidu U., Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 1979 Mar;25(3):415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- Linder K., Oliver J. D. Membrane fatty acid and virulence changes in the viable but nonculturable state of Vibrio vulnificus. Appl Environ Microbiol. 1989 Nov;55(11):2837–2842. doi: 10.1128/aem.55.11.2837-2842.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle A., Killham K., Prosser J. I., Glover L. A. Luminometric measurement of population activity of genetically modified Pseudomonas fluorescens in the soil. FEMS Microbiol Lett. 1992 Dec 1;78(2-3):217–220. doi: 10.1016/0378-1097(92)90029-n. [DOI] [PubMed] [Google Scholar]
- Oliver J. D., Nilsson L., Kjelleberg S. Formation of nonculturable Vibrio vulnificus cells and its relationship to the starvation state. Appl Environ Microbiol. 1991 Sep;57(9):2640–2644. doi: 10.1128/aem.57.9.2640-2644.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattray E. A., Prosser J. I., Killham K., Glover L. A. Luminescence-based nonextractive technique for in situ detection of Escherichia coli in soil. Appl Environ Microbiol. 1990 Nov;56(11):3368–3374. doi: 10.1128/aem.56.11.3368-3374.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Colwell R. R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987 Sep;51(3):365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. J., Dane F., Geiger D., Kloepper J. W. Use of bioluminescence for detection of genetically engineered microorganisms released into the environment. Appl Environ Microbiol. 1992 Jan;58(1):267–273. doi: 10.1128/aem.58.1.267-273.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silcock D. J., Waterhouse R. N., Glover L. A., Prosser J. I., Killham K. Detection of a single genetically modified bacterial cell in soil by using charge coupled device-enhanced microscopy. Appl Environ Microbiol. 1992 Aug;58(8):2444–2448. doi: 10.1128/aem.58.8.2444-2448.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. W., Novitsky T. J., Quinby H. L., Valois F. W. Determination of bacterial number and biomass in the marine environment. Appl Environ Microbiol. 1977 Apr;33(4):940–946. doi: 10.1128/aem.33.4.940-946.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley C., Morgan J. A., Pickup R. W., Jones J. G., Saunders J. R. Differential regulation of lambda pL and pR promoters by a cI repressor in a broad-host-range thermoregulated plasmid marker system. Appl Environ Microbiol. 1989 Apr;55(4):771–777. doi: 10.1128/aem.55.4.771-777.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weger L. A., Dunbar P., Mahafee W. F., Lugtenberg B. J., Sayler G. S. Use of Bioluminescence Markers To Detect Pseudomonas spp. in the Rhizosphere. Appl Environ Microbiol. 1991 Dec;57(12):3641–3644. doi: 10.1128/aem.57.12.3641-3644.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]



