Abstract
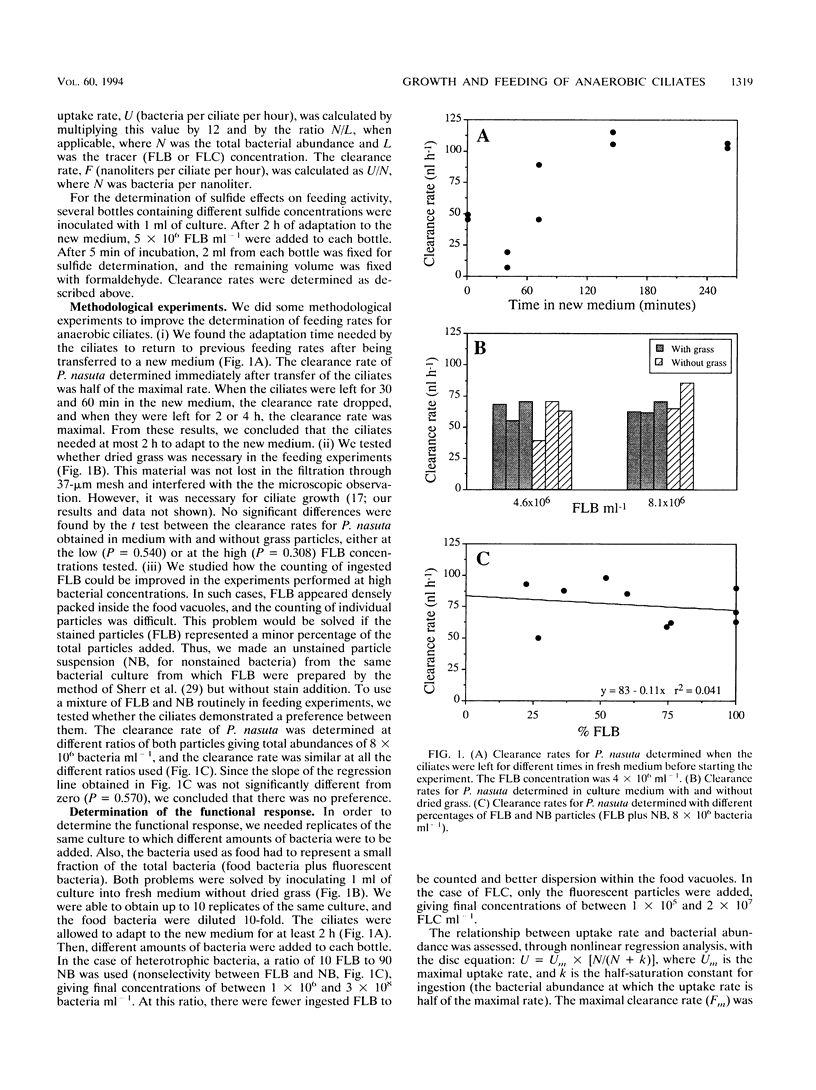
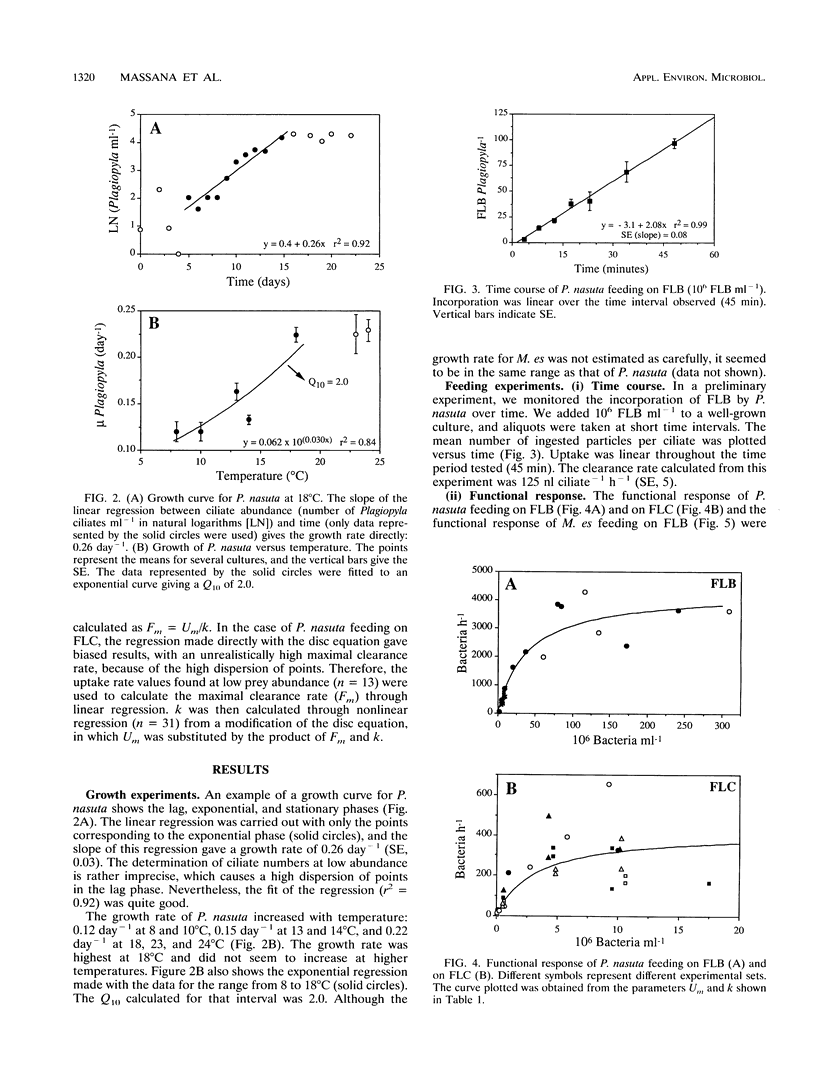
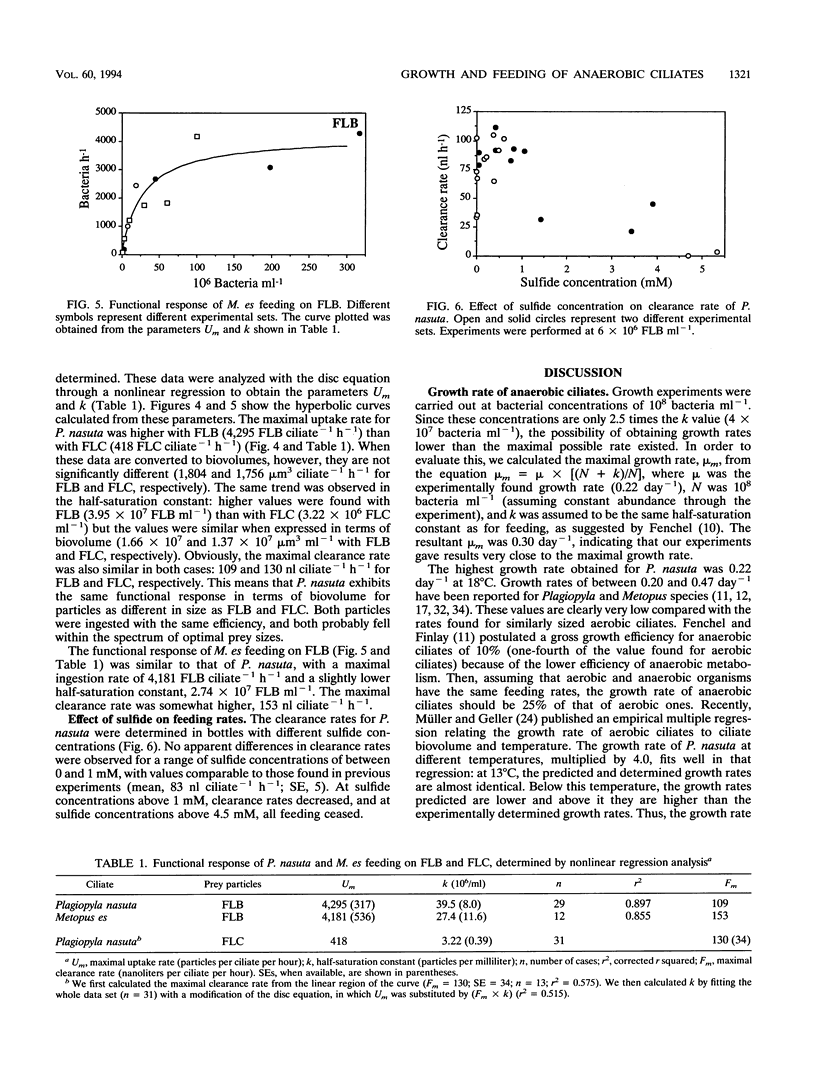
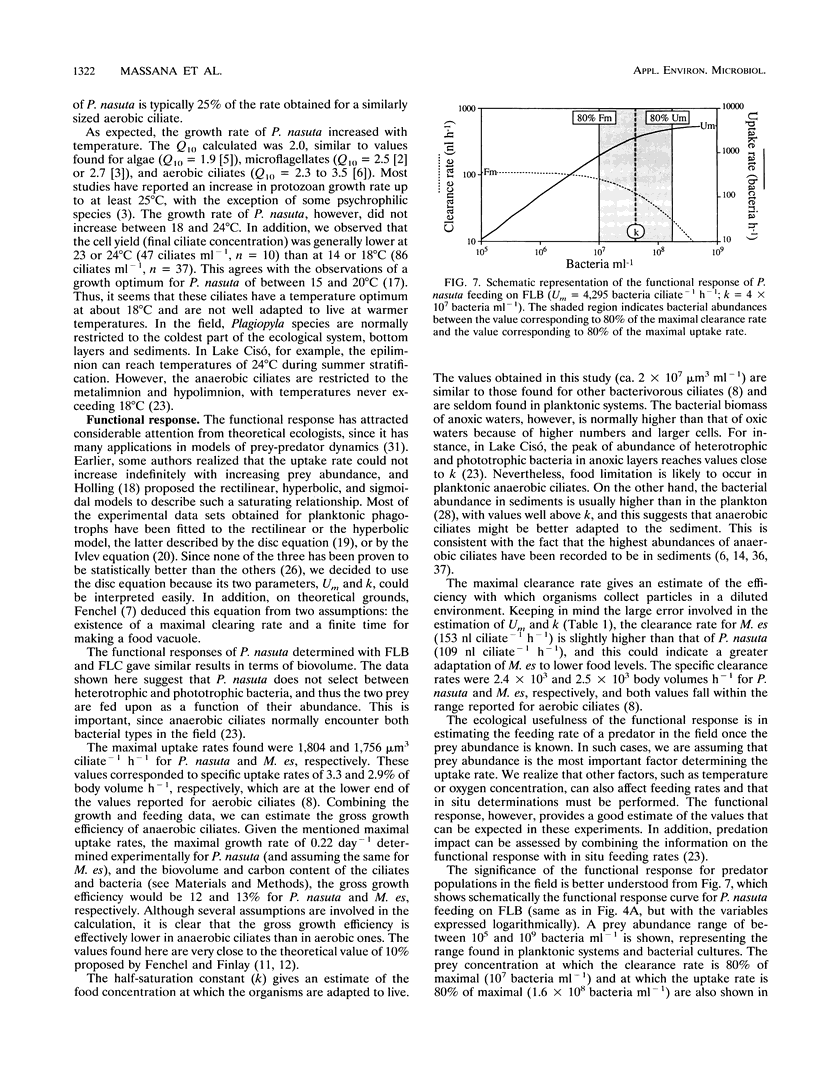
The trophic role of ciliates in anaerobic food webs has not been assessed experimentally. In order to obtain basic information necessary to interpret field situations, we studied the effects of temperature, sulfide concentration, and food abundance on the growth and feeding activities of two anaerobic ciliates, Plagiopyla nasuta and Metopus es. The growth rate of P. nasuta increased with temperature from 8 to 18°C (Q10 = 2.0) and remained constant in the range between 18 and 24°C (0.22 day-1). Sulfide concentrations of between 0 and 1 mM did not affect the feeding activities, but concentrations greater than 2 mM were inhibitory. The functional response of P. nasuta feeding on fluorescently labeled heterotrophic and phototrophic bacteria was investigated. In both cases, the parameters of the functional response were almost identical when expressed in terms of biovolume: the maximal uptake rate (Um) was 1,800 μm3 ciliate-1 h-1 and the half-saturation constant for ingestion (k) was 1.5 × 107 μm3 ml-1. The functional response of M. es feeding on heterotrophic bacteria was found to be similar to that of P. nasuta. These ciliates needed high bacterial abundances in order to maintain their growth (k of about 4 × 107 bacteria ml-1), implying that they will frequently be food limited in planktonic environments. Both the maximal uptake rates and the maximal clearance rates were comparable to those of aerobic ciliates. By combining the growth and feeding data, we estimated gross growth efficiencies of 12 and 13% for P. nasuta and M. es, respectively. These results indicate that the feeding rates of anaerobic ciliates are similar to those of aerobic ciliates. Their slower growth must, therefore, be due to the lower gross growth efficiency (likely due to anaerobic metabolism).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjørnsen P. K. Automatic determination of bacterioplankton biomass by image analysis. Appl Environ Microbiol. 1986 Jun;51(6):1199–1204. doi: 10.1128/aem.51.6.1199-1204.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron D. A., Goldman J. C., Dennett M. R. Effect of temperature on growth, respiration, and nutrient regeneration by an omnivorous microflagellate. Appl Environ Microbiol. 1986 Dec;52(6):1340–1347. doi: 10.1128/aem.52.6.1340-1347.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. W., Peters F. Effects of Temperature on Two Psychrophilic Ecotypes of a Heterotrophic Nanoflagellate, Paraphysomonas imperforata. Appl Environ Microbiol. 1992 Feb;58(2):593–599. doi: 10.1128/aem.58.2.593-599.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doddema H. J., Vogels G. D. Improved identification of methanogenic bacteria by fluorescence microscopy. Appl Environ Microbiol. 1978 Nov;36(5):752–754. doi: 10.1128/aem.36.5.752-754.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massana R., Pedrós-Alió C. Role of anaerobic ciliates in planktonic food webs: abundance, feeding, and impact on bacteria in the field. Appl Environ Microbiol. 1994 Apr;60(4):1325–1334. doi: 10.1128/aem.60.4.1325-1334.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M. Energy metabolism of protozoa without mitochondria. Annu Rev Microbiol. 1988;42:465–488. doi: 10.1146/annurev.mi.42.100188.002341. [DOI] [PubMed] [Google Scholar]
- Sherr B. F., Sherr E. B., Fallon R. D. Use of monodispersed, fluorescently labeled bacteria to estimate in situ protozoan bacterivory. Appl Environ Microbiol. 1987 May;53(5):958–965. doi: 10.1128/aem.53.5.958-965.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



