Abstract
Background
Asthma and obesity are common conditions that are strongly associated. This association might be due to shared genetic or environmental causes.
Objective
We sought to determine whether a shared genetic cause is responsible for the association between asthma and obesity and to estimate the magnitude of shared genetic cause.
Methods
The analyses were performed with 1001 monozygotic and 383 dizygotic same-sex twin pairs within the University of Washington Twin Registry. The presence of asthma was determined by self-report of a physician diagnosis of asthma, and body mass index (BMI) was calculated by using self-reported height and weight. Obesity was defined as a BMI of 30 or greater. The association between asthma and BMI was assessed by means of mixed-effects ordinal regression. Twin correlations examined the association of asthma and obesity. Univariate and bivariate structural equation models estimated the components of variance attributable to genetic and environmental effects.
Results
A strong association between asthma and BMI was identified in the sample population (P < .001). Substantial heritability was detected for asthma (53%) and obesity (77%), which is indicative of additive genetic influences on each disorder. The best-fitting model of shared components of variance indicated that 8% of the genetic component of obesity is shared with asthma.
Conclusion
The covariation between obesity and asthma is predominantly caused by shared genetic risk factors for both conditions.
Keywords: Asthma, obesity, genetic, twin
Asthma and obesity are both common conditions of great public health concern worldwide.1,2 Between 1980 and 1994, the prevalence of self-reported asthma increased by 73.4% in the United States.3 Likewise, the prevalence of obesity increased from 12.5% in 1960 to 22.5% in 19945 and continues to increase.5 These parallel trends in asthma and obesity in developed countries3,4 suggest that shared environmental and genetic factors affect both conditions.1 The presence of shared genetic determinants for more than one condition is known as genetic pleiotropy.6
Asthma and obesity have been consistently related in cross-sectional7–11 and longitudinal12–15 epidemiologic studies. This relationship between asthma and obesity is stronger in women than in men.3,4,12,15,16 Obesity is a significant independent predictor of the persistence of childhood asthma into adulthood.17 Although asthma and obesity follow a polygenic mode of inheritance in which genes with low penetrance are responsible for the genetic susceptibility,2 the extent to which the association is due to genetic factors shared by both conditions is unknown. Notably, linkage analyses of asthma and obesity demonstrate an overlap of chromosomal regions linked to each condition.2
Quantitative genetic analysis of twins can be used to identify a shared cause and ascertain whether genetic or environmental influences predominate.18 Therefore we used data from a community-based American twin registry to (1) measure the association between asthma and obesity, (2) assess the genetic influence on each trait, and (3) estimate the magnitude of shared genetic cause that could explain the association between asthma and obesity.
METHODS
Sample population
The study population consisted of 1001 monozygotic (MZ) and 383 dizygotic (DZ) same-sex twin pairs registered in the University of Washington Twin Registry, which is a community-based sample of twins derived from the driver’s license applications of the Washington State Department of Licensing. Unique to Washington State, all new applicants for a driver’s license are asked if they are a twin. Because Washington State law allows state agencies to share data, a weekly electronic list of all new driver’s license applicants who are twins is transmitted weekly to the University of Washington and forms the basis of the twin registry. These applicants are invited, along with their co-twin, to become members of the University of Washington Twin Registry. The University of Washington Human Subjects Review Committee and the Washington State Attorney General approved the procedures for establishing the twin registry and all data collection involved in this study. Informed consent was obtained from all participants.
Data collection
A brief survey was administered to all registry members by mail or telephone that included age, race-ethnicity, sex, education, and marital status. Zygosity was assigned by using standard questions on childhood similarity that correctly classify twins as MZ or DZ more than 95% of the time.19–21 Health conditions, including asthma, were obtained by using a checklist of self-reported medical problems that asked specifically whether a physician ever diagnosed the condition.
Height and weight were obtained by self-report. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. BMI was divided into 7 categories reported in previous studies10,14,22,23 to examine the relationship between asthma and obesity. The 7 categories represent low (BMI <20.0), normal (BMI ≥20.0 to ≤22.49 and ≥22.50 to ≤24.99), overweight (BMI ≥25.0 to ≤27.49 and ≥27.50 to ≤29.99), and obese (BMI ≥30) BMI.
Statistical analysis
The classical twin study analysis is based on a comparison of phenotypic similarity in MZ and DZ twins. MZ twins have identical genotypes, and DZ twins share, on average, half of their genes. Greater phenotypic similarity, indicated by a higher correlation in MZ than DZ twins, is indicative of a genetic component in the cause of the disease. Structural equation modeling is a general statistical approach useful for estimating genetic and environmental effects in classical twin studies.24 In this approach genetic and environmental effects are modeled as latent variables representing an underlying liability for one or more phenotypes, such as asthma or obesity. Structural equation modeling is a highly flexible analysis that can determine the best-fitting and most parsimonious model for any phenotype and estimate the relative magnitude of the genetic, common environmental, and unique environmental effects. For example, by using structural equation modeling, it is possible to assess whether the MZ and DZ correlations for asthma are best explained by a model that includes genes alone, the common environment alone, or some combination of both genes and the common environment. For multiple phenotypes, such as asthma and obesity, structural equation modeling can be used to estimate how much of the variability in the 2 phenotypes is due to shared genetic, common environmental, and unique environmental effects. Structural equation models for twin studies typically use path diagrams to visually illustrate the relative magnitude of the hypothesized connections between the latent genetic, common environmental, and unique environmental effects with the observed phenotypes.
The distributions of age, race-ethnicity, physician-diagnosed asthma, BMI, and obesity were compared according to sex and zygosity by using χ² and t test statistics. The relationship between asthma and BMI was assessed by using mixed-effects ordinal regression.25 Age was added to the mixed-effects ordinal regression model to determine whether age affected the relationship between asthma and obesity. The association between asthma and obesity in MZ and DZ pairs was initially assessed by 3 types of tetrachoric correlations: phenotypic, twin, and cross-twin, cross-trait. Structural equation modeling was used to estimate the components of phenotypic variance caused by additive genetic (A), common environment (C), and unique environment (E) from the within-pair twin correlations for asthma and obesity in MZ and DZ pairs.24 A model was fit to the twin correlations on the basis of the additive genetic correlation of 1.0 for MZ and 0.5 for DZ twins and a shared environmental correlation of 1.0 for all twins. Parameter estimates for the full ACE model were then estimated. Reduced models were constructed by removing a specific parameter and comparing the goodness of fit of the full and reduced models by using a likelihood ratio χ² test. Parameters were removed from the model if the removal did not result in a significant degradation of model fit. A model parameter was considered significant if its omission resulted in a decrement in fit of the model at the .05 level of significance.
Models were also evaluated by using Akaike Information Criterion26 to compare alternative models. The model with the lowest Akaike Information Criteria was judged to have superior fit over models with larger Akaike Information Criteria values. The proportions of variance for additive genetics, common environment, and unique environment were estimated from the final best-fitting model.
To test for the presence of shared genetic and environmental influence on asthma and obesity, we fit bivariate structural equation models of asthma and obesity. The model started with a full Cholesky decomposition that specifies a general multivariate covariance structure that allows for both shared and specific influences on asthma and obesity. Reduced models were then fit after removing shared or specific influences. The final best-fitting and most parsimonious model was identified by removing factors that did not significantly degrade the fit of the model on the basis of the likelihood ratio χ² test and Akaike Information Criteria. Estimates of the components of variance caused by both shared and specific genetic and environmental influences were calculated from the path coefficients of the best-fitting bivariate model. The narrow genetic correlation was calculated from the path coefficients, as previously described.27
RESULTS
Sample characteristics
The University of Washington Twin Registry has enrolled 1042 pairs of MZ, 406 pairs of same-sex DZ, and 422 pairs of opposite-sex DZ twins. Because of the known sex differences in the association between asthma and obesity,3,4,12,15,16 the analyses were restricted to 1001 MZ and 383 DZ same-sex twin pairs with complete asthma and BMI information (2768 individuals). The prevalence of physician-diagnosed asthma was 14.1%, and that of obesity was 13.5%. The characteristics of this sample according to sex are presented in Table I. The prevalence of asthma was higher in women than men. Conversely, the mean BMI was higher in men than in women (25.1 kg/m² in men vs 24.4 kg/m² in women, P<.001); however, the prevalence of obesity was slightly higher in women than men (14.1% in women and 12.5% in men). When we grouped the study population into 7 categories of BMI, the prevalence of asthma increased with greater BMI in women (P < .001, Fig 1) and marginally in men (P = .07). The association between asthma and BMI was not appreciably changed by adjustment for age (P < .001 in women and P = .06 in men).
TABLE I.
Characteristics of same-sex twins enrolled in the University of Washington Twin Registry
| Men | Women | |
|---|---|---|
| Characteristic | (n = 1010) | (n = 1758) |
| Zygosity | ||
| Monozygotic, n (%) | 736 (72.9) | 1266 (72.0) |
| Dizygotic, n (%) | 274 (27.1) | 492 (28.0) |
| Age, mean y (SD) | 32.3 (14.1) | 33.2 (14.8) |
| Race-ethnicity, n (%)* | ||
| White (non-Hispanic) | 880 (87.1) | 1528 (86.9) |
| African American | 37 (3.7) | 41 (2.3) |
| Asian–Pacific Islander | 42 (4.2) | 67 (3.8) |
| Hispanic | 21 (2.1) | 62 (3.5) |
| Native Indian–Alaska Native | 8 (0.8) | 30 (1.7) |
| Other | 22 (2.2) | 30 (1.7) |
| Physician-diagnosed asthma, n (%)† | 112 (11.1) | 278 (15.8) |
| BMI, mean (SD)† | 25.1 (4.7) | 24.4 (5.1) |
| BMI, n (%)‡ | ||
| <20 | 77 (7.6) | 274 (15.6) |
| 20–22.49 | 253 (25.1) | 520 (29.6) |
| 22.5–24.99 | 239 (23.7) | 386 (22.0) |
| 25–27.49 (overweight) | 205 (20.3) | 234 (13.3) |
| 27.5–29.99 (overweight) | 110 (10.9) | 96 (5.5) |
| 30–39.99 (obese) | 115 (11.4) | 222 (12.6) |
| ≥ 40 (obese) | 11 (1.1) | 26 (1.5) |
P < .05.
P < .001.
P < .0001.
FIG 1.
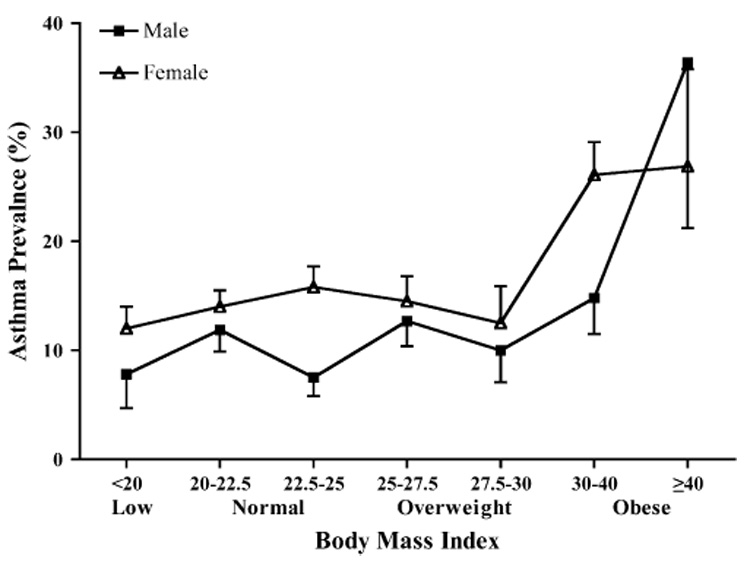
Association between asthma and obesity in the University of Washington Twin Registry. The prevalence of asthma was assessed according to 7 categories of BMI. The association of asthma and obesity was assessed in each sex by using mixed-effects ordinal regression. The prevalence of asthma increased with greater BMI in women (P < .001) and marginally in men (P = .07).
The characteristics of the study population also differed according to zygosity. Compared with MZ twins, DZ twins were older (35.9 vs 31.7 years, P < .0001) and had higher BMI (25.4 vs 24.4 kg/m², P < .0001).
Biometric genetic models
Table II presents 3 types of tetrachoric correlations in MZ and DZ pairs for asthma and obesity: phenotypic, twin, and cross-twin, cross-trait. The within-pair similarity of the trait was measured by using the twin correlation. The higher twin correlation in MZ than DZ twins is indicative of genetic influence on each trait. Phenotypic correlations measure the association of obesity and asthma within individuals in the sample population. The phenotypic correlations indicate that asthma is associated with obesity, although the magnitude of this association is small. The cross-twin, cross-trait correlations assessed the degree of association for 2 traits; for example, the relationship of obesity in twin 1 and asthma in twin 2, as well as obesity in twin 2 and asthma in twin 1. The higher cross-twin, cross-trait correlations in MZ compared with DZ pairs is indicative of shared genes influencing both traits.
TABLE II.
Tetrachoric correlations and 95% CIs for asthma and obesity according to zygosity*
| Twin 1 |
Twin 2 |
|||
|---|---|---|---|---|
| Asthma | Obesity | Asthma | Obesity | |
| Monozygotic (n = 1001) | ||||
| Twin 1 | ||||
| Asthma | 1.000 | |||
| Obesity | 0.13 (0.00–0.27)† | 1.000 | ||
| Twin 2 | ||||
| Asthma | 0.51 (0.40–0.62)‡ | 0.13 (0.00–0.28)§ | 1.000 | |
| Obesity | 0.10 (0.00–0.25)§ | 0.77 (0.68–0.83)‡ | 0.20 (0.05–0.35)† | 1.000 |
| Dizygotic (n = 383) | ||||
| Twin 1 | ||||
| Asthma | 1.000 | |||
| Obesity | 0.17 (0.00–0.38)† | 1.000 | ||
| Twin 2 | ||||
| Asthma | 0.34 (0.14–0.52)‡ | 0.00 (0.00–0.19)§ | 1.000 | |
| Obesity | 0.13 (0.00–0.33)§ | 0.38 (0.18–0.55)‡ | 0.41 (0.21–0.58)† | 1.000 |
The correlations and 95% CIs have a lower bound of zero.
Phenotypic correlation between asthma and obesity.
Twin correlations.
Cross-twin, cross-trait correlation.
Table III shows the results of the univariate structural equation models for asthma and obesity. In the full model 35% of the phenotypic variance of asthma was due to additive genetic effects. By using sequential reduced models based on the likelihood ratio χ² test, the best-fitting model for asthma included both additive genetic effects (53%) and unique environmental effects (47%). Similarly, the full model for obesity indicated that 77% of the phenotypic variation was due to additive genetic effects. The best-fitting model, derived from sequential reduced models, included additive genetic effects (77%) and unique environmental exposures (23%). For both asthma and obesity, common environmental effects could be removed without deterioration in the fit of the models.
TABLE III.
Univariate biometric genetic models for asthma and obesity
| Estimates of variance components† |
Tests of model fit |
||||||
|---|---|---|---|---|---|---|---|
| Model* | Additive genetic (A) | Common environment (C) | Unique environment (E) | χ² | df | P Value | AIC‡ |
| Asthma | |||||||
| ACE | 0.35 (0.00–0.62) | 0.17 (0.00–0.52) | 0.49 (0.38–0.60) | - | - | - | - |
| AE | 0.53 (0.41–0.63) | - | 0.47 (0.37–0.59) | 0.62 | 1 | .43 | −1.38 |
| CE | - | 0.46 (0.36–0.56) | 0.54 (0.44–0.64) | 2.37 | 1 | .12 | 0.37 |
| Obesity | |||||||
| ACE | 0.77 (0.40–0.83) | 0.00 (0.00–0.34) | 0.23 (0.17–0.32) | - | - | - | - |
| AE | 0.77 (0.68–0.83) | - | 0.23 (0.17–0.32) | 0.00 | 1 | 1.00 | −2.00 |
| CE | - | 0.66 (0.58–0.73) | 0.34 (0.27–0.42) | 17.89 | 1 | <.0001 | 15.89 |
ACE refers to a model that includes additive genetics (A), common environment (C), and unique environment (E); AE only includes additive genetics and unique environment; and CE only includes the common and unique environment.
Proportion of variance caused by additive genetics (a²), shared environment (c²), and unique environment (e²) according to each model.
Akaike’s information criterion (AIC) is a global measure of goodness of fit; the best-fitting and most parsimonious models are shown in bold.
Bivariate genetic analysis
Bivariate models of asthma and obesity specified a structural equation model for shared and specific additive genetic, common, and unique environmental components of variance (Table IV). By using sequential reduced models based on the likelihood ratio χ² test and Akaike Information Criterion, the best-fitting and most parsimonious bivariate model included shared additive genetic and shared unique environment influences for asthma and obesity, as well as specific genetic, common environmental, and unique environmental effects for each phenotype (Table V). There was no evidence of a shared common environmental influence on asthma and obesity. The full model with standardized pathway coefficients is illustrated in Fig 2. The narrow genetic correlation between asthma and obesity was 0.29. Therefore additive genetic effects, which are correlated 0.29, accounted for 65% of the covariance between asthma and obesity. On the basis of the best-fitting model shown in Table V, 8% of the genetic component of obesity is shared with asthma, and 5% of the specific environment component is shared. The findings indicate that the association between asthma and obesity is substantially due to shared genetic risk factors for both conditions, also known as genetic pleiotropy.
TABLE IV.
Bivariate structural equation models of asthma and obesity
| Shared component | χ² | df | P value | AIC* |
|---|---|---|---|---|
| ACE | - | - | - | - |
| CE | 1.06 | 1 | .30 | −0.94 |
| AE | 0.00 | 1 | 1.00 | −2.00 |
| AC | 1.13 | 1 | .29 | −0.87 |
| A | 2.20 | 2 | .33 | −1.80 |
| C | 6.85 | 2 | .03 | 2.85 |
| E | 6.59 | 2 | .04 | 2.59 |
| None | 21.26 | 3 | <.0001 | 15.26 |
Akaike’s information criterion (AIC) is a global measure of goodness of fit; the best-fitting and most parsimonious model is shown in bold.
TABLE V.
Unique and shared additive genetic and environmental effects for asthma and obesity
| Proportion of variance (95% CI) |
|||
|---|---|---|---|
| Effect | Additive genetic (A) | Common environment (C) | Unique environment (E) |
| Trait specific | |||
| Asthma | 0.33 (0.01–0.62) | 0.18 (0.00–0.26) | 0.49 (0.38–0.58) |
| Obesity | 0.76 (0.38–0.83) | 0.01 (0.00–0.03) | 0.23 (0.17–0.32) |
| Shared | 0.08 | - | 0.05 |
FIG 2.
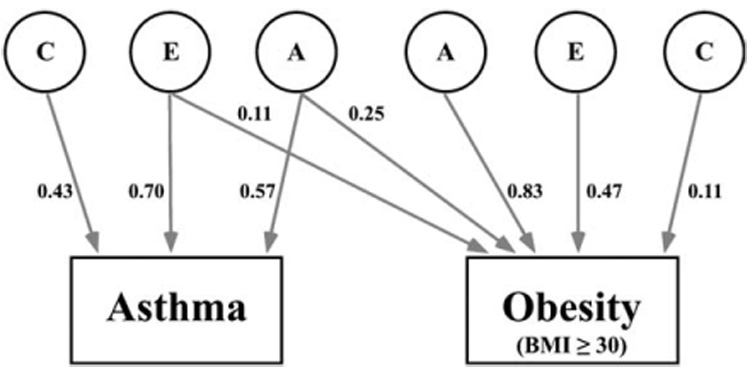
Path model depicting the additive genetic effects that are common to asthma and obesity plus additive genetic (A), unique environmental (E), and common environmental (C) effects that are unique to each phenotype. The parameter estimates are path coefficients, indicating the relative importance of the latent variables A, E, and C to asthma and obesity.
DISCUSSION
Asthma and obesity are common conditions in the United States and many other countries.1,2 Several studies have shown that asthma and obesity are associated, especially in women.3,4,12,15,16 The relationship between asthma and obesity was similarly identified in this community-based sample of twins. The magnitude of the association between asthma and obesity is modest in this and other studies; obesity is only one of the factors that influence the etiologically heterogeneous asthma phenotype. Like other studies, the prevalence of asthma was higher in women compared with that in men, even in the lower weight categories. This suggests that factors other than obesity are responsible for the difference in asthma prevalence between men and women. Although it is clear that obesity, defined as a BMI of 30 or greater, is associated with asthma, the relationship between a BMI of less than 30 and asthma is less clear. Some studies have shown a U-shaped distribution for the relationship between asthma and BMI in men,23,28,29 and others have failed to show a clear relationship between asthma and BMI in men.10,30,31
The association between obesity and asthma could be due to the influence of each phenotype on the other, shared environmental determinants, or shared genetic factors. For example, obesity has a direct mechanical effect on airway smooth muscle that increases bronchial hyperresponsiveness.32 Obesity alters the production of inflammatory mediators, such as TNF-α and IL-6, by constitutive expression in adipocytes.2 Reduced activity is associated with asthma and is likewise associated with body weight.33,34 Dietary factors, such as the intake of certain lipids and antioxidant vitamins, are associated with both asthma and obesity.35,36 In addition to these phenotypic and environmental influences on asthma and obesity, both traits are highly heritable conditions,37–40 a finding we replicated in this community-based sample of twins. In this study the presence and magnitude of the shared genetic influence on asthma and obesity was assessed by using structural equation models demonstrating significant genetic pleiotropy between the 2 conditions.
The additive genetic effects observed in the present study are the first to be reported from an American community-based sample of twins. The size of the additive genetic effect on asthma is similar to that found in twin studies from other countries.37 The consistency of genetic effects across countries is remarkable because the prevalence of asthma varies markedly worldwide.37
Genetic pleiotropy occurs when a gene or set of genes influences 2 or more traits.6 In this study we showed that 8% of the genetic component of obesity is shared with asthma. Although this is the first study to estimate the size of the shared genetic contribution, linkage studies of asthma and obesity have revealed regions of overlap at positions 2p, 5q, 6p, 7p, and 12q.2 Recent studies have demonstrated the biologic plausibility of this genetic association. The β2-adrenergic receptor is expressed on airway smooth muscle, inflammatory cells, and adipose tissue, where endogenous catecholamines influence energy expenditure, airway tone, and airway inflammation.41 Furthermore, insulin levels, which are altered in obesity, can influence β2-adrenergic receptor sensitivity.42 Polymorphisms of the β2-adrenergic receptor, although not associated with asthma per se, are associated with bronchial hyperresponsiveness,43,44 serum IgE,45 and response to treatment with β2-agonists.46–48 Polymorphisms of the β2-adrenergic receptor are also associated with obesity.49,50 A polymorphism at the −308 position in relation to the gene encoding TNF-α is associated with asthma,51,52 BMI,53 and obesity.54
Asthma and obesity are both polygenic disorders, in which the influence at any single locus is likely small.55 Therefore many genetic determinants will ultimately be identified for asthma, some of which will be shared with other polygenic traits. Although we identified significant overlap in the genetic determinants of asthma and obesity, as in other studies of pleiotropy, the magnitude of the shared influence was modest. For example, in a Finnish study of adult twins using comparable methods, the phenotypic variation between atopy and depression was due to shared additive genetic factors and shared unique environmental factors.27 Although the magnitude of the additive genetic effects on atopy and depression were less than in the present study, the narrow genetic correlation between the 2 traits was similar to that described here. Similarly, significant overlap in the genetic determinants of atopy and asthma has been demonstrated among 381 young twins recruited from an Australian volunteer twin registry.56
Common environmental effects are those shared by MZ and DZ twins and reflect influences, such as in utero exposures, socioeconomic class, and diet. Consistent with previous twin studies, the environmental determinants of asthma identified in the present study were unique, rather than common environmental effects.37 The absence of common environmental effects on atopic disease has also been demonstrated in a study of twins reared together and apart.57 Notably, the absence of common environmental effects on asthma in twin studies is inconsistent with epidemiologic studies in which diverse exposures increase (eg, house dust mite58) or decrease (eg, endotoxin59) the risk of asthma. One explanation is that common environmental factors might only influence asthma in the presence of genetic factors. In this situation, environmental effects that are only manifest in the presence of a specific genetic background, also known as gene-by-environment effects, will be concealed in the genetic component of the disease.37
This study has several limitations. First, our measures of physician-diagnosed asthma and BMI were obtained by self-report. However, physician-diagnosed asthma obtained on a questionnaire has a specificity of 0.99 and positive predictive value of 0.82 compared with an asthma diagnosis based on pertinent symptoms, lung function, bronchodilator response, and bronchial hyperresponsiveness.60 Self-reported weight and height might also underestimate actual BMI.61 In this regard the young age of the sample is advantageous because misclassification of asthma37 and obesity61 increase with age. A second concern is the predominance of white subjects and women, thereby limiting the generalizability of our findings to groups with other demographic features. Despite these limitations, the strengths of this study include sophisticated genetic modeling and the use of a community-based twin registry that minimizes biases associated with selected twin samples identified from clinical sources or volunteer registries.
In conclusion, an association between asthma and obesity was identified in a young community-based sample of twins in the United States. By using structural equation modeling, both asthma and obesity were found to be heritable, indicating strong genetic influences on each phenotype. We also found that unique environmental factors that are not shared by siblings also influenced both phenotypes, indicating that certain environmental factors work independently of genetic background. In contrast, common environmental factors, which are shared by siblings, did not influence either phenotype, although these effects might be hidden within the model if gene-by-environment effects are strong. Finally, our analyses demonstrated that a large part of the covariation between obesity and asthma is controlled by genetic factors. This is evidence of genetic pleiotropy, in which a common set of genes increases the susceptibility to both asthma and obesity. We also observed modest evidence for a shared unique environmental determinant for asthma and obesity. These findings also raise the possibility that both asthma and obesity are influenced by gene-by-environment interaction; for example, a specific environment, such as a westernized lifestyle, might be necessary for genetic effects to manifest on the asthma and obesity phenotypes. These gene-by-environment interactions would be concealed within the genetic covariation identified in this study. Diet is an important candidate for future studies of gene-by-environment interaction because it can effect the expression of genes through epigenetic mechanisms.62 Identification of modifiable environmental factors that lead to both obesity and asthma in the genetically susceptible host will have important implications for future efforts to curb the increasing prevalence of obesity and asthma.
Acknowledgments
Supported by National Institutes of Health grants K23HL04231 (TSH), K23HL72923 (MMW), and U19AI38429 (DB).
Abbreviations used
- BMI
Body mass index
- DZ
Dizygotic
- MZ
Monozygotic
Footnotes
Disclosure of potential conflict of interest: T. Hallstrand has received grants from Merck and Co. Medical School grant awarded to the University of Washington in 2000 and is on the speakers’ bureau for Merck and Co. All others—none disclosed.
REFERENCES
- 1.Weiss ST, Shore S. Obesity and asthma: directions for research. Am J Respir Crit Care Med. 2004;169:963–968. doi: 10.1164/rccm.200303-403WS. [DOI] [PubMed] [Google Scholar]
- 2.Tantisira KG, Weiss ST. Complex interactions in complex traits: obesity and asthma. Thorax. 2001;56 suppl 2:ii64–ii73. [PMC free article] [PubMed] [Google Scholar]
- 3.Mannino DM, Homa DM, Pertowski CA, Ashizawa A, Nixon LL, Johnson CA, et al. Surveillance for asthma—United States, 1960–1995. MMWR Surveill Summ. 1998;47:1–27. [PubMed] [Google Scholar]
- 4.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 5.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 6.Kauffmann F, Dizier MH, Pin I, Paty E, Gormand F, Vervloet D, et al. Epidemiological study of the genetics and environment of asthma, bronchial hyperresponsiveness, and atopy: phenotype issues. Am J Respir Crit Care Med. 1997;156 suppl:S123–S129. doi: 10.1164/ajrccm.156.4.12tac9. [DOI] [PubMed] [Google Scholar]
- 7.Shaheen SO, Sterne JA, Montgomery SM, Azima H. Birth weight, body mass index and asthma in young adults. Thorax. 1999;54:396–402. doi: 10.1136/thx.54.5.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figueroa-Munoz JI, Chinn S, Rona RJ. Association between obesity and asthma in 4–11 year old children in the UK. Thorax. 2001;56:133–137. doi: 10.1136/thorax.56.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Mutius E, Schwartz J, Neas LM, Dockery D, Weiss ST. Relation of body mass index to asthma and atopy in children: the National Health and Nutrition Examination Study III. Thorax. 2001;56:835–838. doi: 10.1136/thorax.56.11.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Dales R, Tang M, Krewski D. Obesity may increase the incidence of asthma in women but not in men: longitudinal observations from the Canadian National Population Health Surveys. Am J Epidemiol. 2002;155:191–197. doi: 10.1093/aje/155.3.191. [DOI] [PubMed] [Google Scholar]
- 11.Celedon JC, Palmer LJ, Litonjua AA, Weiss ST, Wang B, Fang Z, et al. Body mass index and asthma in adults in families of subjects with asthma in Anqing, China. Am J Respir Crit Care Med. 2001;164:1835–1840. doi: 10.1164/ajrccm.164.10.2105033. [DOI] [PubMed] [Google Scholar]
- 12.Castro-Rodriguez JA, Holberg CJ, Morgan WJ, Wright AL, Martinez FD. Increased incidence of asthmalike symptoms in girls who become overweight or obese during the school years. Am J Respir Crit Care Med. 2001;163:1344–1349. doi: 10.1164/ajrccm.163.6.2006140. [DOI] [PubMed] [Google Scholar]
- 13.Guerra S, Sherrill DL, Bobadilla A, Martinez FD, Barbee RA. The relation of body mass index to asthma, chronic bronchitis, and emphysema. Chest. 2002;122:1256–1263. doi: 10.1378/chest.122.4.1256. [DOI] [PubMed] [Google Scholar]
- 14.Camargo CA, Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med. 1999;159:2582–2588. doi: 10.1001/archinte.159.21.2582. [DOI] [PubMed] [Google Scholar]
- 15.Beckett WS, Jacobs DR, Jr, Yu X, Iribarren C, Williams OD. Asthma is associated with weight gain in females but not males, independent of physical activity. Am J Respir Crit Care Med. 2001;164:2045–2050. doi: 10.1164/ajrccm.164.11.2004235. [DOI] [PubMed] [Google Scholar]
- 16.Hancox RJ, Milne BJ, Poulton R, Taylor DR, Greene JM, McLachlan CR, et al. Sex differences in the relation between body mass index and asthma and atopy in a Birth cohort. Am J Respir Crit Care Med. 2004;171:440–445. doi: 10.1164/rccm.200405-623OC. [DOI] [PubMed] [Google Scholar]
- 17.Guerra S, Wright AL, Morgan WJ, Sherrill DL, Holberg CJ, Martinez FD. Persistence of asthma symptoms during adolescence: role of obesity and age at onset of puberty. Am J Respir Crit Care Med. 2004;170:78–85. doi: 10.1164/rccm.200309-1224OC. [DOI] [PubMed] [Google Scholar]
- 18.Martin N, Boomsma D, Machin G. A twin-pronged attack on complex traits. Nat Genet. 1997;17:387–392. doi: 10.1038/ng1297-387. [DOI] [PubMed] [Google Scholar]
- 19.Becker A, Busjahn A, Faulhaber HD, Bahring S, Robertson J, Schuster H, et al. Twin zygosity. Automated determination with microsatellites. J Reprod Med. 1997;42:260–266. [PubMed] [Google Scholar]
- 20.Eisen S, Neuman R, Goldberg J, Rice J, True W. Determining zygosity in the Vietnam Era Twin Registry: an approach using questionnaires. Clin Genet. 1989;35:423–432. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- 21.Torgersen S. The determination of twin zygosity by means of a mailed questionnaire. Acta Genet Med Gemellol (Roma) 1979;28:225–236. doi: 10.1017/s0001566000009077. [DOI] [PubMed] [Google Scholar]
- 22.Jarvis D, Chinn S, Potts J, Burney P. Association of body mass index with respiratory symptoms and atopy: results from the European Community Respiratory Health Survey. Clin Exp Allergy. 2002;32:831–837. doi: 10.1046/j.1365-2222.2002.01380.x. [DOI] [PubMed] [Google Scholar]
- 23.Kim S, Camargo CA., Jr Sex-race differences in the relationship between obesity and asthma: the behavioral risk factor surveillance system, 2000. Ann Epidemiol. 2003;13:666–673. doi: 10.1016/s1047-2797(03)00054-1. [DOI] [PubMed] [Google Scholar]
- 24.Neale MC, Cardon LR. Dordrecht, The Netherlands: Kluwer Academic; 1992. Methodology for genetic studies of twins and families. [Google Scholar]
- 25.Hedeker D, Gibbons RD. MIXOR: a computer program for mixed-effects ordinal regression analysis. Comput Methods Programs Biomed. 1996;49:157–176. doi: 10.1016/0169-2607(96)01720-8. [DOI] [PubMed] [Google Scholar]
- 26.Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:327–332. [Google Scholar]
- 27.Wamboldt MZ, Hewitt JK, Schmitz S, Wamboldt FS, Rasanen M, Koskenvuo M, et al. Familial association between allergic disorders and depression in adult Finnish twins. Am J Med Genet. 2000;96:146–153. doi: 10.1002/(sici)1096-8628(20000403)96:2<146::aid-ajmg4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 28.Luder E, Ehrlich RI, Lou WY, Melnik TA, Kattan M. Body mass index and the risk of asthma in adults. Respir Med. 2004;98:29–37. doi: 10.1016/j.rmed.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Litonjua AA, Sparrow D, Celedon JC, DeMolles D, Weiss ST. Association of body mass index with the development of methacholine airway hyperresponsiveness in men: the Normative Aging Study. Thorax. 2002;57:581–585. doi: 10.1136/thorax.57.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hancox RJ, Milne BJ, Poulton R, Taylor DR, Greene JM, McLachlan CR, et al. Sex differences in the relation between body mass index and asthma and atopy in a birth cohort. Am J Respir Crit Care Med. 2005;171:440–445. doi: 10.1164/rccm.200405-623OC. [DOI] [PubMed] [Google Scholar]
- 31.Schachter LM, Peat JK, Salome CM. Asthma and atopy in overweight children. Thorax. 2003;58:1031–1035. doi: 10.1136/thorax.58.12.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fredberg JJ, Inouye DS, Mijailovich SM, Butler JP. Perturbed equilibrium of myosin binding in airway smooth muscle and its implications in bronchospasm. Am J Respir Crit Care Med. 1999;159:959–967. doi: 10.1164/ajrccm.159.3.9804060. [DOI] [PubMed] [Google Scholar]
- 33.Rasmussen F, Lambrechtsen J, Siersted HC, Hansen HS, Hansen NC. Low physical fitness in childhood is associated with the development of asthma in young adulthood: the Odense schoolchild study. Eur Respir J. 2000;16:866–870. doi: 10.1183/09031936.00.16586600. [DOI] [PubMed] [Google Scholar]
- 34.Huovinen E, Kaprio J, Laitinen LA, Koskenvuo M. Social predictors of adult asthma: a co-twin case-control study. Thorax. 2001;56:234–236. doi: 10.1136/thorax.56.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soutar A, Seaton A, Brown K. Bronchial reactivity and dietary antioxidants. Thorax. 1997;52:166–170. doi: 10.1136/thx.52.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh RB, Beegom R, Rastogi SS, Gaoli Z, Shoumin Z. Association of low plasma concentrations of antioxidant vitamins, magnesium and zinc with high body fat per cent measured by bioelectrical impedance analysis in Indian men. Magnes Res. 1998;11:3–10. [PubMed] [Google Scholar]
- 37.Los H, Postmus PE, Boomsma DI. Asthma genetics and intermediate phenotypes: a review from twin studies. Twin Res. 2001;4:81–93. doi: 10.1375/1369052012191. [DOI] [PubMed] [Google Scholar]
- 38.Kaprio J, Eriksson J, Lehtovirta M, Koskenvuo M, Tuomilehto J. Heritability of leptin levels and the shared genetic effects on body mass index and leptin in adult Finnish twins. Int J Obes Relat Metab Disord. 2001;25:132–137. doi: 10.1038/sj.ijo.0801526. [DOI] [PubMed] [Google Scholar]
- 39.Allison DB, Kaprio J, Korkeila M, Koskenvuo M, Neale MC, Hayakawa K. The heritability of body mass index among an international sample of monozygotic twins reared apart. Int J Obes Relat Metab Disord. 1996;20:501–506. [PubMed] [Google Scholar]
- 40.Pietilainen KH, Kaprio J, Rissanen A, Winter T, Rimpela A, Viken RJ, et al. Distribution and heritability of BMI in Finnish adolescents aged 16y and 17y: a study of 4884 twins and 2509 singletons. Int J Obes Relat Metab Disord. 1999;23:107–115. doi: 10.1038/sj.ijo.0800767. [DOI] [PubMed] [Google Scholar]
- 41.Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, et al. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297:843–845. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- 42.Hupfeld CJ, Dalle S, Olefsky JM. Beta-Arrestin 1 down-regulation after insulin treatment is associated with supersensitization of beta 2 adrenergic receptor Galpha s signaling in 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 2003;100:161–166. doi: 10.1073/pnas.0235674100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D’Amato M, Vitiani LR, Petrelli G, Ferrigno L, di Pietro A, Trezza R, et al. Association of persistent bronchial hyperresponsiveness with beta2-adrenoceptor (ADRB2) haplotypes. A population study. Am J Respir Crit Care Med. 1998;158:1968–1973. doi: 10.1164/ajrccm.158.6.9804126. [DOI] [PubMed] [Google Scholar]
- 44.Hall IP, Wheatley A, Wilding P, Liggett SB. Association of Glu 27 beta 2-adrenoceptor polymorphism with lower airway reactivity in asthmatic subjects. Lancet. 1995;345:1213–1214. doi: 10.1016/s0140-6736(95)91994-5. [DOI] [PubMed] [Google Scholar]
- 45.Dewar JC, Wilkinson J, Wheatley A, Thomas NS, Doull I, Morton N, et al. The glutamine 27 beta2-adrenoceptor polymorphism is associated with elevated IgE levels in asthmatic families. J Allergy Clin Immunol. 1997;100:261–265. doi: 10.1016/s0091-6749(97)70234-3. [DOI] [PubMed] [Google Scholar]
- 46.Israel E, Drazen JM, Liggett SB, Boushey HA, Cherniack RM, Chinchilli VM, et al. The effect of polymorphisms of the beta(2)-adrenergic receptor on the response to regular use of albuterol in asthma. Am J Respir Crit Care Med. 2000;162:75–80. doi: 10.1164/ajrccm.162.1.9907092. [DOI] [PubMed] [Google Scholar]
- 47.Martinez FD, Graves PE, Baldini M, Solomon S, Erickson R. Association between genetic polymorphisms of the beta2-adrenoceptor and response to albuterol in children with and without a history of wheezing. J Clin Invest. 1997;100:3184–3188. doi: 10.1172/JCI119874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Israel E, Chinchilli VM, Ford JG, Boushey HA, Cherniack R, Craig TJ, et al. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet. 2004;364:1505–1512. doi: 10.1016/S0140-6736(04)17273-5. [DOI] [PubMed] [Google Scholar]
- 49.Large V, Hellstrom L, Reynisdottir S, Lonnqvist F, Eriksson P, Lannfelt L, et al. Human beta-2 adrenoceptor gene polymorphisms are highly frequent in obesity and associate with altered adipocyte beta-2 adrenoceptor function. J Clin Invest. 1997;100:3005–3013. doi: 10.1172/JCI119854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meirhaeghe A, Helbecque N, Cottel D, Amouyel P. Beta2-adrenoceptor gene polymorphism, body weight, and physical activity. Lancet. 1999;353:896. doi: 10.1016/S0140-6736(99)00251-2. [DOI] [PubMed] [Google Scholar]
- 51.Albuquerque RV, Hayden CM, Palmer LJ, Laing IA, Rye PJ, Gibson NA, et al. Association of polymorphisms within the tumour necrosis factor (TNF) genes and childhood asthma. Clin Exp Allergy. 1998;28:578–584. doi: 10.1046/j.1365-2222.1998.00273.x. [DOI] [PubMed] [Google Scholar]
- 52.Chagani T, Pare PD, Zhu S, Weir TD, Bai TR, Behbehani NA, et al. Prevalence of tumor necrosis factor-alpha and angiotensin converting enzyme polymorphisms in mild/moderate and fatal/near-fatal asthma. Am J Respir Crit Care Med. 1999;160:278–282. doi: 10.1164/ajrccm.160.1.9808032. [DOI] [PubMed] [Google Scholar]
- 53.Fernandez-Real JM, Gutierrez C, Ricart W, Casamitjana R, Fernandez-Castaner M, Vendrell J, et al. The TNF-alpha gene Nco I polymorphism influences the relationship among insulin resistance, percent body fat, and increased serum leptin levels. Diabetes. 1997;46:1468–1472. doi: 10.2337/diab.46.9.1468. [DOI] [PubMed] [Google Scholar]
- 54.Herrmann SM, Ricard S, Nicaud V, Mallet C, Arveiler D, Evans A, et al. Polymorphisms of the tumour necrosis factor-alpha gene, coronary heart disease and obesity. Eur J Clin Invest. 1998;28:59–66. doi: 10.1046/j.1365-2362.1998.00244.x. [DOI] [PubMed] [Google Scholar]
- 55.Sandford AJ, Pare PD. The genetics of asthma. The important questions. Am J Respir Crit Care Med. 2000;161 suppl:S202–S206. doi: 10.1164/ajrccm.161.supplement_2.a1q4-11. [DOI] [PubMed] [Google Scholar]
- 56.Clarke JR, Jenkins MA, Hopper JL, Carlin JB, Mayne C, Clayton DG, et al. Evidence for genetic associations between asthma, atopy, and bronchial hyperresponsiveness: a study of 8- to 18-yr-old twins. Am J Respir Crit Care Med. 2000;162:2188–2193. doi: 10.1164/ajrccm.162.6.9904057. [DOI] [PubMed] [Google Scholar]
- 57.Hanson B, McGue M, Roitman-Johnson B, Segal NL, Bouchard TJ, Jr, Blumenthal MN. Atopic disease and immunoglobulin E in twins reared apart and together. Am J Hum Genet. 1991;48:873–879. [PMC free article] [PubMed] [Google Scholar]
- 58.Platts-Mills TA, Vervloet D, Thomas WR, Aalberse RC, Chapman MD. Indoor allergens and asthma: report of the Third International Workshop. J Allergy Clin Immunol. 1997;100 suppl:S2–S24. doi: 10.1016/s0091-6749(97)70292-6. [DOI] [PubMed] [Google Scholar]
- 59.Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869–877. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 60.Kilpelainen M, Terho EO, Helenius H, Koskenvuo M. Validation of a new questionnaire on asthma, allergic rhinitis, and conjunctivitis in young adults. Allergy. 2001;56:377–384. doi: 10.1034/j.1398-9995.2001.056005377.x. [DOI] [PubMed] [Google Scholar]
- 61.Nieto-Garcia FJ, Bush TL, Keyl PM. Body mass definitions of obesity: sensitivity and specificity using self-reported weight and height. Epidemiology. 1990;1:146–152. [PubMed] [Google Scholar]
- 62.Vercelli D. Genetics, epigenetics, and the environment: switching, buffering, releasing. J Allergy Clin Immunol. 2004;113:381–386. doi: 10.1016/j.jaci.2004.01.752. [DOI] [PubMed] [Google Scholar]


