Abstract
Suspension arrays present a promising tool for multiplexed assays in large-scale screening applications. A simple and robust platform for quantitative multiprotein immunoanalysis has been developed with the use of magnetic Co:Nd:Fe2O3/luminescent Eu:Gd2O3 core/shell nanoparticles (MLNPs) as a carrier. The magnetic properties of the MLNPs allow their manipulation by an external magnetic field in the separation and washing steps in the immunoassay. Their optical properties enable the internal calibration of the detection system. The multiplexed sandwich immunoassay involves dual binding events on the surface of the MLNPs functionalized with the capture antibodies. Secondary antibodies labeled with conventional organic dyes (Alexa Fluor) are used as reporters. The amount of the bound secondary antibody is directly proportional to the concentration of the analyte in the sample. In our approach, the fluorescence intensity of the reporter dye is related to the luminescence signal of the MLNPs. In this way, the intrinsic luminescence of the MLNPs serves as an internal standard in the quantitative immunoassay. The concept is demonstrated for a simultaneous immunoassay for three model proteins (human, rabbit and mouse IgGs). The method uses a standard bench plate reader. It can be applied to disease diagnostics and for the detection of biological threats.
Keywords: luminescence, nanoparticles, lanthanide oxide, magnetic, multiplex immunoassay
Introduction
Multiplexed techniques are essential to satisfy the growing demands of many fields of bioanalytics, including immunology, drug screening, disease diagnosis and defense against biological threats. The ability to measure simultaneously multiple proteins in a single assay offers several advantages, such as higher throughput compared to single-target systems, savings in reagents and consumables, decreased sampling errors, and easy inclusion of internal controls. Current multiplexed analysis systems are divided into two classes: flat-surface (biochips) [1, 2] and suspension arrays [3]. Microbead-based assays offer several attractive aspects such as enhanced signal due to large surface-to-volume ratio; fluid-phase kinetics (which are faster than the solid-phase kinetics of planar arrays); and greater precision (due to measurements of hundreds of beads for each analyte). Flow cytometry is most commonly used to read out suspension arrays [3, 4]. Multiplexed sandwich immunoassays are the most advanced assays formats among the different protein microarray applications [5].
Most efforts that have been directed toward multianalyte immunoassays have focused on fluorescent detection using different reporters [6], such as conventional fluorophores [7, 8], silica nanoparticles [9], micro/nanobeads [10, 11] and quantum dots [12]. The widespread use of these systems in routine analysis is still impaired by the lack of suitable measurement platforms for fast and accurate multiplexed detection in a laboratory. Multicolor detection and analysis is often obstructed by the requirement of complicated excitation and/or detection schemes [7], challenging data collection and analysis (signal deconvolution) [12] and high background signals. In addition, fluorescence techniques rely on measurement of relative fluorescence units and require calibration to obtain reliable and comparable quantitative data.
The preparation of magnetic fluorescent particles, such as polystyrene magnetic beads with entrapped organic dyes/quantum dots (QDs) [13, 14] or a shell of QDs [15]; iron oxide particles coated with dye-doped silica shells [16]; silica nanoparticles embedded with iron oxide and QDs [17-20] have recently been reported. However, their application is limited mostly to cellular separation and imaging, drug delivery and therapy. Only a few papers have reported the use of dual-functional nanoparticles for multiplexed quantitative bioanalysis [10, 21]. Recently we have demonstrated the use of cost effective spray pyrolysis synthesis of bifunctional magnetic/luminescent core/shell nanoparticles with cores of paramagnetic Co:Nd:Fe2O3 and shells of luminescent Eu:Gd2O3 [22]. We have demonstrated their application in a new immunoassay format with an internal luminescent standard eliminating the experimental error due to particle handling. Due to their small size, the magnetic luminescent nanoparticles offer larger surface area-to-volume ratio than currently used microbeads, resulting in good reaction homogeneity and fast reaction kinetics.
In this report we present a new detection format for multiplexed analysis based on the use of magnetic luminescent Co:Nd:Fe2O3/Eu:Gd2O3 nanoparticles (MLNPs). Multiplexed sandwich immunoassays for three model proteins were performed on the surface of the MLNPs. The magnetic properties of the MLNPs allowed their manipulation by an external magnetic field without the need of centrifugation and filtration. Their optical characteristics (sharp emission, photostability, long life time) facilitated the implementation of an internal calibration in the detection system. This introduced a unique internal quality control and easy quantification in the multiplexed immunoanalysis. The method developed here enables a direct, simple and quantitative multiplex protein analysis using conventional organic dyes and a bench plate reader (simple laboratory equipment) that can be applied for disease diagnostics and for detection of biological threats.
Materials and methods
Reagents
Goat anti-mouse, anti-rabbit and anti-human (H+L) antibodies were obtained from Abcam (Cambridge, MA). The antibodies were highly pre-adsorbed i.e. cross-reactivity among antibodies was minimal. Goat anti-mouse IgG conjugated to Alexa Fluor 660 (4 dye molecules/mol protein), goat anti-rabbit IgG conjugated to Alexa Fluor 350 (5 dye molecules/mol protein) and goat anti-human IgG conjugated to Alexa Fluor 488 (6 dye molecules/mol protein) were purchased from Molecular Probes (Eugene, OR). Mouse, rabbit and human IgGs, and bovine serum albumin (BSA) were from Sigma (St. Luis, MO). Phosphate buffer saline (PBS) was 10 mmol l−1 phosphate buffer (pH=7.5), 0.8% saline. Phosphate buffer (PB) was 25 mmol l−1 (pH=7).
Apparatus
The magnetic bead assays and separations were performed on a MagneSil™ magnetic separation unit (Promega, Madison, WI). A Spectramax M2 microplate reader (Molecular Devices, Sunnyvale, CA) was used for fluorescence detection in the immunoassay. Black 96-well plates from Greiner Bio-one (Monroe, North Carolina) were used for fluorescence measurements.
Coating of magnetic luminescent nanoparticles (MLNPs) with capture antibodies
The MLNPs (1 mg) were suspended in 1 ml of 25 mmol l−1 PB using ultrasonic bath. A solution of 2 mg ml −1 capture antibody (anti-rabbit, mouse, human and/or sheep IgG) (15 μl, 35 μl and 50 μl for 30, 70 and 100 μg capture antibody/mg MLNPs coatings, respectively) was added to the particle suspension and incubated in a rotating mill overnight at room temperature. The particles were extracted from the solution on the magnetic rack and washed 3 times with PB.
Sandwich Fluoroimmunoassays
The surface of the antibody functionalized MLNPs (1 mg) was blocked with 1 ml of 0.2%BSA/PBS for 1h. Each sandwich fluoroimmunoassay was performed with 0.5 mg MLNPs in a 0.5 ml total sample volume. The analyte solution (rabbit, mouse and/or human IgG, 0.25 ml) was added to 0.25 ml of MLNP suspension to give final analyte concentration in the range of 0−120 μg ml−1 and mixed for 1h. After isolation with the magnet, the MLNPs bearing the immunocomplex were washed 3 times with 0.2%BSA/PBS and incubated with 0.5 ml of 25 μg ml−1 antibody solution (anti-rabbit IgG-Alexa 350, anti-mouse IgG-Alexa 660, anti-human IgG-Alexa 488) for 1h. The resulting sandwich-conjugated MLNPs were magnetically extracted and washed 3 times. Then the nanoparticles were re-suspended in 100 μl PBS and the fluorescence was read on a plate reader. Both Eu:Gd2O3 and Alexa Fluor 350 were excited at 350 nm and their emission spectra were detected in the interval 430−650 nm. Alexa Fluor 488 and 660 were excited at 485 nm and 630 nm, respectively, and the emission spectra were detected in the intervals 510−600 nm and 680−800 nm. The maximum emissions at 610 nm, 450 nm, 525 nm and 690 nm, respectively, were used for quantitative analysis. Negative controls were prepared by using sheep IgG–coated magnetic nanoparticles. Samples were run in triplicates.
Results and discussion
Magnetic luminescent nanoparticles (MLNPs)
We have used flame spray pyrolysis as a low-cost and flexible synthesis method for the preparation of novel magnetic luminescent nanoparticles (MLNPs) consisting of magnetic cores of iron oxide doped with cobalt and neodymium (Co:Nd:Fe2O3) encapsulated in luminescent shells of europium-doped gadolinium oxide (Eu:Gd2O3) [22]. The MLNPs used in this work have diameters in the range of 200 − 400 nm. The saturation magnetization of the nanoparticles is ∼4 emu/g; they can be easily separated from solution by commercial magnetic extractors commonly used in bioanalysis. In addition, the MLNPs offer a large Stokes shift (UV excitation and red emission peak centered at 615 nm), long fluorescence life time (about 1−2 ms) and inherent photostability. In Figure 1 the fluorescence emission spectrum of the MLNPs is compared to those of commercial magnetic polystyrene microbeads dyed with organic fluorophores. The MLNPs have significantly narrower fluorescent emission (full-width half-maximum, or FWHM less than 20 nm) than fluorescent dyes, making them suitable for bioassays with multicolor detection. In addition, due to the long life time emission of the Eu3+ ion, the signal of the MLNPs can be measured in a time-gated detection mode, independently of other fluorophores.
Figure 1.
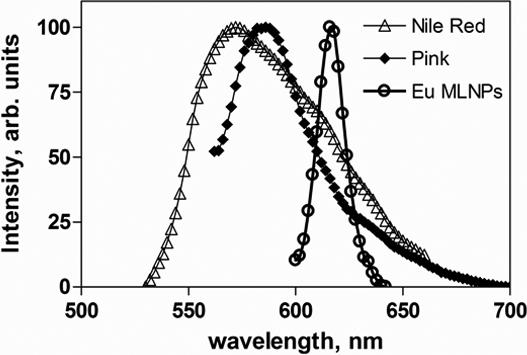
Emission spectra of magnetic luminescent (Co:Nd:Fe2O3/Eu:Gd2O3) nanoparticles (MLNPs) excited at 350 nm and commercial (Spherotech) microbeads: Nile Red (4 μm; 535nm/570nm) and Pink (1 μm; 560nm/580nm).
Detection principle
The MLNP-based immunoassay for the simultaneous detection of multiple proteins is presented in Figure 2. The new fluorescent immunoassay involves a dual binding event on the surface of the MLNPs for each analyte. The target proteins are captured by the antibodies immobilized on the surface of the MLNPs. After incubation with reporter antibodies (dye-labeled secondary antibodies) and consecutive magnetic separation, the MLNP-immunocomplex is subject to fluorescent detection. Recognition of each protein yields a distinct fluorescent peak, whose position and intensity reflects the identity and the concentration of the corresponding analyte. For quantitative evaluation the intensity of this fluorescent peak (Ireporter) is related to the intensity of the MLNPs emission (IEu). The ratio Ireporter/IEu is directly proportional to the analyte concentration. In this way, the intrinsic luminescence of the magnetic nanoparticles serves as an internal standard in the quantitative immunoassay. This approach eliminates the experimental errors due to possible variability in the amount of particles due to weighing, magnetic particle extraction, and washing between incubations. The measured signal is relative instead of absolute, with the Eu signal as a measure of the amount of particles (and hence primary antibodies) used in the assay. In contrast to the direct fluorescent measurements, the ratio Ireporter/IEu will not be affected even by a substantial particle loss in any of the assay steps. This approach gives real quantitative meaning to the fluorescent signal intensity. The effect of the internal calibration on assay precision has already been demonstrated in our recent work [22]. Here we employed the power of the internal standard calibration to develop a model multiplexed system for the simultaneous detection of IgG molecules (rabbit, mouse and human). As fluorescent reporters we used Alexa Fluor dyes because their antibody conjugates are commercially available and have excellent fluorescent properties, such as brightness, photostability and pH insensitivity [23, 24].
Figure 2.
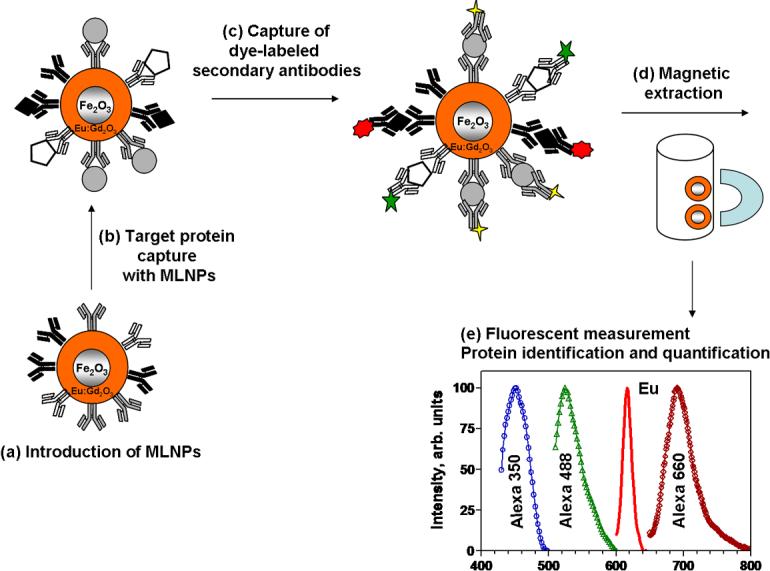
Multiplexed fluoroimmunoassay with. magnetic luminescent nanoparticles (MLNPs) (a) Introduction of antibody-modified nanomagneto-phosphors; (b) binding of the protein analytes on the surface of the nanomagneto-phosphors; (c) capture of the dye-labeled secondary (reporter) antibodies; (d) magnetic extraction; (e) fluorescence detection of reporters and nanomagneto-phosphors
Individual assays
Single assays were performed for each of the three analytes to ensure that the antibody pairs would be useful in a multicolor multi-analyte study. In these assays, only the appropriate capture antibody and unmixed analyte (rabbit, human and mouse IgG) were applied, and the signal was detected by the corresponding secondary antibody (anti-rabbit IgG-Alexa 350, anti-human IgG-Alexa 488 and anti-mouse IgG-Alexa 660, respectively). The MLNPs were coated with the specific capture antibody using the direct coating method based on spontaneous physical adsorption according to our previously reported procedure [22, 25] . This method of protein coating has been reported also for dye-doped silica particles [26], silole nanocrystals [27], and quantum dots [28, 29]. Although it is believed that the protein adsorption on the nanoparticle surface is due to hydrophobic and/or electrostatic forces, the exact mechanism is not yet fully understood.
Figures 3A, C and E represent the standard curves obtained for each analyte (rabbit, human and mouse IgG) in a single immunoassay. The standard curves are expressed as a ratio between the fluorescent intensity of each Alexa dye versus the intensity of the Eu signal of the MLNPs. The corresponding spectra of the Alexa label and the Eu signal of the MLNPs are presented in Fig. 3B, D and F, respectively. Well-defined peaks proportional to the concentration of the corresponding analyte were observed. The best signal-to-noise ratio was observed for the human IgG assay (S/N∼15), followed by the rabbit assay (S/N∼13). The mouse immunoassay has S/N∼7. We can explain these differences with reference to the different properties of the three Alexa dyes (extinction coefficient, quantum yield, degree of labeling). In all cases, no response was observed for the corresponding control experiment with non-specific capture (sheep) antibody. This reflects the effective magnetic separation that, combined with blocking of the nanoparticles surface with BSA and effective washing, eliminates nonspecific adsorption effects. The assays were optimized in terms of the amount of capture antibody on the surface of the MLNPs and the reporter (secondary) antibody concentration. As an example, Fig 3A shows the standard curves for rabbit IgG when different amounts of capture antibody (30, 70 and 100 μg mg−1 MLNP) were used for a fixed secondary antibody concentration (25 μg ml−1). It can be observed that a high S/N ratio can be achieved at a coating concentration of 70 μg mg−1 MLNP. No further increase in S/N was seen at 100 μg mg−1 MLNP, thus 70 μg mg−1 MLNP concentration was chosen for the multiplexed assay.
Figure 3.
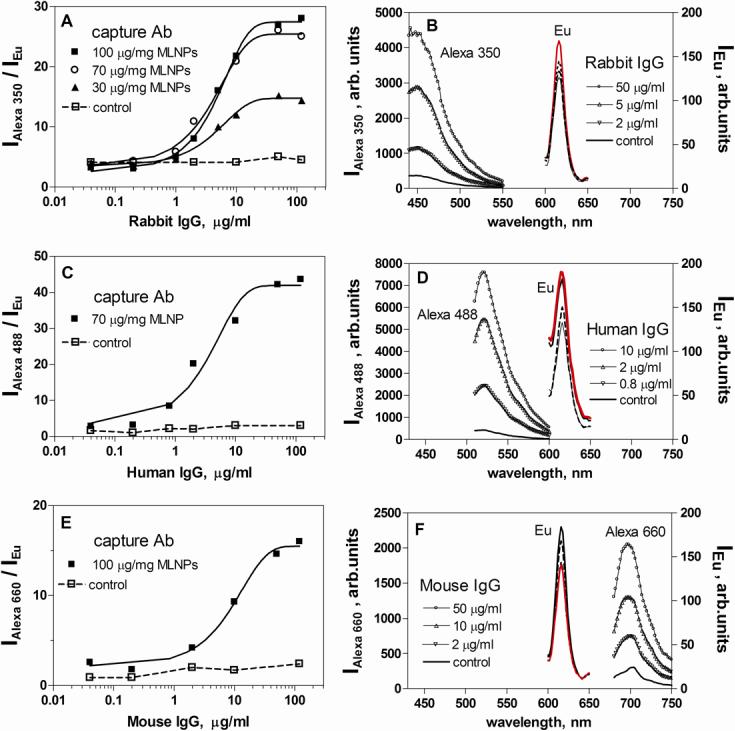
Single analyte immunoassays for rabbit (A, B), human (C, D) and mouse (E, F) IgG. The standard curves (A, C, E) represent the analyte concentration dependence of the ratio between the fluorescence intensity of the Alexa dye reporter (IAlexa) and the intensity of the Eu MLNPs (IEu). Standard curves are run for different coatings of capture antibody (Ab) (30, 70, 100 μg/mg MLNPs). The corresponding emission spectra of the Alexa dye reporters and the Eu MLNPs are presented in B, D and F for different analyte concentrations.
Multiplexed assay
Simultaneous detection of three analytes was performed by an all-in-one reaction. As the nanoparticles provide an enormous surface for the immobilization of capture antibodies, they could be coated with the three types of capture antibodies (equimolar amount – 70 μg mg−1 MLNP). In this way, only one reagent needs to be used as a MLNP probe for multiplexed analysis. After extraction of the three analytes (rabbit, human and mouse IgG) from the sample, the MLNPs were incubated with an equimolar mixture of the secondary antibodies (25 μg ml−1 each). The calibration graph for the multiplexed fluoroimmunoassay is presented in Fig. 4. It can be observed that the multiplexed standard curves are very similar to those for the corresponding single analyte assays. A negative control experiment showed negligible non-specific interactions. The highest sensitivity was obtained for the human IgG assay with limit of detection (LOD) of 0.2 μg ml−1 (LOD is defined as 10% of the maximum signal), followed by rabbit IgG with LOD of 1 μg ml−1 and mouse IgG with 2 μg ml−1. Additionally, the LOD for the three assays were estimated as the concentration corresponding to the signal of the blank control (the mean value of zero analyte concentration plus 3 times the standard deviation of 6 blank measurements): 0.3 μg ml−1 human IgG, 1.2 μg ml−1 rabbit IgG and 1.8 μg ml−1 mouse IgG. In these proof-of-principle experiments, the limits of detection were acceptable for multiplexed detection of the model analytes. Further improvement in sensitivity could be achieved using other reporters and optimizing the reaction conditions for a specific target protein. Cross-reactivity was not observed because each capture and detecting antibody had been pre-adsorbed against the remaining serum proteins (e.g. the antibody used in the rabbit assay was pre-adsorbed against mouse, human and bovine proteins). We used tunable excitation: each dye was individually excited at a specific wavelength, thus avoiding the need for deconvolution. Fluorescence read-out is performed in seconds resulting in a very short analysis time. Because of the internal calibration (the average fluorescence per Eu nanoparticle per well and not the total fluorescence per well is considered), the total number of nanoparticles per well does not need to be rigorously controlled. In the fluorescence measurements, many MLNPs are interrogated and the signal obtained is statistically representative, resulting in better precision [22]. We would like to note that signal amplification of the reporter was observed due to the large surface of the MLNPs per well. The same format in a heterogeneous fluorescent immunoassay was not possible on the surface of a microtiter plate because very weak fluorescence was detected. Because generic reagents such as dye-labeled antibodies were used, the assay can be easily performed with commercially available products and with simple instrumentation such as magnetic extractors and plate readers. QDs or other lanthanide particles can be used as secondary labels if one excitation source is required.
Figure 4.
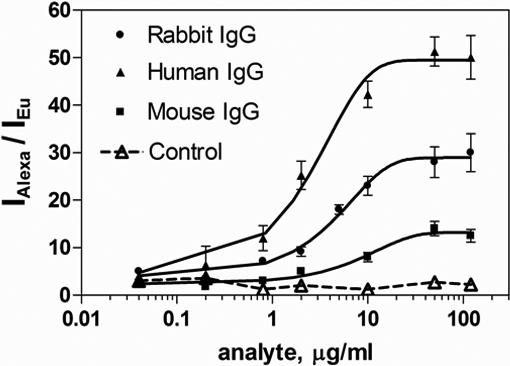
Multiplexed fluoroimmunoassay for model proteins (rabbit, human, mouse IgGs). MLNPs coated with three types of capture antibodies (equimolar amount − 70 μg mg−1 MLNP) are used for the simultaneous detection of three proteins (all-in-one reaction). The standard curves for each analyte represent the analyte concentration dependence of the ratio between the fluorescence intensity of the Alexa dye reporter (IAlexa) and the intensity of the Eu MLNPs (IEu). The negative control experiment is performed with MLNPs coated with non-specific capture antibody.
In conclusion, magnetic luminescent Co:Nd:Fe2O3/Eu:Gd2O3 nanoparticles (MLNPs) can be used as carriers for multiplexed nanobead-assays for multiprotein detection with internal calibration. MLNPs have three main functions: (1) a probe for the specific extraction of multiple target analytes from a sample; (2) a carrier in the quantitative immunoassays with magnetic separation; and (3) an internal standard in the fluorescence measurement of multiple reporters. The MLNPs serve as an internal control for the total analysis including extraction and assay performance. In this method numerous beads are evaluated in a well, thereby increasing the redundancy of results and decreasing the chance of both false positive and false negatives. Simple instrumentation such as plate readers can be used. The multiplexed assay format could be easily performed in 96- or 384- well microplate with a complete automation of all steps of the protocol. Our approach represents a new platform for a multiplexed immunoassay with the potential for integration into high throughput protein arrays or miniaturized devices for clinical diagnostics. The internal calibration concept presented in this work would be crucial for detection systems where a variable excitation source such as a laser is used. Furthermore, MLNPs with different emission wavelengths can be easily obtained by controlled doping of different lanthanide ions (e.g. Eu and Tb) into the host material, making them very attractive as a “bar-coding” tool. The ability to use the MLNPs for both separation and detection makes them a valuable tool for a wide range of biotechnology applications, such as carriers with magnetic orientation and as a fluorescence tracer for potent drug targeting.
Acknowledgments
The authors wish to acknowledge the support of the Superfund Basic Research Program with Grant 5P42ES04699 from the National Institute of Environmental Health Sciences, NIH and the Cooperative State Research, Education, and Extension Service, US Department of Agriculture, under Award No. 05-35603-16280, and the Western Center for Agricultural Safety & Health under the National Institute of Occupational Safety and Health award PHS OH07550.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhu H, Snyder M. Protein chip technology. Curr. Opin. Chem. Biol. 2003;7:55–63. doi: 10.1016/s1367-5931(02)00005-4. [DOI] [PubMed] [Google Scholar]
- 2.Sievertzon M, Nilsson P, Lundeberg J. Improving reliability and performance of DNA microarrays. Expert Rev. Molec. Diagnostics. 2006;6:481–492. doi: 10.1586/14737159.6.3.481. [DOI] [PubMed] [Google Scholar]
- 3.Nolan JP, Sklar LA. Suspension array technology: evolution of the flat-array paradigm. Trends Biotechnol. 2002;20:9–12. doi: 10.1016/s0167-7799(01)01844-3. [DOI] [PubMed] [Google Scholar]
- 4.Wilson R, Cossins AR, Spiller DG. Encoded microcarriers for high-throughput multiplexed detection. Angew. Chem., Int. Ed. 2006;45:6104–6117. doi: 10.1002/anie.200600288. [DOI] [PubMed] [Google Scholar]
- 5.Templin MF, Stoll D, Bachmann J, Joos TO. Protein microarrays and multiplexed sandwich immunoassays: What beats the beads? Comb. Chem. High Throughput Screening. 2004;7:223–229. doi: 10.2174/1386207043328814. [DOI] [PubMed] [Google Scholar]
- 6.Schaferling M, Nagl S. Optical technologies for the read out and quality control of DNA and protein microarrays. Anal. Bioanal. Chem. 2006;385:500–517. doi: 10.1007/s00216-006-0317-5. [DOI] [PubMed] [Google Scholar]
- 7.Swartzman EE, Miraglia SJ, Mellentin-Michelotti J, Evangelista L, Yuan PM. A homogeneous and multiplexed immunoassay for high-throughput screening using fluorometric microvolume assay technology. Anal. Biochem. 1999;271:143–151. doi: 10.1006/abio.1999.4128. [DOI] [PubMed] [Google Scholar]
- 8.Biagini RE, Sammons DL, Smith JP, MacKenzie BA, Striley CAF, Robertson SA, Snawder JE, Quinn CP. Simultaneous measurement of specific serum IgG responses to five select agents. Anal. Bioanal. Chem. 2005;382:1027–1034. doi: 10.1007/s00216-005-3204-6. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Yang CY, Tan WH. Dual-luminophore-doped silica nanoparticles for multiplexed signaling. Nano Lett. 2005;5:37–43. doi: 10.1021/nl048417g. [DOI] [PubMed] [Google Scholar]
- 10.Moser C, Mayr T, Klimant I. Microsphere sedimentation arrays for multiplexed bioanalytics. Anal. Chim. Acta. 2006;558:102–109. [Google Scholar]
- 11.Wang L, Cole KD, Gaigalas AK, Zhang YZ. Fluorescent nanometer microspheres as a reporter for sensitive detection of simulants of biological threats using multiplexed suspension arrays. Bioconjugate Chem. 2005;16:194–199. doi: 10.1021/bc0498020. [DOI] [PubMed] [Google Scholar]
- 12.Goldman ER, Clapp AR, Anderson GP, Uyeda HT, Mauro JM, Medintz IL, Mattoussi H. Multiplexed toxin analysis using four colors of quantum dot fluororeagents. Anal. Chem. 2004;76:684–688. doi: 10.1021/ac035083r. [DOI] [PubMed] [Google Scholar]
- 13.Mulvaney SP, Mattoussi HM, Whitman LJ. Incorporating fluorescent dyes and quantum dots into magnetic microbeads for immunoassays. Biotechniques. 2004;36:602–609. doi: 10.2144/04364BI01. [DOI] [PubMed] [Google Scholar]
- 14.Gaponik N, Radtchenko IL, Sukhorukov GB, Rogach AL. Luminescent polymer microcapsules addressable by a magnetic field. Langmuir. 2004;20:1449–1452. doi: 10.1021/la035914o. [DOI] [PubMed] [Google Scholar]
- 15.Wang DS, He JB, Rosenzweig N, Rosenzweig Z. Superparamagnetic Fe2O3 Beads-CdSe/ZnS quantum dots core-shell nanocomposite particles for cell separation. Nano Lett. 2004;4:409–413. [Google Scholar]
- 16.Levy L, Sahoo Y, Kim KS, Bergey EJ, Prasad PN. Nanochemistry: Synthesis and characterization of multifunctional nanoclinics for biological applications. Chem. Mater. 2002;14:3715–3721. [Google Scholar]
- 17.Yi DK, Selvan ST, Lee SS, Papaefthymiou GC, Kundaliya D, Ying JY. Silica-coated nanocomposites of magnetic nanoparticles and quantum dots. J. Am. Chem. Soc. 2005;127:4990–4991. doi: 10.1021/ja0428863. [DOI] [PubMed] [Google Scholar]
- 18.Salgueirino-Maceira V, Correa-Duarte MA, Spasova M, Liz-Marzan LM, Farle M. Composite silica spheres with magnetic and luminescent functionalities. Adv. Funct. Mater. 2006;16:509–514. [Google Scholar]
- 19.Santra S, Bagwe RP, Dutta D, Stanley JT, Walter GA, Tan W, Moudgil BM, Mericle RA. Synthesis and characterization of fluorescent, radio-opaque, and paramagnetic silica nanoparticles for multimodal bioimaging applications. Adv. Mater. 2005;17:2165–2169. [Google Scholar]
- 20.Sathe TR, Agrawal A, Nie SM. Mesoporous silica beads embedded with semiconductor quantum dots and iron oxide nanocrystals: Dual-function microcarriers for optical encoding and magnetic separation. Anal. Chem. 2006;78:5627–5632. doi: 10.1021/ac0610309. [DOI] [PubMed] [Google Scholar]
- 21.Eastman PS, Ruan WM, Doctolero M, Nuttall R, De Feo G, Park JS, Chu JSF, Cooke P, Gray JW, Li S, Chen FQF. Qdot nanobarcodes for multiplexed gene expression analysis. Nano Lett. 2006;6:1059–1064. doi: 10.1021/nl060795t. [DOI] [PubMed] [Google Scholar]
- 22.Dosev D, Nichkova M, Dumas R, Gee SJ, Hammock BD, Liu K, Kennedy IM. Magnetic/luminescent core/shell particles synthesized by spray pyrolysis and their application in immunoassays with internal standard. Nanotechnology. 2007;18 doi: 10.1088/0957-4484/18/5/055102. 055102 (6pp) 055102 (6pp) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berlier JE, Rothe A, Buller G, Bradford J, Gray DR, Filanoski BJ, Telford WG, Yue S, Liu JX, Cheung CY, Chang W, Hirsch JD, Beechem JM, Haugland RP. Quantitative comparison of long-wavelength Alexa Fluor dyes to Cy dyes: Fluorescence of the dyes and their bioconjugates. J. Histochem. Cytochem. 2003;51:1699–1712. doi: 10.1177/002215540305101214. [DOI] [PubMed] [Google Scholar]
- 24.Panchuk–Voloshina Nataliya, Haugland Rosaria P., Bishop–Stewart Janell, Bhalgat Mahesh K., Millard Paul J., Mao Fei, Leung Wai-Yee, Haugland RP, Dyes Alexa. a Series of New Fluorescent Dyes that Yield Exceptionally Bright, Photostable Conjugates. J. Histochem. Cytochem. 1999;47:1179–1188. doi: 10.1177/002215549904700910. [DOI] [PubMed] [Google Scholar]
- 25.Nichkova M, Dosev D, Perron R, Gee SJ, Hammock BD, Kennedy IM. Eu3+-doped Gd2O3 nanoparticles as reporters for optical detection and visualization of antibodies patterned by microcontact printing. Anal. Bioanal. Chem. 2006;384:631–637. doi: 10.1007/s00216-005-0246-8. [DOI] [PubMed] [Google Scholar]
- 26.Tapec R, Zhao XJJ, Tan WH. Development of organic dye-doped silica nanoparticles for bioanalysis and biosensors. J. Nanosci. Nanotechno. 2002;2:405–409. doi: 10.1166/jnn.2002.114. [DOI] [PubMed] [Google Scholar]
- 27.Chan CPY, Bruemmel Y, Seydack M, Sin KK, Wong LW, Merisko-Liversidge E, Trau D, Renneberg R. Nanocrystal biolabels with releasable fluorophores for immunoassays. Anal. Chem. 2004;76:3638–3645. doi: 10.1021/ac0353740. [DOI] [PubMed] [Google Scholar]
- 28.Goldman ER, Anderson GP, Tran PT, Mattoussi H, Charles PT, Mauro JM. Conjugation of luminescent quantum dots with antibodies using an engineered adaptor protein to provide new reagents for fluoroimmunoassays. Anal. Chem. 2002;74:841–847. doi: 10.1021/ac010662m. [DOI] [PubMed] [Google Scholar]
- 29.Gao XH, Chan WCW, Nie SM. Quantum-dot nanocrystals for ultrasensitive biological labeling and multicolor optical encoding. J. Biomed. Opt. 2002;7:532–537. doi: 10.1117/1.1506706. [DOI] [PubMed] [Google Scholar]


