Abstract
Nitric oxide subserves diverse physiologic roles in the nervous system. NO is produced from at least three different NO synthase (NOS) isoforms: neuronal NOS (nNOS), endothelial NOS, and immunologic NOS (iNOS). We show that nNOS is the predominant isoform constitutively expressed in glia. NO derived from nNOS in glia inhibits the transcription factor nuclear factor κB (NFκB) as NOS inhibitors enhance basal NFκB activation. Pyrrolidine dithiocarbamate (PDTC) is an inhibitor of NFκB in most cells; however, we show that PDTC is also a potent scavenger of NO through formation of mononitrosyl iron complexes with PDTC. In Jurkat cells, a human T-cell lymphoma cell line, tumor necrosis factor-α (TNF-α) induces NFκB activation that is inhibited by PDTC. Contrary to the results in Jurkat cells, PDTC did not inhibit tumor necrosis factor-α-induced NFκB activation in astrocytes; instead PDTC itself induces NFκB activation in astrocytes, and this may be related to scavenging of endogenously produced NO by the PDTC iron complex. In astrocytes PDTC also dramatically induces the NFκB-dependent enzyme, iNOS, supporting the physiologic relevance of endogenous NO regulation of NFκB. NFκB activation in glia from mice lacking nNOS responds more rapidly to PDTC compared with astrocytes from wild-type mice. Our data suggest that nNOS in astrocytes regulates NFκB activity and iNOS expression, and indicate a novel regulatory role for nNOS in tonically suppressing central nervous system, NFκB-regulated genes.
Nitric oxide is a potent messenger molecule with diverse physiologic activities, including regulation of vascular tone, neurotransmission, and killing of microorganisms and tumor cells (1–3). NO is produced from l-arginine (l-Arg) by the enzyme NO synthase (NOS). A family of related NOS proteins are the products of different genes and include neuronal NOS (nNOS, type 1), immunologic NOS (iNOS, type 2), and endothelial NOS (eNOS, type 3) (3). nNOS occurs in discreet neuronal populations in the brain and also is localized to the sarcoplasmic reticulum of skeletal muscle (4). eNOS primarily has endothelial cell localizations, but also is localized to a variety of other tissue types, including CA1 pyramidal cells of the hippocampus (5). Both nNOS and eNOS are constitutively expressed and are calcium-calmodulin-dependent enzymes (3, 4). iNOS is expressed in response to cytokines, lipopolysaccharide (LPS), and a host of other agents (6, 7). iNOS has been localized to a variety of cell types upon appropriate immunologic stimulation (6, 7). The key to regulation of NO production by iNOS is through regulation of transcription (8, 9). Characterization of the promoter region of the gene for iNOS reveals a complex pattern of regulation (8–12). Upstream from the transcription start site are distinct regulatory regions, including LPS-related response elements, binding sites for NFκB, and γ-interferon motifs (8–11). Recent studies indicate that NO transcriptionally inhibits iNOS mRNA expression in astrocytes (13). However, the mechanism by which NO transcriptionally regulates iNOS expression has not been clarified. Preliminary studies showed that exogenously applied NO inhibits the activation of NFκB (14, 15). Thus, we wondered whether NO inhibition of NFκB could regulate the expression of iNOS. We now report that endogenous NO regulates the transcription factor NFκB, and through this regulation modulates the expression of iNOS. Moreover, we show that type I (nNOS) in glia tonically suppresses NFκB activity and transcriptionally regulates iNOS expression.
MATERIALS AND METHODS
Cell Cultures.
Primary mixed glial cell cultures were prepared from postnatal day 0–3 Lewis rats as described (16). Briefly, the cortex was dissected under a microscope in Brooks–Logan dissecting solution. After dissection, the cortical tissue was placed in 0.25% trypsin solution at 37°C for 30 min. The trypsin solution was removed, and DMEM (GIBCO/BRL) with 20% fetal bovine serum (FBS) (GIBCO/BRL) and 2 mM l-glutamine (GIBCO/BRL) was added to the cortical tissue suspension. The cells were dissociated by trituration through 9-inch Pasteur pipettes until the solution was cloudy. The suspended cells were plated on 75-cm2 flasks coated with polyornithine and placed in an 8% CO2 humidified 37°C incubator. After 2 days in culture, the medium was changed to DMEM, 10% FBS, and 2 mM glutamine. The medium was changed twice per week, and the cultures were allowed to mature to confluence (approximately 1 week) before being used for the experiments. To examine the role of nNOS in NFκB activation, primary mixed glial cell cultures also were prepared from day 0–3 pups of wild-type mice and mutant mice lacking the gene for nNOS (17). Jurkat cells (American Type Culture Collection) were grown in RPMI 1640 medium (GIBCO/BRL) containing 10% FBS. Jurkat cells were plated on 75-cm2 flasks and grown in a 5% CO2 humidified 37°C incubator. Cultures were treated with the various agents as indicated in the text and figure legends.
Measurement of Nitrite Formation.
To remove any trace of phenol red, the cell cultures were washed gently with Griess medium. Griess medium consisted of DMEM without glucose, glutamine, phenol red, sodium bicarbonate, and sodium pyruvate with the following additions: 0.4 mM MgSO4, 20 mM NaHCO3, 12 mM D-glucose, 0.5 mM pyruvic acid, and 0.4 mM CaCl2. After the exposure period, culture media were harvested for the colorimetric determination of nitrite concentration by comparison to nitrite standards (16).
Northern Blot Analysis.
Total cellular RNA was isolated from cells with the guanidinium thiocyanate-phenol-chloroform method (18). Northern blot analysis was done as described (19). Equal amounts of total RNA (5 μg/lane) were separated by denaturing agarose gel electrophoresis and transferred to positively charged nylon membranes (Hybond-N+, Amersham). The membrane was hybridized with a 32P-labeled random-primed probe made to rat iNOS and β-actin. Hybridized filters were washed at 65°C in 0.1× standard saline citrate (1× SSC = 0.15 M sodium chloride/0.015 m sodium citrate, pH 7) and 0.1% SDS. The membrane then was exposed to a PhosphorImager (Molecular Dynamics) screen, and the bands were quantitated.
Western Blot Analysis.
Cell culture plates were washed twice with ice-cold PBS. Cells were harvested by being scraped into ice-cold lysis buffer [50 mM Tris·HCl, pH 7.4/1 mM 2-mercaptoethanol/1 mM phenylmethylsulfonyl fluoride (PMSF)/1 mM benzamidine/10 μg/ml pepstatin A/1 μg/ml aprotinin/1 mM EDTA]. The cell lysate was transferred to microcentrifuge tubes and incubated on ice for 60 min and centrifuged at 15,000 rpm for 20 min at 4°C. The supernatant fluid (total cell lysate) was used for Western blot analysis. Western blot analysis was carried out using 50 μg of the total cell lysates. Proteins were electroblotted from SDS-polyacrylamide gels onto Immobilon-P membranes. The membrane was blocked with 5% skim milk in PBS for 1 h at room temperature. Affinity-purified rabbit polyclonal antisera to iNOS and mouse polyclonal antiserum to nNOS and eNOS were from Transduction Laboratories (Lexington, KY). The blots were incubated with primary antibodies overnight at 4°C in PBS buffer containing 3% BSA. The blots were washed four times with 5% skim milk in PBS and then incubated with secondary antibody (1:5,000 dilution) coupled to horseradish peroxidase. Immunodetection was accomplished using a Lumiglo Substrate Kit (Kirkegaard & Perry Laboratories) for chemiluminescent detection.
Electrophoretic Mobility Shift Assay and Supershift Analysis.
The 26-mer oligonucleotide from the major histocompatibility complex class I promoter, including the consensus binding site for NFκB (5′-GATCCAGAGGGGACTTTCCGAGAGGA-3"), was used for gel shifts (20) (Santa Cruz Biotechnology). The NFκB binding oligonucleotide was end-labeled with [γ-32P]ATP and T4 polynucleotide kinase (New England Biolabs), and purified by G-50 Sephadex columns (Pharmacia). Confluent cultured glial cells and Jurkat cells were harvested with ice-cold hypoosmotic buffer (10 mM Hepes, pH 7.8/10 mM KCl/2 mM MgCl2/0.1 mM EDTA/10 μg/ml aprotinin/0.5 μg/ml leupeptin/3 mM PMSF/3 mM DTT) after stimulation. The cells in the hypotonic buffer were incubated for 17 min on ice. Nonidet P-40 was added, and the nuclei were pelleted by centrifugation at 15,000 rpm for 5 min in a microcentrifuge. The supernatants containing the cytoplasmic proteins were removed and stored at −70°C. The pelleted nuclei were resuspended in a high-salt buffer [50 mM Hepes, pH 7.4/50 mM KCl/300 mM NaCl/0.1 mM EDTA/10% (vol/vol) glycerol/3 mM DTT/3 mM PMSF] to solubilize DNA binding proteins. The resuspended nuclei were gently shaken for 30 min at 4°C. The extracts were spun in a microcentrifuge at 15,000 rpm for 10 min, and the clear supernatants containing nuclear protein were aliquoted and stored at −70°C. Binding reactions were performed at room temperature for 15 min using 6–8 μg of nuclear protein and 0.25 ng (25,000 cpm) of labeled oligonucleotide in 30 μl of binding buffer containing 10 mM Tris·HCl, pH 7.5/50 mM NaCl/50 mM KCl/1 mM MgCl2/1 mM EDTA/5 mM DTT/5% glycerol/2 μg of poly(dI-dC) (Pharmacia). DNA-protein complexes were separated from unbound probe on native 6.0% polyacrylamide gels (Bio-Rad) at 200–250 V for 2 h. The resultant gel was vacuum-dried and exposed to a PhosphorImager screen. Cold competition was performed using consensus (5′-GGGGACTTTCCC-3′) or mutant NFκB subunits p65 and p50 (Santa Cruz Biotechnology). Supershift experiments were performed by adding NFκB p65 or p50 antibody (1 or 2 μl) to the binding mixture immediately after the addition of the radiolabeled NFκB probe. The reaction mixture was incubated for 20 min at room temperature, and the complexes were resolved as described above.
Statistical Analysis.
Data were analyzed using one-way ANOVA, followed by least significant difference post hoc analysis to determine statistical significance. Differences were considered significant at P < 0.05.
Chemicals.
LPS, PDTC, l-Arg, bathophenanthroline disulfonic acid (BPS), PMSF, benzamidine, leupeptin, pepstatin A, and aprotinin were obtained from Sigma. (±)-(E)-Ethyl-2[(E)-hydroxyimino]-5-nitro-3-hexenamide (NOR-3), l-NG-(1-iminoethyl)ornithine, and N-monomethylarginine (l-NMMA) were from Alexis (San Diego, CA). Human TNF-α was from Intergen (Purchase, NY).
RESULTS
Endogenous NO Inhibits NFκB Activation.
To evaluate the role of endogenously produced NO effects on NFκB transcription we examined the effects of NOS inhibitors on electrophoretic mobility shift assays in astrocytes (Fig. 1). l-NMMA (500 μM), a competitive NOS inhibitor, begins to enhance NFκB binding at 4 h, and at 24 h there is a dramatic increase in NFκB binding. The substrate for NOS, l-Arg (5 mM), completely reverses the enhancement of NFκB binding by l-NMMA (Fig. 1). The structurally unrelated inhibitor l-NG-(1-iminoethyl)ornithine (250 μM) also enhances NFκB binding in a manner similar to l-NMMA (data not shown). To confirm that NO inhibits NFκB transcription we treated astrocytes with the potent NFκB activator LPS (Fig. 1B). LPS induces NFκB binding at 2 h with maximal induction at 4 h, which is sustained over the course of the 24-h treatment with LPS. The highly selective NO donor, NOR-3, potently inhibits NFκB activation at all time points (Fig. 1B). NOR-3 depleted of NO by incubating NOR-3 in culture media for several hours has no effect on LPS-induced NFκB activation (data not shown).
Figure 1.
NO regulates NFκB activity as determined by electrophoretic mobility shift assays. (A) Cultured glia were incubated with l-NMMA (500 μM), and NFκB-binding activity was measured at 0, 2, 4, 8, 16, and 24 h after treatment. l-NMMA begins to enhance NFκB activity at 4 h, and at 24 h there is a dramatic increase in NFκB-binding activity. Excess l-Arg (5 mM) completely reversed l-NMMA-induced NFκB activation. (B) LPS (60 ng/ml) strongly enhances NFκB activity in glial cultures. LPS enhances NFκB activation at 2 h and continues up to 24 h after treatment with LPS. NOR-3 (100 μM) potently inhibits NFκB activation at all time points. Representative blots are shown for experiments that were performed at least three independent times with similar results.
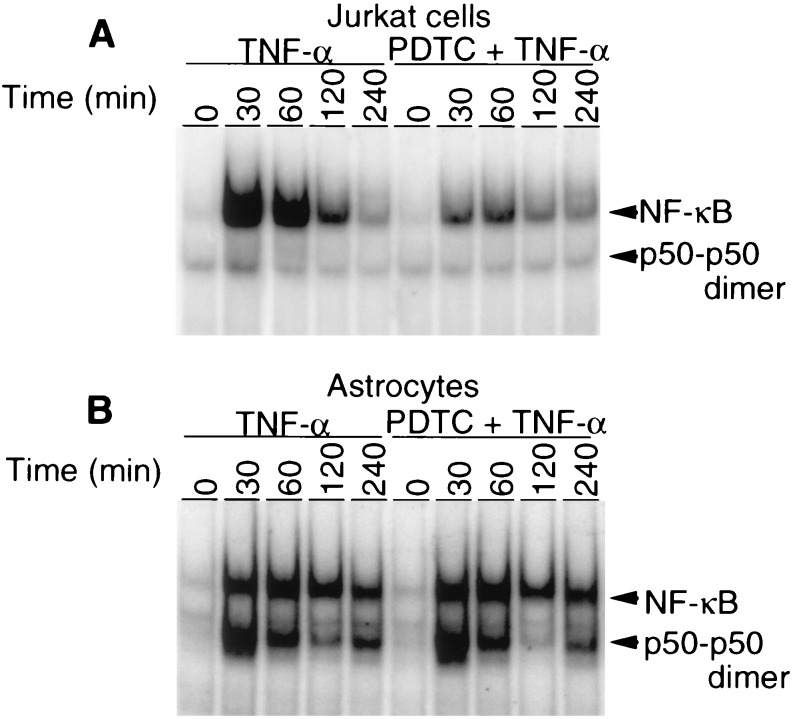
PDTC Differentially Regulates NFκB Expression in Lymphocytes and Astrocytes.
PDTC is a potent inhibitor of NFκB activation in intact cells (20). However, PDTC is also a potent scavenger of NO through formation of mononitrosyl iron complexes with PDTC (21, 22). Thus, we wondered whether PDTC would differentially regulate NFκB activation in cells that constitutively express NOS at low levels (23) versus cells that express NOS at relatively higher levels. The human T-cell line, Jurkat cells, express eNOS constitutively at very low levels (23), whereas astrocytes contain relatively higher levels of constitutive NOS (24). Similar to previous reports, TNF-α in Jurkat cells induces NFκB activation at 30 min, which diminishes over time to modest increases over baseline at 240 min (Fig. 2) (25). PDTC dramatically inhibits NFκB activation at all time points (Fig. 2A). In contrast, PDTC does not inhibit TNF-α-induced NFκB in astrocytes (Fig. 2B). Astrocytes in response to TNF-α have sustained activation of NFκB and also appear to contain higher levels of P50 dimer than Jurkat cells (Fig. 2).
Figure 2.
PDTC differentially regulates NFκB expression in Jurkat cells and glial cultures as determined by electrophoretic mobility shift assays. (A) In Jurkat cells, TNF-α (300 units per ml) enhances NFκB activity, which peaks at 30 min. PDTC (100 μM) inhibits TNF-α-induced NFκB activation at all time points. Representative blots are shown for experiments that were performed at least three independent times with similar results. (B) TNF-α (300 units per ml) induces NFκB activation in cultured glia. PDTC (100 μM) fails to inhibit TNF-α (300 units per ml)-induced NFκB activation. Note the higher levels of P50 dimer in glia compared with Jurkat cells.
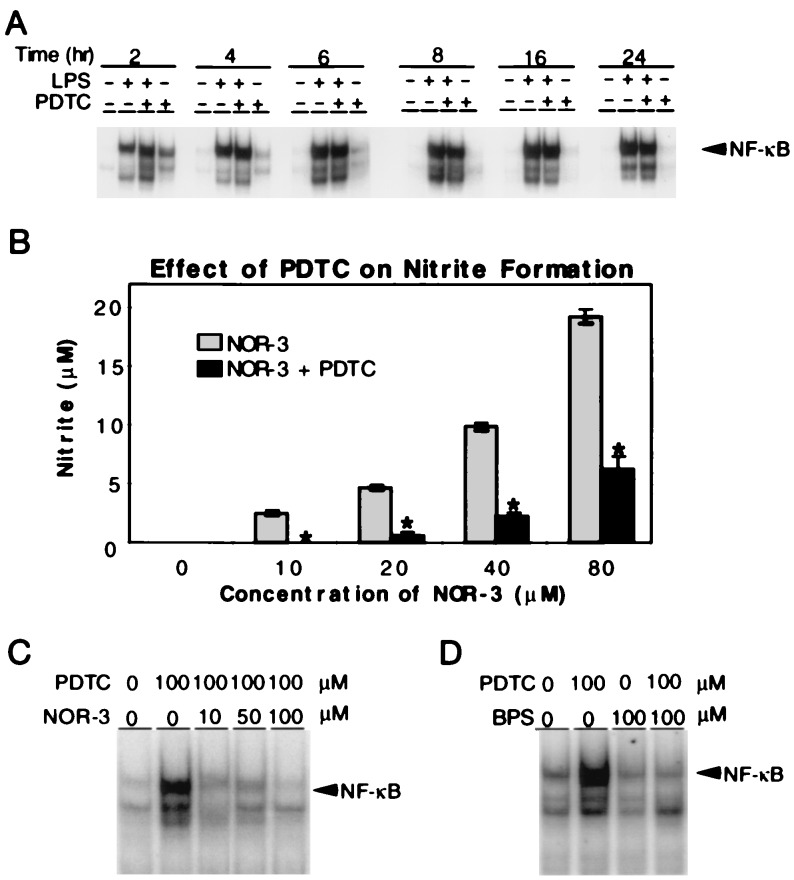
PDTC Induces NFκB Activation in Astrocytes.
Because PDTC fails to inhibit TNF-α-induced NFκB activation in astrocytes, this prompted us to further evaluate the effects of PDTC on NFκB activation in astrocytes. LPS potently activated NFκB binding in astrocytes at 2 h, and NFκB binding continues to increase up to 24 h after LPS administration. Interestingly, at 2 h PDTC enhances LPS-mediated activation of NFκB. The enhancement of LPS-stimulated NFκB binding by PDTC is present at 4 h and 6 h (Fig. 3A). At 8 to 24 h PDTC modestly inhibits LPS-stimulated NFκB binding (Fig. 3A). There are no differences in cell viability among control, LPS, PDTC plus LPS, and PDTC groups (data not shown). Strikingly, PDTC alone activates NFκB binding at 2 h with effects diminishing at 6 h of PDTC treatment (Fig. 3A). Confirmation of the specificity of NFκB activation by PDTC is the complete elimination of NFκB binding by competition with unlabeled oligonucleotide and the failure of a mutant oligonucleotide to compete for NFκB binding (data not shown). Furthermore, anti-P65 and anti-P50 antibodies to NFκB subunits abolish the NFκB band and cause further gel retardation (supershift) (data not shown).
Figure 3.
PDTC enhances NFκB binding in glia by scavenging NO. (A) Electrophoretic mobility shift assays show that LPS (60 ng/ml) potently activates NFκB binding in astrocytes at 2 h, and this activation continues to 24 h. PDTC (100 μM) enhances LPS-mediated NFκB activation for the first 6 h after LPS administration. At 8 to 24 h PDTC modestly inhibits LPS-stimulated NFκB binding. Notably, PDTC (100 μM) alone activates NFκB binding at 2 h with effects diminishing at 6 h of PDTC treatment. (B) PDTC (100 μM) significantly inhibits NOR-3-mediated nitrite formation at all concentrations of NOR-3. Results represent the mean of five independent determinations ± the SD. ∗, P < 0.01 as compared with NOR-3 alone. (C) Electrophoretic mobility shift assays indicate that NOR-3 (10–100 μM) blocks PDTC (100 μM) enhancement of NFκB activity by effectively counteracting the NO-scavenging capabilities of PDTC. (D) Electrophoretic mobility shift assays demonstrate that BPS (100 μM) effectively inhibits PDTC (100 μM) enhancement of NFκB activity in astrocytes. A, C, and D are representative of at least three independent experiments.
We investigated the potential mechanism by which PDTC enhances NFκB binding in astrocytes (Fig. 3B). PDTC is an effective scavenger of NO formation as indicated by its ability to inhibit nitrite formation by NOR-3 (Fig. 3B). PDTC enhancement of NFκB is dose dependently blocked by NOR-3, which effectively counteracts the NO-scavenging capabilities of PDTC (Fig. 3C). PDTC is thought to scavenge NO through forming mononitrosyl iron complexes with PDTC (21, 22). The noncell permeable iron chelator BPS effectively inhibits PDTC enhancement of NFκB binding (Fig. 3D), confirming the role of PDTC iron complexes in the enhancement of NFκB binding in astrocytes. BPS alone has minimal effects on NFκB binding in astrocytes (Fig. 3D); however, we cannot exclude the possibility that BPS is acting as a scavenger of NO. Although our results suggest that PDTC is enhancing NFκB activity through scavenging of NO through the formation of mononitrosyl iron complexes, we cannot exclude the possibility that it is inhibiting superoxide dismutase or inhibiting the Fenton reaction.
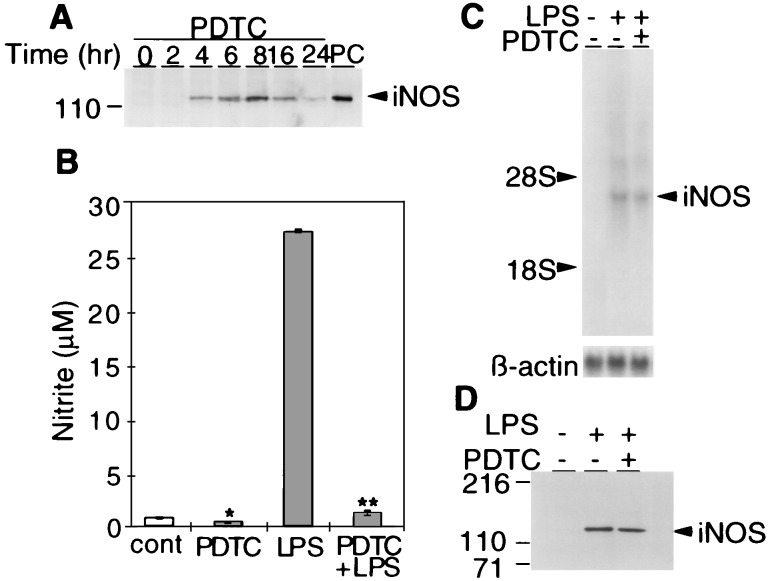
Constitutive NOS Regulates iNOS Expression.
To explore the physiologic relevance of NO-regulated NFκB transcription we examined the effects of PDTC on the NFκB-regulated gene, iNOS (8–12) (Fig. 4A). PDTC dramatically induces iNOS expression in astrocytes with iNOS protein being detectable at 4 h after PDTC treatment. Maximal iNOS protein levels are present at 8 h and begin to diminish at 16 h with almost complete loss of iNOS levels after 24 h of PDTC treatment (Fig. 4A). To further examine the role of PDTC regulation of NFκB and iNOS we examined the effects of PDTC on LPS-stimulated NO formation by iNOS (Fig. 4 B–D). Similar to previous observations LPS potently stimulates NO formation in astrocyte cultures (Fig. 4B). Consistent with the notion that PDTC scavenges NO and enhances NFκB activity is the ability of PDTC to diminish nitrite levels in LPS-stimulated cultures without affecting the levels of iNOS mRNA or protein (Fig. 4 B-D).
Figure 4.
Regulation of iNOS expression by constitutive NOS. (A) Western blot analysis indicates that PDTC (100 μM) dramatically induces iNOS expression in glial cultures with iNOS protein being detectable at 4 h and peaking at 8 h after PDTC treatment. PC, positive control. (B) PDTC (100 μm) alone significantly suppresses basal nitrite formation compared with control (∗, P < 0.05). PDTC also significantly inhibits LPS (24 h)-stimulated nitrite formation compared with LPS alone (∗∗, P < 0.01). Results represent the mean of five independent determination ± SD. (C) Northern blot analysis shows that PDTC did not affect the level of iNOS mRNA induced by LPS (24 h). (D) Western blot analysis shows that PDTC did not affect the level of iNOS protein induced by LPS (24 h). A, C, and D are representative of at least three independent experiments.
nNOS Regulates NFκB Transcription in Astrocytes.
To ascertain the source of NO that accounts for regulation of NFκB transcription we conducted Western blot analysis on glial cultures. Glia constitutively express nNOS and contain barely detectable levels of eNOS, and at baseline iNOS is undetectable (Fig. 5A). Because nNOS is the major isoform that is constitutively expressed, we examined the effects of PDTC modulation of NFκB transcription in cultures derived from mice lacking the gene for nNOS and compared it to cultures derived from wild-type animals. As previously shown, PDTC induces NFκB activation in wild-type cultures at 120 min. In contrast, PDTC in nNOS astrocyte cultures massively induces NFκB after 30 min of treatment, and at 2 h NFκB is dramatically induced when compared with wild-type animals (Fig. 5C). Thus, NO derived from the neuronal form of NOS seems to be a major regulator of NFκB transcription in glial cultures.
Figure 5.
nNOS regulates NFκB activation in glial cultures. (A) Western blot analysis indicates that glia constitutively express nNOS and contain barely detectable levels of eNOS, and at baseline iNOS is undetectable. (B) Electrophoretic mobility shift assays demonstrate that in wild-type glial cultures PDTC (100 μM) induces NFκB activation at 120 min. (C) Electrophoretic mobility shift assays show that in nNOS− glial cultures PDTC massively induces NFκB activation after 30 min of treatment, and the induction continues until 2 h. Blots are representative of at least two independent experiments.
DISCUSSION
Our findings indicate that NO derived from type 1 (nNOS) in glia inhibits NFκB activation, confirming previous reports that exogenous NO can regulate and inhibit NFκB activity (14, 15). Furthermore, we show that endogenously derived NO inhibits NFκB-binding activity, because competitive NOS inhibitors enhance NFκB binding, and excess substrate L-Arg reverses the enhancement of NFκB binding by NOS inhibitors in intact cells. PDTC, a well known inhibitor of NFκB binding in nonneuronal tissue (20), enhances NFκB binding in astrocytes through scavenging of NO. This activation of NFκB induces iNOS expression in astrocytes. The source of endogenously derived NO that regulates NFκB binding in astrocytes appears to be nNOS as astrocytes predominantly express nNOS and contain negligible quantities of eNOS and iNOS. NFκB binding is also more effectively induced at an earlier time point by the NO scavenging effects of PDTC in mutant mice lacking nNOS when compared with wild-type mice.
One pathway toward NFκB binding activity is its translocation to the nucleus through degradation of inhibitory protein κB (IκB) (26, 27). Although it is not well clarified, IκB degradation is regulated by serine phosphorylation and ubiquination (27). Recent studies suggest that exogenously applied NO may inhibit NFκB binding in endothelial cells through stabilization of IκB-α or through increased transcription of IκB-α (14). Alternatively NO may directly inhibit NFκB through S-nitrosylation of the cysteine-62 residue of p50 (28). PDTC is a potent inhibitor of NFκB binding in nonneuronal tissue such as Jurkat cells (25). In contrast, PDTC fails to inhibit NFκB binding in astrocytes after either TNF-α- or LPS-induced NFκB binding. PDTC suppression of NFκB binding in nonneuronal tissues is thought to be due to inhibition of the release of IκB through either its metal chelating or antioxidative properties. The molecular mechanisms underlying the differential regulation of NFκB in astrocytes versus lymphocytes is not known. It is possible that the inability of PDTC to enhance NFκB binding in lymphocytes may be related to the relatively low levels of constitutive expression of NOS in this cell line. Alternatively, the relatively low levels of P50 in Jurkat cells (25) in contrast to the high levels of P50 in astrocytes may account for the differential regulation of NFκB binding by PDTC.
nNOS is primarily localized to neurons throughout the peripheral and central nervous system (29). Recent studies indicate that nNOS is also localized in skeletal muscle, pancreatic islets, endometrium, and respiratory and gastrointestinal epithelium (4). nNOS in neurons may play a role in neurotransmitter release, neural development, synaptic plasticity, and regulation of gene expression. Previous reports indicated constitutive expression of NOS in astrocytes (24); however, the isoform and physiologic role of NOS in astrocytes was not clarified. We show that nNOS is the predominant isoform in astrocytes, implicating nNOS in a new physiologic role. nNOS in astrocytes constitutively generates NO to repress the NFκB redox-sensitive transcription factor and regulates the expression of NFκB-sensitive genes such as iNOS. Because NFκB regulates the transcription of several “inflammatory” genes such as iNOS, interleukin-6, TNF-α, and major histocompatibility complex class I and II (30), constitutive NO derived from nNOS in astrocytes may tonically inhibit inflammatory processes through gene regulation. Consistent with this notion are our observations that scavenging of NO and inhibition of NOS activates NFκB binding and induces iNOS. Furthermore, NFκB activation is readily induced in astrocytes from mice lacking the gene for nNOS. Thus, NO derived from nNOS in glia subserves a novel redox-signaling role through regulation of NFκB activity. NO may be regulating NFκB through both intracellular and intercellular signaling. In the nervous system astrocytes may be regulating NFKB through NO acting intercellularly. However, the major source of NO in vivo would be derived from nNOS containing neurons, which could regulate NFκB through cell-to-cell signaling. Because NO is a freely diffusible messenger molecule, NO could regulate NFκB through intracellular interactions in nonneuronal tissue as well. These observations may have clinical relevance in which inhibition of NOS is contemplated in diseases such as multiple sclerosis (31) and severe AIDS dementia (32) where iNOS is elevated.
Acknowledgments
This work was supported by U.S. Public Health Service Grants NS 01578 (T.M.D.) and NS 22643 (T.M.D. and V.L.D.) and by the American Foundation for AIDS Research (V.L.D.).
ABBREVIATIONS
- NOS
nitric oxide synthase
- nNOS
neuronal NOS
- eNOS
endothelial NOS
- iNOS
immunologic NOS
- NFκB
nuclear factor κB
- PDTC
pyrrolidine dithiocarbamate
- LPS
lipopolysaccharide
- TNF-α
tumor necrosis factor-α
- FBS
fetal bovine serum
- PMSF
phenylmethylsulfonyl fluoride
- l-Arg
l-arginine
- BPS
bathophenanthroline disulfonic acid
- l-NMMA
N-monomethylarginine
- NOR-3
(±)-(E)-ethyl-2-[(E)-hydroxyimino]-5-nitro-3-hexeneamide
References
- 1.Moncada S, Higgs A. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 2.Nathan C. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 3.Bredt D S, Snyder S H. Annu Rev Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- 4.Yun H Y, Dawson V L, Dawson T M. Crit Rev Neurobiol. 1996;10:291–316. doi: 10.1615/critrevneurobiol.v10.i3-4.20. [DOI] [PubMed] [Google Scholar]
- 5.Dinerman J L, Dawson T M, Schell M J, Snowman A, Snyder S H. Proc Natl Acad Sci USA. 1994;91:4214–4218. doi: 10.1073/pnas.91.10.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathan C, Xie Q-W. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 7.Morris S M, Billiar T R. Am J Physiol. 1994;266:E829–E839. doi: 10.1152/ajpendo.1994.266.6.E829. [DOI] [PubMed] [Google Scholar]
- 8.Lowenstein C J, Alley E W, Raval P, Snowman A M, Snyder S H. Proc Natl Acad Sci USA. 1993;89:6711–6715. [Google Scholar]
- 9.Xie Q-W, Whisnant R, Nathan C. J Exp Med. 1993;177:1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin E, Nathan C, Xie Q-W. J Exp Med. 1994;180:977–984. doi: 10.1084/jem.180.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie Q-W, Nathan C. J Leukocyte Biol. 1994;56:576–582. doi: 10.1002/jlb.56.5.576. [DOI] [PubMed] [Google Scholar]
- 12.DeVera M E, Shapiro R A, Nussler A K, Mudgett J S, Simmons R L, Morris S M, Billiar T R, Geller D A. Proc Natl Acad Sci USA. 1996;93:1054–1059. doi: 10.1073/pnas.93.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park S K, Lin H L, Murphy S. Biochem Biophys Res Comm. 1994;201:762–768. doi: 10.1006/bbrc.1994.1766. [DOI] [PubMed] [Google Scholar]
- 14.Peng H B, Libby P, Liao J K. J Biol Chem. 1995;270:14214–14219. doi: 10.1074/jbc.270.23.14214. [DOI] [PubMed] [Google Scholar]
- 15.Colasanti M, Persichini T, Menegazzi M, Mariotto S, Giordano E, Caldarera C M, Sogos V, Lauro G M, Suzuki H. J Biol Chem. 1995;270:26731–26733. doi: 10.1074/jbc.270.45.26731. [DOI] [PubMed] [Google Scholar]
- 16.Dawson V L, Brahmbhatt H P, Mong J A, Dawson T M. Neuropharmacology. 1994;33:1425–1430. doi: 10.1016/0028-3908(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 17.Huang P L, Dawson T M, Bredt D S, Snyder S H, Fishman M C. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- 18.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. pp. 7.46–7.48. [Google Scholar]
- 20.Schreck R, Meier B, Mannel D N, Droge W, Baeuerle P A. J Exp Med. 1992;175:1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarte B, Stanford J, LaPrice W J, Uhrich D L, Lockhart T E, Gelerinter E, Duffy N V. Inorg Chem. 1978;17:3361–3365. [Google Scholar]
- 22.Mikoian V D, Kubrina L N, Vanin A F. Biofizika. 1994;39:915–918. (Abstr. English). [PubMed] [Google Scholar]
- 23.Reiling N, Kroncke R, Ulmer A J, Gerdes J, Flad H-D, Hauschildt S. Eur J Immunol. 1996;26:511–516. doi: 10.1002/eji.1830260302. [DOI] [PubMed] [Google Scholar]
- 24.Murphy S, Simmons M L, Agullo L, Garci A, Feinstein D L, Galea E, Reis D J, Minc-Golomb D, Schwartz J P. Trends Neurosci. 1993;16:323–328. doi: 10.1016/0166-2236(93)90109-y. [DOI] [PubMed] [Google Scholar]
- 25.Thompson J E, Phillips R J, Erdjument-Bromage H, Tempst P, Ghosh S. Cell. 1995;80:573–582. doi: 10.1016/0092-8674(95)90511-1. [DOI] [PubMed] [Google Scholar]
- 26.Henkel T, Machleidt T, Alkalay I, Kronke M, Ben-Neriah Y, Baeuerle P A. Nature (London) 1993;365:182–185. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- 27.Thanos D, Maniatis T. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 28.Matthews J R, Botting C H, Panico M, Morris H R, Hay R T. Nucleic Acids Res. 1996;24:2236–2242. doi: 10.1093/nar/24.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dawson T M, Snyder S H. J Neurosci. 1994;14:5147–5159. doi: 10.1523/JNEUROSCI.14-09-05147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lenardo M J, Baltimore D. Cell. 1989;58:227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- 31.Bo L, Dawson T M, Wesselingh S, Mork S, Choi S, Kong P A, Pardo C, Hanley D, Trapp B D. Ann Neurol. 1994;36:778–786. doi: 10.1002/ana.410360515. [DOI] [PubMed] [Google Scholar]
- 32.Adamson D C, Wildemann B, Sasaki M, Glass J D, McArthur J C, Christov V I, Dawson T M, Dawson V L. Science. 1996;274:1917–1921. doi: 10.1126/science.274.5294.1917. [DOI] [PubMed] [Google Scholar]