Over 75,000 radical prostatectomies were performed in the USA last year for the treatment of prostate cancer. Most of these were performed by radical retropubic prostatectomy (RRP), the gold standard for treatment of this disease. However, the quest for increased efficacy and decreased morbidity is having as profound an impact on the treatment of prostate cancer as in any other area of medicine.
There are two unique factors at work in the search for decreased morbidity in prostate cancer treatment. The first is the high prevalence, since prostate cancer is diagnosed in 1 in 6 men during their lifetime. The second involves the well-known side effects of incontinence and erectile dysfunction, whose impact is often more crippling psychologically than physically.
The original minimally invasive treatment for prostate cancer, interstitial brachytherapy, has now been joined by novel technologies such as high-intensity focused ultrasound, cryotherapy, and, most recently, by another form of radiation delivery, Cyberknife. These technologies will likely all find a place in our armamentarium, but even the most seasoned of these treatments (brachytherapy) still has limitations that prevent it from replacing surgical removal of the prostate.
For these reasons, both patients and urologic surgeons have continued to seek out less invasive surgical options. In 1991, Clayman et al performed the first laparoscopic radical prostatectomy (1). In the USA, enthusiasm over this procedure was mitigated by prolonged operative times, a steep learning curve, and a failure to demonstrate major advantages over open surgery. In Europe, however, the experience continued, and investigators began to present laparoscopic prostatectomy outcomes that were comparable to those of open surgery with roughly equivalent operative times (2, 3). However, the procedure never gained widespread acceptance, likely due to the technical challenges of traditional laparoscopic instrumentation.
In 2001, the Henry Ford Hospital described the first robotic prostatectomy (4), and shortly thereafter surgeons there published short-term outcome data comparing RRP with robotically assisted laparoscopic radical prostatectomy (RALP) (5, 6). Since that time the urology community has seen unparalleled growth in this procedure. Roughly 8500 RALP procedures were performed in 2004 and 18,000 in 2005; it has been estimated that 35% of all prostatectomies performed in 2007 will be performed robotically.
The DA VINCI SURGICAL SYSTEM
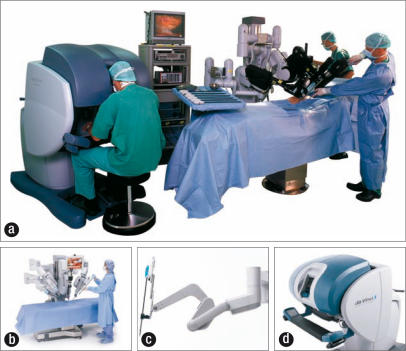
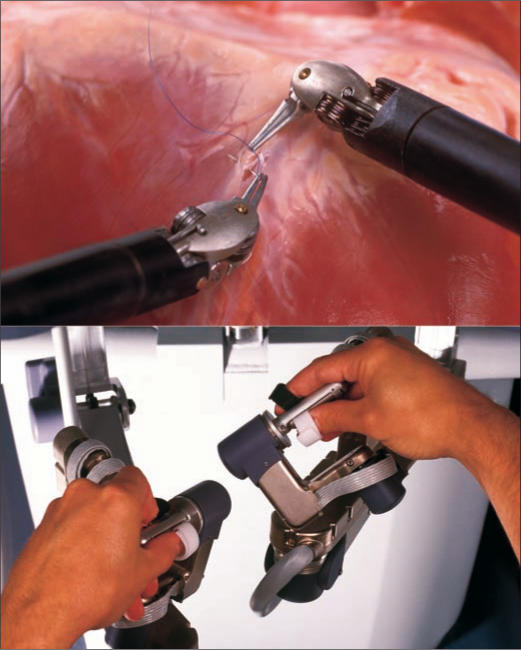
The Da Vinci computer-enhanced system consists of two main components: the surgical cart and the surgeon's console (Figure 1). At the console, the surgeon manipulates both hand and foot controls that direct the arms of the surgical cart. This is a true “master-slave” system, as all motions are direct translations of the surgeon's actions, although movement scaling can be set to normal, fine, or ultrafine depending on the precision required (Figure 2). There is no measurable delay between the surgeon's movements and those of the surgical cart, and tremor filtering removes unwanted movements. The instruments controlled by the robot have seven degrees of freedom, giving them a greater range of motion than the human wrist.
Figure 1.
(a) The Da Vinci surgical system. (b) The surgical cart houses four robotic arms that control the laparoscope and surgical tools. (c) A robotic arm. (d) The surgeon's console. Photos courtesy of Intuitive Surgical, Inc.
Figure 2.
The surgeon's hand movements at the console are translated to the surgical field. Photos courtesy of Intuitive Surgical, Inc.
A final technologic advancement over traditional laparoscopic surgery is the imaging system. Traditional laparoscopic cameras have a single camera and light source, giving a two-dimensional view. The Da Vinci system laparoscope consists of two separate cameras and light sources housed in a single instrument. This give a three-dimensional stereo-optic and magnified image of the operative field.
OUTCOMES OF RALP
Although the number of RALP procedures has rapidly increased, outcome data are based on short-term follow-up, with even the most mature series just now reporting 5-year data.
Operative time
One of the main barriers to acceptance of traditional laparoscopic radical prostatectomy is the prolonged operative times. By facilitating suturing and tissue dissection, the robotic approach has demonstrated shorter operative times than traditional laparoscopy. While initial operative times have been as long as 6 to 8 hours, operative time improves significantly with surgical experience. Several series have shown that average operative times decrease to around 140 to 180 minutes after 150 cases (7, 8). While there are some variations in defining actual operating time (set-up, docking, pelvic lymph node dissection, etc.), these times are roughly equivalent to open surgical times (6, 8–10).
Blood loss and transfusion
The blood loss and transfusion rates of RALP also vary among investigators, but blood loss remains consistently below that seen in open prostatectomy series (8–11). This is the result of early ligation of the dorsal venous complex and tamponade of venous sinuses from the pneumoperitoneum. In one study, mean discharge hematocrit was 38% with RALP vs 33% with RRP (11). As a consequence, transfusion rates are lower as well, with many series reporting rates <1% (5, 7–11). This rate is significantly lower than even the lowest reported transfusion rates of 5% to 10% with open prostatectomy (12, 13).
Convalescence and length of stay
Few would dispute that patients who undergo RALP recover more quickly than those who undergo an open procedure. However, convalescence is difficult to objectively measure, and length of hospital stay is often used as a surrogate marker. Length of stay with RALP is reported as 1.08 to 1.5 days, while comparable open series range from 2.2 to 3.5 days (9, 10).
An additional marker of recovery is the amount of time the urethral catheter remains in place postoperatively. While the duration varies significantly by surgeon and institution, it is typically much shorter with the robotic procedure. The decreased duration is a consequence of the vesicourethral anastomosis being performed in a running fashion rather than an interrupted fashion, which decreases anastomotic urine leaks and allows for earlier removal. At our institution, the catheter is typically in place for 1 week with RALP vs 2 to 3 weeks with RRP.
Oncologic efficacy
Prostatectomy is first and foremost an operation to cure cancer, and no short-term advantages are worth compromising oncologic efficacy. Only long-term data analyzing recurrence rates and survival data comparing these two approaches will definitively answer this question. However, positive surgical margins have been shown to be an independent predictor of cancer recurrence and a direct result of surgical technique when controlling for tumor grade and stage. Surgical margin status is often used as a measurable, short-term surrogate marker for oncologic outcomes. Positive surgical margin rates for RRP range from 0% to 77%, with an average of 28% for all stages based on a review of published series (14). Positive margin rates for RALP have been reported as 0% to 36.4% (5, 7–9). Again, surgical experience seems to play a role in decreasing positive surgical margins, with clinically significant improvement noted after 30 cases (15). Based on these data, RALP seems to be at least equivalent to RRP with regard to positive margin rates when controlling for tumor stage. However, long-term survival data are needed for a true comparison of the procedure's efficacy.
Urinary incontinence
Postprostatectomy incontinence occurred at very high rates in early series, but refinements in surgical technique have led to significant improvement over the last 20 years. The vast majority of men regain urinary control and do not require pads for urinary leakage after RRP. Walsh et al reported continence after RRP (as defined by no pad usage) returning at 3 months in 54% of men, at 6 months in 80% of men, and at 12 months in 93% of men (16).
Because RALP results in less bleeding and provides magnification leading to excellent visualization of the apex, it allows preservation of urethral length and decreased manipulation of the delicate urethral sphincter. These two factors are thought to play a major role in return of continence after prostatectomy. Continence rates of 33% at 1 week, 63% at 1 month, and 81% at 3 months after RALP were reported in one study (7). Patel et al reported similar findings, with 47%, 82%, and 89% continence rates at 1, 3, and 6 months, respectively, and an overall continence rate of 98% at 12 months (8). These data suggest that RALP continues the excellent overall continence rates seen after RRP, with a trend towards earlier return of control.
Erectile dysfunction
Preservation of erectile function is dependent on multiple factors, but the most important surgical factor is the preservation of the cavernosal nerves. This preservation is dependent on the precise dissection and separation of these nerve bundles from either side of the prostate in an atraumatic and athermic fashion. Recovery of these nerves and improvement in erections is a prolonged process that can continue for several years. The increases in magnification, improved visualization from decreased blood loss, and less traumatic, antegrade nerve preservation approach would seem to provide RALP an advantage in nerve preservation over RRP.
However, evaluation and comparison of erectile function is complicated by numerous factors. Results correlate closely with patient age, preoperative erectile function, unilateral or bilateral nerve sparing, tumor stage, and the use of adjunctive therapies such as oral phosphodiesterase inhibitors and vasoactive injections. Additional problems exist with variations in definitions of “successful” return of erectile function. These factors make comparisons difficult between series and techniques. Good results have been reported with RALP; Menon et al demonstrated that at 6 months, 64% of men under age 60 were able to have successful intercourse compared with 38% of men over age 60 (10). This result compares favorably with Walsh et al's 54% success rate at 6 months after RRP (16).
CONCLUSION
RALP is a minimally invasive option for the treatment of prostate cancer that has proven short-term advantages over RRP with regard to blood loss, transfusion rates, and convalescence. While long-term data for concerns such as urinary incontinence, erectile dysfunction, and oncologic cure are still lacking, the early data are encouraging. As experience continues, we expect to see continued improvement in patient outcomes.
References
- 1.Schuessler WW, Schulam PG, Clayman RV, Kavoussi LR. Laparoscopic radical prostatectomy: initial short-term experience. Urology. 1997;50(6):854–857. doi: 10.1016/S0090-4295(97)00543-8. [DOI] [PubMed] [Google Scholar]
- 2.Guillonneau B, Vallancien G. Laparoscopic radical prostatectomy: the Montsouris experience. J Urol. 2000;163(2):418–422. doi: 10.1016/s0022-5347(05)67890-1. [DOI] [PubMed] [Google Scholar]
- 3.Rassweiler J, Seemann O, Schulze M, Teber D, Hatzinger M, Frede T. Laparoscopic versus open radical prostatectomy: a comparative study at a single institution. J Urol. 2003;169(5):1689–1693. doi: 10.1097/01.ju.0000062614.56629.41. [DOI] [PubMed] [Google Scholar]
- 4.Pasticier G, Rietbergen JB, Guillonneau B, Fromont G, Menon M, Vallancien G. Robotically assisted laparoscopic radical prostatectomy: feasibility study in men. Eur Urol. 2001;40(1):70–74. doi: 10.1159/000049751. [DOI] [PubMed] [Google Scholar]
- 5.Menon M, Tewari A. Robotic radical prostatectomy and the Vattikuti Urology Institute technique: an interim analysis of results and technical points. Urology. 2003;61(4 Suppl 1):15–20. doi: 10.1016/s0090-4295(03)00116-x. [DOI] [PubMed] [Google Scholar]
- 6.Menon M, Tewari A, Baize B, Guillonneau B, Vallancien G. Prospective comparison of radical retropubic prostatectomy and robot-assisted anatomic prostatectomy: the Vattikuti Urology Institute experience. Urology. 2002;60(5):864–868. doi: 10.1016/s0090-4295(02)01881-2. [DOI] [PubMed] [Google Scholar]
- 7.Ahlering TE, Skarecky D, Lee D, Clayman RV. Successful transfer of open surgical skills to a laparoscopic environment using a robotic interface: initial experience with laparoscopic radical prostatectomy. J Urol. 2003;170(5):1738–1741. doi: 10.1097/01.ju.0000092881.24608.5e. [DOI] [PubMed] [Google Scholar]
- 8.Patel VR, Tully AS, Holmes R, Lindsay J. Robotic radical prostatectomy in the community setting—the learning curve and beyond: initial 200 cases. J Urol. 2005;174(1):269–272. doi: 10.1097/01.ju.0000162082.12962.40. [DOI] [PubMed] [Google Scholar]
- 9.Ahlering TE, Woo D, Eichel L, Lee DI, Edwards R, Skarecky DW. Robot-assisted versus open radical prostatectomy: a comparison of one surgeon's outcomes. Urology. 2004;63(5):819–822. doi: 10.1016/j.urology.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 10.Tewari A, Srivasatava A, Menon M. A prospective comparison of radical retropubic and robot-assisted prostatectomy: experience in one institution. BJU Int. 2003;92(3):205–210. doi: 10.1046/j.1464-410x.2003.04311.x. [DOI] [PubMed] [Google Scholar]
- 11.Farnham SB, Webster TM, Herrell SD, Smith JA. Intraoperative blood loss and transfusion requirements for robotic-assisted radical prostatectomy versus radical retropubic prostatectomy. Urology. 2006;67(2):360–363. doi: 10.1016/j.urology.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 12.Nuttall GA, Cragun MD, Hill DL, Morris TJ, Decker PA, Blute ML, Patterson DE, Warner DO. Radical retropubic prostatectomy and blood transfusion. Mayo Clin Proc. 2002;77(12):1301–1305. doi: 10.4065/77.12.1301. [DOI] [PubMed] [Google Scholar]
- 13.Lepor H, Nieder AM, Ferrandino MN. Intraoperative and postoperative complications of radical retropubic prostatectomy in a consecutive series of 1,000 cases. J Urol. 2001;166(5):1729–1733. [PubMed] [Google Scholar]
- 14.Wieder JA, Soloway MS. Incidence, etiology, location, prevention and treatment of positive surgical margins after radical prostatectomy for prostate cancer. J Urol. 1998;160(2):299–315. [PubMed] [Google Scholar]
- 15.Atug F, Castle EP, Srivastav SK, Burgess SV, Thomas R, Davis R. Positive surgical margins in robotic-assisted radical prostatectomy: impact of learning curve on oncologic outcomes. Eur Urol. 2006;49(5):866–871. doi: 10.1016/j.eururo.2006.02.054. discussion 871-872. [DOI] [PubMed] [Google Scholar]
- 16.Walsh PC, Marschke P, Ricker D, Burnett AL. Patient-reported urinary continence and sexual function after anatomic radical prostatectomy. Urology. 2000;55(1):58–61. doi: 10.1016/s0090-4295(99)00397-0. [DOI] [PubMed] [Google Scholar]