Polyarteritis nodosa (PAN) is a form of systemic necrotising vasculitis involving predominantly small‐ and medium‐sized muscular arteries. Aetiological factors are usually unknown in the causation of polyarteritis nodosa, though some cases may be due to hepatitis B virus, cytomegalovirus, drug hypersensitivity, and malignancies. Hairy cell leukaemia (HCL) is characterised by pancytopenia, splenomegaly and inaspirable bone marrow. The coexistence of the two diseases is highly unlikely by chance, and it is hypothesised that an aetiological relationship exists between the two conditions.
Case report
A 37‐year‐old man presented with a one‐week history of fever and oliguria. On examination, he had generalised lymphadenopathy and hepatosplenomegaly. Other systemic examination was normal.
Laboratory tests revealed pancytopenia; erythrocyte sedimentation rate (ESR) was 56 mm in the first hour. Bone marrow aspirate was dry‐tap. Trephine biopsy showed hypercellular marrow spaces with characteristic features of hairy cell leukaemia. These lymphoid cells expressed CD25 and CD103. He died from the respiratory illness.
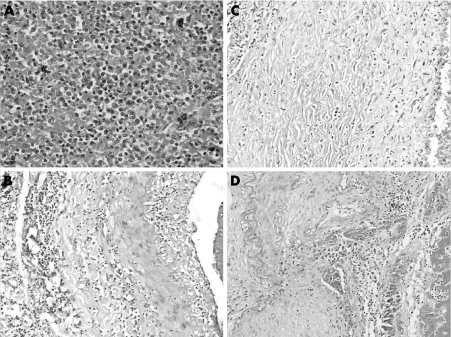
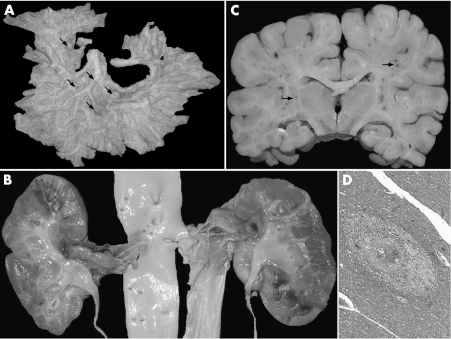
An autopsy was performed after obtaining informed consent. Bone marrow and spleen (weight 1470 g) showed morphological features of HCL (fig 1A). Grossly, mesenteric (fig 2A), renal (fig 2B), and coronary arteries showed multiple segmental aneurysmal dilatations measuring 0.4–1.8 cm in diameter. Kidneys (weight 190 g) showed geographic infarcts. Microscopically, there were variable changes including neutrophilic infiltration, mononuclear cell infiltrate, fibroblastic proliferation in the intima, fibrinoid necrosis, aneurysm formation, fragmentation of the internal elastic lamina, obliterative lumina, calcification and thrombus formation, indicating changes of acute, sub‐acute and healed stages of PAN (fig 1B–D). No granulomatous reaction or glomerulonephritis was noticed. Ultrastructurally, glomeruli did not reveal any electron‐dense deposits. Immunostain for hepatitis B surface and core antigen on liver was negative. Lungs showed acute necrotising pneumonia. Internal lymph nodes were enlarged, matted, and fleshy. Microscopically, they were completely effaced by the leukaemia infiltrate. Brain (weight 1390 g) was unremarkable externally. There were diffuse petechial haemorrhages in white matter, internal capsule, middle cerebral peduncle and pons (fig 2C). Microsopically, perivascular haemorrhages and oedema (fig 2D), lymphocytic infiltrate with secondary myelin loss and oedema were seen eliciting formation of micro‐glial nodules. No fibrin deposition was noticed in the small vessels. These features suggested disseminated vasculomyelinopathy.
Figure 1 (A) Spleen shows immature lymphoid cells admixed with plasmacytoid cells in the red pulp. (B) Renal artery showing active stage of polyarteritis nodosa (PAN) displaying moderate degree of infiltration by inflammatory cells in the intima and media with fibrinoid necrosis and fragmentation of internal elastic membrane. (C) Subacute stage of PAN showing fibroblastic proliferation with sclerosis of media. (D) Chronic stage of PAN showing reduplication of internal elastic lamina, beaded mass, recanalised lumina, calcification, lymphomononuclear infiltrate in media and organised thrombus in the lumen.
Figure 2 (A) Mesenteric arterial arcade showing multiple beaded appearance at the bifurcation (arrows). (B) Renal arteries showing segmental thrombosed and aneurysmal dilatation. Renal ostia are normal. Kidneys reveal geographic areas of fresh and old infarcts. (C) Coronal slice of brain showing diffuse petechial haemorrhages in the white matter (arrows). (D) Central white matter showing perivascular oedema and secondary myelin loss.
Discussion
HCL is one of the remote causes of PAN. Hughes et al first noted the association between HCL and PAN in 1979.1 HCL is occasionally associated with other systemic autoimmune disorders including rheumatoid disease and secondary autoimmune haemolytic anaemia.2
Various mechanisms have been proposed for the pathogenesis of PAN in HCL.3 They include hepatitis B antigenaemia, direct invasion of blood vessel wall by the leukaemic cells, cross‐reactivity of antibodies that target surface determinants on hairy cells with epitopes on endothelial cells, and splenectomy. Hasler et al reviewed previously reported cases of PAN associated with HCL in 1995.4
Disseminated vasculomyelinopathy, initially coined by Poser, includes the different clinical and pathological forms of hyperergic reaction of the nervous system to various infections and vaccinations and to other exogenous or endogenous antigenic stimuli.5
In asymptomatic patients, the increased ESR should alert the clinician about this association, when it is unexplainable due to anaemia. The easily accessible diagnostic methods—arteriography and skin/renal biopsy—could help in management of the condition.
Footnotes
Competing interests: None declared.
References
- 1.Hughes G R V, Elkon K B, Spiller R.et al Polyarteritis nodosa and hairy‐cell leukemia. Lancet 1979i678. [DOI] [PubMed] [Google Scholar]
- 2.Mainwaring C J, Walewska R, Snowden J.et al Fatal cold anti‐i autoimmune haemolytic anaemia complicating hairy cell leukaemia. Br J Haematol 2000109641–643. [DOI] [PubMed] [Google Scholar]
- 3.Gabriel S E, Conn D L, Phyliky R L.et al Vasculitis in hairy cell leukemia: review of literature and consideration of possible pathogenic mechanisms. J Rheumatol 1986131167–1172. [PubMed] [Google Scholar]
- 4.Hasler P, Kistler H, Gerber H. Vasculitides in hairy cell leukemia. Semin Arthritis Rheum 199525134–142. [DOI] [PubMed] [Google Scholar]
- 5.Poser C M. Disseminated vasculomyelinopathy. Acta Neurol Scand 196545(Suppl 37)1–44. [Google Scholar]