Abstract
Background
Despite policies advocating centralised transfusion services based on voluntary donors, the hospital‐based replacement donor system is widespread in sub‐Saharan Africa.
Aims
To evaluate the cost of all laboratory resources needed to provide a unit of safe blood in rural Malawi using the family replacement donor system
Methods
Full economic costs of all laboratory tests used to screen potential donors and to perform cross‐matching were documented in a prospective, observational study in Ntcheu district hospital laboratory.
Results
1729 potential donors were screened and 11 008 tests were performed to ensure that 1104 units of safe blood were available for transfusion. The annual cost of all transfusion‐related tests (in 2005 US$) was $17 976, equivalent to $16.28 per unit of transfusion‐ready blood. Transfusion‐related tests used 53% of the laboratory's total annual expenditure of $33 608.
Conclusions
This is the first study to provide prospective economic costs of all laboratory tests associated with the family replacement donor system in a district hospital in Africa. Results show that despite potential economies of scale, a unit of blood from the centralised system costs about three times as much as one from the hospital‐based “replacement” system. Factors affecting these relative costs are complex but are in part due to the cost of donor recruitment in centralised systems. In the replacement system the cost of donor recruitment is entirely borne by families of patients needing a blood transfusion.
Keywords: blood transfusion; cost; Africa; Malawi, laboratory
Anaemia is a major public health problem in countries in sub‐Saharan Africa, so demand for blood transfusions is high. The World Health Organization (WHO) recommends that all blood for transfusion should come from voluntary unpaid donors. In wealthy countries intensive public education campaigns and well‐organised mobile donor sessions mean that over 97% of transfused blood comes from voluntary donors. Blood collection and screening is centralised at regional centres that are independent of individual hospitals. These centres recruit voluntary blood donors, screen and group blood, process it into components and have distribution networks to provide regular supplies to hospitals in their region.
In most of the poorest African countries, transfusion services are decentralised in individual hospitals. Patients who need a transfusion have to find their own “replacement” blood donors, usually family members. These potential donors are screened in the local hospital which provides a “basic adequate” transfusion service (defined by the WHO as screening of donors for HIV, hepatitis B and syphilis, and determining the blood groups and cross‐match compatibility of donor and recipient samples).1 Overall 80% of blood for transfusion in sub‐Saharan Africa comes from these hospital‐based systems using replacement donors.2 Centralised, national systems are the internationally preferred model because they are associated with a lower risk of transfusion‐transmitted infections than hospital‐based systems.3 Centralised transfusion services are logistically complex and more expensive than hospital‐based systems, so they have only been implemented in a few sub‐Saharan countries. They usually rely on support from external funders, so there are concerns about sustainability.4
The spread of the HIV epidemic, a reluctance of individuals to donate blood and the high prevalence of transfusion‐transmitted infections, means that it is becoming increasingly difficult for poorer countries to provide safe blood for transfusion. In sub‐Saharan Africa the prevalence of markers for transfusion‐transmitted infections in potential donors is 0.5–16% for HIV,5 3–22% for hepatitis B,3 2–7% for hepatitis C6 and 1–21% for syphilis.7 It has been estimated that 5–10% of HIV infections were attributable to HIV contaminated blood.8 There is increasing pressure on African countries to address the problems of donor availability and infection risk by adopting a centralised transfusion system, but there is minimal evidence to show whether this system, or individual components of the system, are feasible, cost‐effective or sustainable compared to the hospital‐based system currently in place in most African countries. The centralised model depends on good communications and transport systems and, in wealthy countries, provides blood to a clinical service that is generally able to predict its transfusion requirements. In contrast, in poor countries, particularly those where malaria transmission is intense, over 80% of blood is given for emergencies, there are marked seasonal and micro‐geographic variations in blood requirements, and transport and communication systems are unreliable.
Very little is known about the full economic cost of laboratory tests needed to screen and cross‐match blood ready for transfusion in sub‐Saharan Africa. Almost all economic studies of transfusion services in sub‐Saharan Africa have focused on HIV‐related components of the service, and have ignored the costs of tests for screening for other infections and checking compatibility. Several reviews and studies concerning the cost of strategies to avert transfusion‐associated HIV transmission in Africa have been published,9,10,11 as well as examples of modifications to existing strategies such as deferring donors before HIV testing,12 and optimising hierarchy of HIV test methods.13,14 For example, the cost of a unit of blood from a centralised system was estimated to be $25 in Uganda15 and $40 in Rwanda16 ($39.60 and $57.68 respectively, adjusted to 2005 US$), and from hospital‐based systems in Zambia and Tanzania as $12.829 and $12.404 respectively ($18.49 and $17.36 respectively, adjusted to 2005 US$). None of these studies included the costs of blood grouping or cross‐matching, and in some cases it is not clear whether they included screening tests for anaemia, hepatitis B or syphilis, which are essential before blood can be issued for transfusion.
Although establishing a centralised transfusion system is a long‐term goal of many poorer countries, these systems are more expensive than the existing hospital‐based model and may not be appropriate or affordable in all contexts in sub‐Saharan Africa. Countries without external support for their transfusion services need to adopt an evidence‐based, pragmatic approach to providing adequate, safe blood supplies that they can afford and sustain. However, there is insufficient data about the cost of individual components of the hospital‐based transfusion system to be able to make meaningful comparisons with the centralised system. Consequently policy makers are not able to make evidence‐based decisions about which components to keep and which to reject or adapt. We have carried out a prospective analysis of the full economic laboratory costs of a rural, district hospital‐based transfusion service in Malawi and compared our results with those of centralised blood transfusion services operating in poor countries in sub‐Saharan Africa.
Methods
This prospective study was conducted over one year in Ntcheu district hospital in the central area of Malawi. Malawi is ranked as 165 out of 177 using the Human Development Index and is therefore one of the poorest, least developed countries in the world and a per capita annual income of US$160.17 Ntcheu hospital serves a population of 500 000 and has 250 beds in medical, surgical, obstetric and paediatric wards. As part of a project to improve the effectiveness of the hospital laboratory, quality assurance processes had already been established for all tests. Between 92% and 97% of transfusion‐related tests (blood grouping 95%, HIV screening 96%, hepatitis B virus screening 92%, syphilis screening 97%) were concordant with independently conducted testing using recommended reference methods.18
Donor selection and screening
After counselling potential donors and questioning to elicit evidence of ill health, laboratory technicians obtained a venous blood sample for haemoglobin estimation (by colorimetric haemiglobincyanide method), HIV screening (HIV‐SPOT and Capillus HIV test kits), hepatitis B screening (Serodia PA method), syphilis screening (VDRL (Venereal Disease Research Laboratory) test card and carbon antigen) and microscopy of thick peripheral blood film for malaria parasites. As almost all transfusions were emergencies, there was very little batching of tests. If the haemoglobin level was >120 g/l and these tests were all negative, a unit of blood was taken into a blood bag and donors were observed for 5–10 min before leaving. In accordance with hospital policy, unsuitable donors were referred by the technicians to counsellors or clinicians depending on the results of the screening tests. Standard tube methods were used to determine the blood group of the donor and recipient and to cross‐match the blood.19
Cost estimations
Annual expenditure data were collected from the Ministry of Health's central medical stores, local suppliers and manufactures, and from the expenditure at Ntcheu district hospital. The value of annual resources used for blood transfusion services was estimated using the ingredients approach, which identifies all components of blood transfusion, including salaries, utilities, building costs, equipment and supplies related to blood collection, screening, blood grouping and cross‐matching and assigns them their economic cost; these costs were aggregated to obtain the total cost of safe blood. The total cost of a unit of safe blood issued was calculated from the total cost of the transfusion service divided by the total number of units of blood issued. Costs are presented as recurrent (ie, items that were consumed over less than one year) and capital costs (ie, items that were consumed over more than one year). Original costs in Malawian Kwacha were converted to 2005 US$.i
Recurrent costs
Recurrent costs comprised staff salaries, supplies and overheads such as electricity and water. The cost of annual staff salaries was based on the mid‐point salary of a middle‐grade laboratory technician. The total time taken to carry out all the tests involved in providing one unit of safe blood was calculated from timesheets completed by the technicians, covering all their daily working activities. Safe blood was defined as blood that had tested negative for markers for HIV, hepatitis B virus, syphilis and malaria, had a haemoglobin level of at least 120 g/l and was group and cross‐match compatible with the recipient's blood sample. The time spent on transfusion‐related activities was used to apportion salary costs. The quantities of supplies required to perform each test were documented by the technicians and cross‐checked with laboratory records of use of supplies and reagents. The costs of the supplies were obtained from the Ministry of Health's Central Medical Stores or the laboratory's usual local suppliers. Overhead costs were derived from the hospital's total annual overhead costs using the proportion of total hospital floor space used for blood transfusion services.
Capital costs
Purchasing prices of equipment were obtained from Malawi's Central Medical Stores and from manufacturers. Building costs were estimated using the rate per square metre of floor area from the UK's Department for International Development Central Africa Building Advisory Team ($365 sq/m in 2001) and apportioned to blood transfusion services according to the space used for transfusion services. Capital costs were annualised using a standard formula that takes account of the expected productive lifetime of equipment, and the 12% annual discount rate used by the Malawian Ministry of Finance.
Results
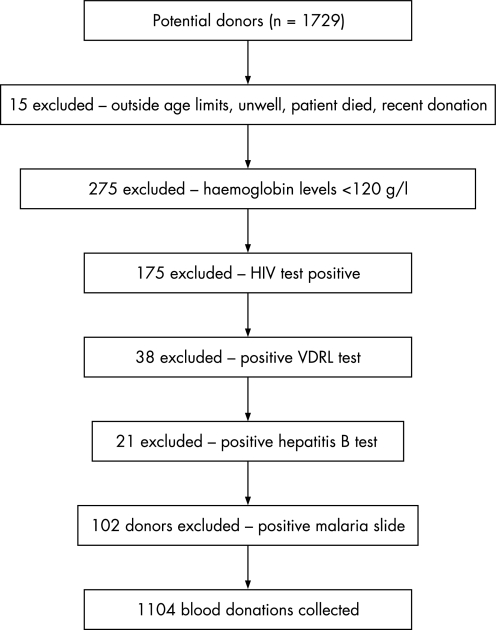
In one year, 1729 individuals attended Ntcheu hospital laboratory as potential donors. A total of 610 (35.6%) potential donors were rejected because they had haemoglobin levels <120 g/l or positive screening tests (fig 1).
Figure 1 Reasons for donor exclusion, Ntcheu district hospital, Malawi. VDRL, Venereal Disease Research Laboratory.
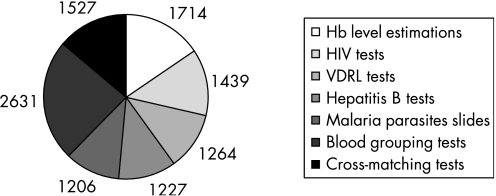
The mean age of the 1104 individuals eligible to donate blood was 29 years; 67% of donors were male. The total number of transfusion‐associated laboratory tests performed was 11 005; these included 5136 (46.5%) tests for screening for infection, 1714 (15.6%) haemoglobin estimations on donors and 4158 (37.8%) tests associated with determining blood groups and cross‐matching donor and patient samples (fig 2).
Figure 2 Number of tests associated with transfusion services in Ntcheu district hospital. VDRL, Venereal Disease Research Laboratory.
The total expenditure of the Ntcheu District Laboratory was $33 608, of which $17 976 (54%) was for blood transfusion services. The remainder of the expenditure was on tuberculosis and malaria microscopy (20%), haemoglobin estimations (6% non‐transfusion related), syphilis testing (4%), HIV testing, blood glucose, white cell count and microscopy of urine, cerebrospinal fluid, pleural and ascitic fluid (<5% each). The cost per unit of safe blood was $16.28 (table 1).
Table 1 Cost of transfusion service in Ntcheu district hospital, Malawi based on actual inputs in 2005 US$.
| Item | Recurrent costs | Capital costs | Total costs | |||
|---|---|---|---|---|---|---|
| Supplies | Staff costs | Overheads | Equipment | Buildings | ||
| Blood collection | $4553.99 | $163.54 | $86.30 | $0.00 | $325.00 | |
| Haemoglobin estimation | $222.82 | $154.26 | $68.56 | $229.19 | $201.86 | |
| Infection screening | $4300.71 | $371.36 | $247.28 | $1006.17 | $551.65 | |
| Blood grouping | $1383.31 | $180.00 | $112.00 | $472.14 | $187.00 | |
| Cross‐matching | $800.11 | $250.00 | $210.00 | $432.00 | $467.00 | |
| General equipment | $1000.00 | |||||
| Total | $11260.94 | $1119.16 | $724.14 | $3139.50 | $1732.51 | |
| Total recurrent/capital costs | $13104.24 | $4872.01 | ||||
| Total costs | $17976.25 | |||||
| Total/unit blood | $16.28 | |||||
Recurrent costs comprised 73% of the total annual cost of the transfusion service; 63% ($11 260.94) of recurrent costs was spent on supplies and 6% ($1119.16) on salaries. Capital costs comprised 27% of the annual cost of the transfusion service with 64% ($3139.50) spent on equipment and 36% ($1732.51) on buildings.
Discussion
To our knowledge this is the first prospective study to accurately measure the cost of all the resources required by a district hospital laboratory in rural sub‐Saharan Africa to prepare a safe unit of blood for transfusion. The transfusion service in Ntcheu consumed 53% of the laboratory's total budget and each unit of blood issued cost $16.28. The cost of a unit of blood from this hospital‐based, replacement donor system in Malawi is similar to the $15.64/unit and $16.66/unit (in 2005 US$) figures reported from two retrospective studies in Africa of hospital‐based transfusion systems.4,9 There is not enough detail provided in these studies about test methods, costs of equipment and reagents, depreciation of equipment, overheads and staff time to enable a direct comparison with our results. The aim of the Zambian study9 was to highlight the benefits of HIV screening so information about other transfusion‐related tests was not provided. The Tanzanian study4 differed from our study in that it was based in an urban referral hospital, volunteer donation accounted for almost 31% of suitable units of blood, transfusion tests were semi‐automated and the costs of testing for hepatitis B and cross‐matching were not included.
Malawi has begun to introduce a centralised blood transfusion system and the recurrent cost of a unit of blood from this system is US$56 (personal communication, Dr JC Emmanuel, Project Manager, Malawi National Blood Service). Blood obtained and screened through these systems is associated with less risk of transfusion‐transmitted infections, better blood stocks and economies of scale for purchase and maintenance of supplies and equipment, and for training, supervision and quality monitoring. A unit of blood produced by a centralised system costs 2–3 times as much as one from a hospital‐based system,4 predominantly due to the higher costs of quality management systems and donor recruitment. Centralised systems aim to recruit voluntary donors through community education campaigns and well‐trained and supervised outreach teams.15 In the hospital‐based system the patient's family, not the transfusion service, bears responsibility for finding a donor. Almost nothing is known about how this responsibility impacts on patients' families, but the loss of work time and other indirect opportunity costs must place a significant financial, social and emotional burden on already impoverished households.
Increasing rates of HIV infection and malaria resistance are accelerating the demand for safe blood and simultaneously reducing the number of potential donors. The provision of safe blood, particularly through the use of voluntary rather than replacement donors, is a cost‐effective means of controlling HIV transmission where prevalence is high.20,21 A better understanding of factors that motivate donors in different cultural contexts is needed if the trend of falling numbers of donors is to be reversed, especially as the ultimate goal of transfusion services, including those in poor countries, is to increase repeat donations by voluntary donors.
The two transfusion systems—rural, hospital‐based and national, centralised—represent two ends of the spectrum of transfusion service models. Neither model is optimal for poor countries in Africa. It is difficult to standardise and ensure the quality and supply of safe blood in the hospital‐based model, and patients' families bear significant hidden costs associated with donor recruitment. The centralised model is expensive and has not yet proved itself to be effective at reaching rural areas and or to be sustainable in impoverished countries with weak health systems. African governments are under increasing pressure to adopt the centralised model of transfusion services, but this model may not be economically viable in poor countries4 because of inadequate heath budgets, limited population coverage and lack of facilities and skills.22 Some countries such as Kenya and Cote D'Ivoire operate a hybrid system which combines a locally‐sourced donor system with regular supplies from a central service.5,23
Even though transfusion services use over half of a district hospital laboratory's budget, published evidence about the cost‐effectiveness of different transfusion strategies, which is essential to guide decisions about the design and resource requirements of transfusion services, is sparse and outdated. The costs from our study cannot be extrapolated directly to other settings as they will vary with local laboratory supply mechanisms and salary scales, and are influenced by demand for blood for transfusion and prevalence rates of transfusion‐transmitted infections. However, the proportions of the various components relative to the overall costs are likely to be transferable between countries of similar economic status. Our study increases knowledge about the laboratory costs of transfusion services in Africa and provides a workable model for collecting high quality economic information that can be used by health providers to underpin decisions about appropriate and cost‐effective transfusion services. It also highlights the unrecognised but substantial economic burden that the hospital‐based system places on the families of patients who have to take responsibility for recruiting blood donors.
Take‐home messages
Although emergency supplies of safe blood are essential to save lives, little is known about the economics of transfusion services in sub‐Saharan Africa.
Centralised transfusion services using voluntary donations are safer, but 2–3 times more expensive than the widespread hospital‐based systems that utilise family replacement donors.
In rural Malawi, blood from a hospital‐based system cost $16/unit; transfusion services consume 53% of the laboratory's budget. Blood from a centralised system in sub‐Saharan Africa costs around $50/unit. (Costs in 2005 US$.)
The cost difference is predominantly due to the quality management and donor recruitment processes in the centralised service. In the hospital‐based system the cost of donor recruitment is hidden because it is borne entirely by the families of patients needing a blood transfusion.
Footnotes
iUS$1 (2005) is equivalent to US$1.07, £0.50 or €0.80 (2007).
Competing interests: None declared.
References
- 1.World Health Organization Guidelines for the organisation of a blood transfusion service. Geneva: WHO, 1992
- 2.Tapko J B. Blood safety: a strategy for the African region. The 4th Arab Congress and the 3rd African Congress of Blood Transfusion, Tunis. World Health Organization, Africa Regional Office 200267–74.
- 3.Sarkodie F, Adarkwa M, Adu‐Sarkodie Y.et al Screening for viral markers in volunteer and replacement blood donors in West Africa. Vox Sang 200180142–147. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs B, Mercer A. Feasibility of hospital‐based blood banking: a Tanzania case study. Health Policy Plan 199914354–362. [DOI] [PubMed] [Google Scholar]
- 5.Allain J P, Owusu‐Ofori S, Bates I. Blood transfusion in sub‐Saharan Africa. Transfusion Alternatives in Transfusion Medicine 20046(1)16–23. [Google Scholar]
- 6.Jeannel F, Fretz C, Troare Y.et al Evidence for high genetic diversity and long term endemicity of hepatitis C virus genotypes 1 and 2 in West Africa. J Med Virol 19985592–97. [PubMed] [Google Scholar]
- 7.Gloyd S, Chai S, Mercer M. Antenatal syphilis in sub‐Saharan Africa: missed opportunities for mortality reduction. Health Policy Plan 200116(1)29–34. [DOI] [PubMed] [Google Scholar]
- 8.Piot P, Laga M, Ryder R.et al The global epidemiology of HIV infection: continuity, heterogeneity and change. J Acquir Immune Defic Syndr 19903403–412. [PubMed] [Google Scholar]
- 9.Foster S, Buvé A. Benefits of HIV screening of blood transfusion in Zambia. Lancet 1995346225–227. [DOI] [PubMed] [Google Scholar]
- 10.Creese A, Floyd K, Alban A.et al Cost‐effectiveness of HIV/AIDS interventions in Africa: a systematic review of the evidence. Lancet 20023591635–1643. [DOI] [PubMed] [Google Scholar]
- 11.Walker D. Cost and cost‐effectiveness of HIV/AIDS prevention strategies in developing countries: is there an evidence base? Health Policy Plan 200318(1)4–17. [DOI] [PubMed] [Google Scholar]
- 12.McFarland W, Kahn J G, Katzenstein A.et al Deferral of blood donors with risk factors for HIV infection saves lives and money in Zimbabwe. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology 19959183–192. [PubMed] [Google Scholar]
- 13.Spielberg F, Kabeya M C, Quinn T C.et al Performance and cost‐effectiveness of a dual rapid assay system for screening and confirmation of HIV type 1 seropositivity. J Clin Microbiol 199028303–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laleman G, Kambale M, van Kerckhoven I.et al A simplified and less expensive strategy for confirming anti HIV‐1 screening results in a diagnostic laboratory in Lubumbashi, Zaire. Ann Soc Belge Med Trop 199171287–294. [PubMed] [Google Scholar]
- 15.Watson Williams E J, Kataaha P K. Revival of the Ugandan Blood Transfusion System 1989: an example of international cooperation. Transfus Sci 199011179–184. [DOI] [PubMed] [Google Scholar]
- 16.van Dam C J, Sondag‐Thull D, Fransen L. The provision of safe blood—policy issues in the prevention of human immunodeficiency virus transmission. Tropical Doctor 19922220–23. [DOI] [PubMed] [Google Scholar]
- 17.Malawi Statistics EU relations with Malawi: country overview. http://web.worldbank.org (accessed 10 August 2007)
- 18.Essential Medical Laboratory Services (EMLS) Project, Malawi 1998–2002, LSTM & MoHP. Final report. Lilongwe, Malawi: Ministry of Health and Population, 2002
- 19.Rowley M, Milkins C. Laboratory aspects of blood transfusion. In: Lewis SM, Bain BJ, Bates I, eds. Practical haematology, 10th edn. Philadelphia, PA: Churchill Livingstone Elsevier 2006
- 20.Gerard C, Sondag‐Thull D, Watson‐Williams E J, Fransen L. eds. Safe blood in developing countries. Luxembourg: European Commission 199595
- 21.Henscher M, Jefferys E.Study on the financing and organisation of blood transfusion services in sub‐Saharan Africa. Final report. Brussels: European Commission, DG VII/AIDS Task Force, 14 May, 1998
- 22.Mburu F M. Health delivery standards: vested interests in health planning. Soc Sci Med 1994391375–1384. [DOI] [PubMed] [Google Scholar]
- 23.Marum L. Monitoring and evaluation of blood safety in Kenya. In: Implementation of the New Blood Safety Policy. Proceedings of a Consultative Technical Meeting, Nairobi, Kenya, 29–30 April 2002. Nairobi, Kenya: Kenya National Blood Transfusion Service, 2002