Abstract
Background
Primary biliary cirrhosis (PBC) is an autoimmune liver disease targeting the intrahepatic small bile ducts showing chronic non‐suppurative destructive cholangitis (CNSDC). Recent studies suggest that naturally‐occurring CD4+CD25high regulatory T cells (Tregs) expressing Forkhead box P3 (Foxp3) play an active role in immunological self‐tolerance.
Aims
To investigate whether Foxp3+Tregs are involved in the pathogenesis of PBC.
Methods
Foxp3+Tregs was detected immunohistochemically in livers from patients with PBC (n = 27), chronic viral hepatitis (CVH) (n = 15), and normal subjects (n = 10). The distribution of Tregs in portal tracts was semi‐quantitatively evaluated in each groups. Levels of Foxp3, IL‐10, TGFβ, IFNγ and TNFα mRNA was evaluated in PBC (n = 15) and control livers (n = 21) using semi‐quantitative reverse transcriptase‐PCR.
Results
In PBC and CVH livers, the amounts of infiltrating Foxp3+Tregs in portal tracts were in parallel with the degree of portal inflammation irrespective of disease. The infiltration of Foxp3+Tregs into portal tracts with CNSDC in PBC was foremost in comparison with inflamed portal tracts in CVH or those without CNSDC in PBC (p<0.05). Focally, Tregs infiltrated into the biliary epithelial layer at the site of CNSDC. The level of Foxp3, IL‐10 and TGFβ mRNA expression was high in PBC compared with normal livers (p<0.05). IFNγ and TNFα mRNA was high in early PBC and CVH livers.
Conclusion
Results of this evaluation of Foxp3+Tregs do not suggest that the reduced regulatory function accounts for the development of CNSDC in PBC.
Keywords: primary biliary cirrhosis, regulatory T cells, Foxp3, IL‐10, TGFβ, autoimmunity
Primary biliary cirrhosis (PBC) is an organ specific autoimmune disease and is histologically characterised as a cholangitis of small bile ducts (chronic non‐suppurative destructive cholangitis; CNSDC) eventually followed by their extensive loss from the liver.1,2,3,4 This is supported by the presence of anti‐mitochondrial antibodies and autoreactive T and B cells, in conjunction with frequent association with other autoimmune disease.5,6,7 Recent data suggest that autoreactive T cell response plays a major role in the pathophysiology of bile duct lesions in PBC.7,8 In particular, loss of tolerance to self antigen, particularly pyruvate dehydrogenase E2 component related to or located in the small bile ducts may be a central event in CNSDC in PBC.
Recently, accumulating data suggest that naturally‐occurring CD4+CD25high regulatory T cells (Tregs) play an active part in establishing and maintaining immunological self‐tolerance and also have a role in negative control of various immune responses to non‐self antigens.9,10,11 The disturbance of Tregs may be responsible for the development and progression of several autoimmune diseases.12,13,14,15 Most of the known surface markers of these Tregs, including CD25, are also up‐regulated in activated conventional CD4+CD25high T cells without regulatory function.9,10,11 Recently, Forkhead box P3 (Foxp3) has been reported as one of the best markers of naturally‐occurring Tregs, and Foxp3 is crucially related to the development and function of Tregs.9,10,11,16 There have been few reports regarding the significance of Foxp3+Tregs in PBC, so far.17,18,19 Lan et al recently reported the relative decrease of Foxp3+Tregs in PBC and suggested that Foxp3+Tregs may play a role in the loss of tolerance in PBC,17 although their results have not been confirmed by other groups.
In order to clarify the significance of naturally occurring Tregs in the pathogenesis of the bile duct lesion of PBC, we investigated the distribution of Foxp3+Tregs in liver tissues of PBC patients and controls with reference to histopathological findings of portal tracts and cholangiopathy. Furthermore, we examined the level of mRNA expression of Foxp3, interleukin‐10 (IL‐10) and transforming growth factor (TGF) β (immunosuppressive cytokines secreted by Tregs),11 interferon (IFN) γ (a hallmark cytokine of T helper 1 (Th1) immune response) and tumour necrosis factor (TNF) α (proinflammatory cytokine) in PBC and control livers. IFNγ and TNFα are reportedly up‐regulated and involved in the pathophysiology of PBC.20,21,22,23
Materials and methods
Patients
A total of 52 liver tissue specimens (all were wedge biopsied or surgically‐resected) were collected from the liver disease file of our laboratory and affiliated hospitals. This is a retrospective study and when possible, the frozen tissues for mRNA evaluation were obtained. There was no difference in the clinical background between the patient groups with and without frozen tissue. To obtain enough number of portal tracts (at least 5) for evaluation, we used only wedge or excision tissues. There were no selection criteria for the patients group of wedge biopsy and those of needle biopsy. The liver specimens consisted of 27 PBC, 15 chronic viral hepatitis (CVH) livers and 10 “histologically normal” livers. All PBC were from patients fulfilling the clinical, serological and histological characteristics consistent with the diagnosis of PBC.3 PBC livers were staged histologically (stages 1/2, n = 17; stages 3/4, n = 10).3,24 Eight and seven CVH were regarded as F0–2 and F3/4, respectively.25 Three and 12 CVH cases were serologically positive for hepatitis B surface B antigen and anti‐hepatitis C viral antibody, respectively. None of the patients received treatment with ursodeoxycholic acid and interferon before histological diagnosis. “Histologically normal” livers were obtained from surgically resected livers for traumatic hepatic rupture or metastatic liver tumour.
Preparation of liver tissues
Liver tissue samples were fixed in 10% neutral buffered formalin, and embedded in paraffin. More than 20 serial sections, 4 μm thick, were cut from each block. Several were processed routinely and the remainder were processed for the following immunohistochemistry. For the analysis of mRNA expression, frozen liver tissue specimens from the patients with PBC (n = 15) (stages 1/2, n = 8; stages 3/4, n = 7), CVH (n = 9) (F1/2, n = 2; F3/4, n = 7) and “histologically normal” livers (n = 12) were used.
Classification of intrahepatic biliary tree
The intrahepatic biliary tree is classified into the intrahepatic large and small bile ducts by their size and distribution in the portal tracts.4,26 Septal and interlobular bile ducts are collectively termed small bile ducts. Bile ductules were evaluated separately and not included in the small bile ducts. In this study, the small bile ducts were mainly examined.
Types of portal tract histopathology
Portal tracts in the liver specimens were largely classified into three types: A, inflamed portal tracts containing CNSDC; B, chronically inflamed portal tracts without CNSDC; and C, non‐inflamed portal tracts.
Immunohistochemistry
Foxp3 was detected using mouse monoclonal anti‐Foxp3 (Abcam, Cambridge, UK) and Envision+ solution (Dako, Glostrup, Denmark) as described previously.27 Deparaffinised sections were pretreated in a microwave oven in EDTA buffer (pH 9) at 95°C for 20 min. A similar dilution of the control mouse IgG (Dako) was applied instead of the primary antibody as negative control. Foxp3 is detected in the nuclei of round cells regarded as naturally‐occurring Tregs, when present. Since the monoclonal antibody we used in this study was raised against the C‐terminus residues (400–431) of Foxp3, it reacts with both “normal” Foxp3 and its splice variant that lacks exon2 (residues 72–106).28,29 Foxp3 is preferentially expressed by CD4+CD25+Tregs30 and human Tregs reportedly coexpress equal amounts of two isoforms of Foxp3.28 Although CD8+T cells as well as CD8+/CD28 suppressor cells are reported to express the spliced variants of Foxp3,29 most Foxp3+ cells were CD4‐positive and there was no CD8+ Foxp3+ cell in our preliminary study (data not shown).
Semiquantitative analysis of Foxp3‐expressing inflammatory cells
The extent of infiltration of Foxp3‐expressing cells in individual portal tracts was graded as follows: 0, portal tract containing <2 Foxp3‐expressing cells; 1, portal tract containing 2–5 Foxp3‐expressing cells; 2, portal tract containing 6–10 Foxp3‐expressing cells; and 3, portal tract containing >10 Foxp3‐expressing cells.
Extraction of RNA and semi‐quantitative reverse‐transcriptase (RT)‐PCR
Total RNA was extracted from the liver tissues or cells with a QIAGEN RNeasy Mini kit (QIAGEN, Hilden, Germany) according to the recommendations of the manufacturer. After cDNA was synthesised, semi‐quantitative RT‐PCR was carried out as described previously.31 Table 1 shows primers used for RT‐PCR. Gel images were evaluated using NIH image and the expression was normalised as a ratio using human G3PDH as a housekeeping gene. Data were expressed as mean (SD).
Table 1 Primers for RT‐PCR.
| Gene | Genbank no | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) | Product size |
|---|---|---|---|---|
| Foxp3 | NM014009 | TCATCCGCTGGGCCATCCTG | GTGGAAACCTCACTTCTTGGTC | 385bp |
| IL‐10 | NM000572.2 | ATGCCCCAAGCTGAGAACCAAGACCCA | TCTCAAGGGGCTGGGTCAGCTATCCCA | 352bp |
| TGFβ | NM000660.3 | CAGAAATACAGCAACAATTCCTGG | TTGCAGTGTGTTATCCCTGCTGTC | 190bp |
| TNFα | NM000594.2 | GAGTGACAAGCCTGTAGCCCATGTTGTAGCAT | GCAATGATCCCAAAGTAGACCTGCCCAGACT | 444bp |
| IFNγ | NM000619.2 | GCATCGTTTTGGGTTCTCTTGGCTCTTACTGC | CTCCTTTTTCGCTTCCCCTGTTTTAGCTGCTGG | 427bp |
| G3PDH | BC023632 | GAACGGGAAGCTCACTGGCATGGC | TGAGGTCCACCACCCTGTTGCTG | 311bp |
Statistical analysis
Statistical analysis for the differences in the human study used the Wilcoxon rank sum test. Statistical analysis of the differences in the in vitro assays used Student's t test. The difference was considered as significant when p<0.05.
Results
Distribution of Foxp3+Tregs in PBC and control livers
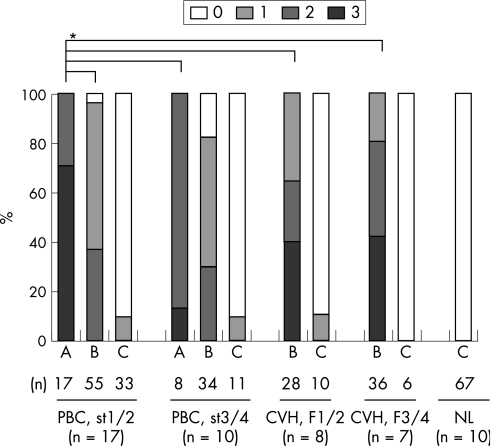
The amounts of Foxp3+Tregs infiltrated in portal tracts were graded according to individual types of portal tracts in PBC and control livers (fig 1).
Figure 1 Degrees of regulatory T cells (Tregs) expressing Forkhead box P3 (Foxp3+Tregs) infiltration in portal tracts depending on three types portal tract histopathology in primary biliary cirrhosis (PBC) and control livers. Foxp3+Tregs infiltrated more extensively into portal tracts with chronic inflammation (types A and B) compared with non‐inflamed portal tracts (type C) in PBC and chronic viral hepatitis (CVH). Foxp3+Tregs infiltrated more extensively into portal tracts with (chronic non‐suppurative destructive cholangitis (CNSDC) (type A) in PBC compared with portal tracts without CNSDC in PBC and CVH (p<0.05). A, chronically inflamed portal tracts containing CNSDC; B, chronically inflamed portal tracts without CNSDC; C, non‐inflamed portal tracts. Extent of infiltration: 0, portal tract containing <2 Foxp3‐expressing cells; 1, portal tract containing 2–5 Foxp3‐expressing cells; 2, portal tract containing 6–10 Foxp3‐expressing cells; 3, portal tract containing >10 Foxp3‐expressing cells. NL, normal liver. *p<0.05.
Normal and CVH livers
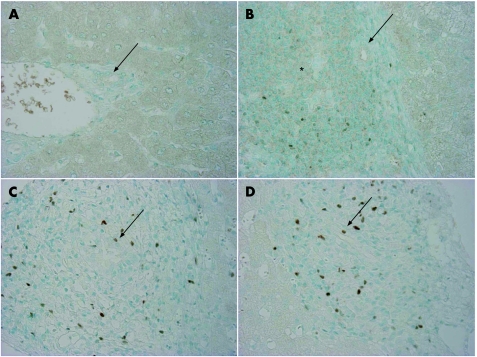
There were few Foxp3+Tregs in portal tracts and in hepatic parenchyma in normal livers (fig 2A). Foxp3+Tregs infiltrated mainly in portal tracts in CVH livers; the extent of Foxp3+Tregs infiltration was in parallel with the extent of chronic inflammation (fig 1). Foxp3+Tregs was scattered around follicle‐like accumulation of lymphoid cells in CVH livers (fig 2B).
Figure 2 Distribution of regulatory T cells (Tregs) expressing Forkhead box P3 (Foxp3+Tregs) in primary biliary cirrhosis (PBC) and control livers. (A) There were no or few Foxp3+Tregs in portal tracts and hepatic parenchyma in normal livers. Arrow indicates the small bile duct. Normal liver, ×200. (B) Tregs infiltrated around follicle‐like accumulation of lymphoid cells (*) was frequently observed in portal tracts. Arrow indicates a small bile duct. CVH, type C. ×200. (C) Foxp3+Tregs showing positive nuclear staining infiltrated extensively into portal tracts with chronic non‐suppurative destructive cholangitis (CNSDC) in PBC. Tregs infiltrated occasionally into the biliary epithelial layer at the site of CNSDC (arrow). PBC liver, stage 1. ×400. (D) Accumulation of Tregs was noted at the interface of portal tracts. Tregs infiltrated occasionally into the biliary epithelial layer at the site of CNSDC (arrow). PBC liver, stage 1, ×400.
PBC livers
Foxp3+Tregs infiltrated mainly in portal tracts (fig 2C,D); the number of Foxp3+Tregs was negligible in hepatic parenchyma. The extent of Foxp3+Tregs infiltration was in parallel with the extent of chronic inflammation as seen in CVH livers (fig 1). Foxp3+Tregs infiltrated more extensively into portal tracts with CNSDC in PBC, when compared with those without CNSDC in PBC and CVH (p<0.05) (fig 1). Foxp3+Tregs infiltrated occasionally into the biliary epithelial layer at the site of CNSDC (fig 2C,D). The extent of Foxp3+Tregs in inflamed portal tracts with CNSDC in early stage of PBC was higher than in late stage of PBC.
Increased mRNA expression of Foxp3, IL‐10 and TGFβ in PBC livers
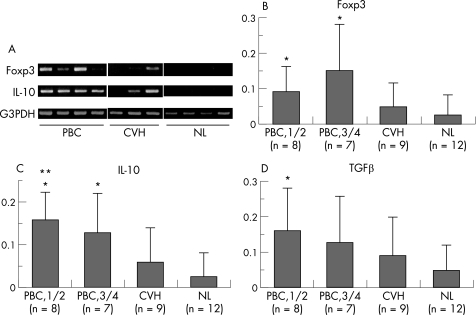
Figure 3A shows the representative gel image and the semi‐quantitative levels of Foxp3, IL‐10 and TGFβ in PBC and control livers.
Figure 3 Semi‐quantitative reverse transcriptase (RT)‐PCR for Foxp3, IL‐10 and TGFβ mRNA in primary biliary cirrhosis (PBC) and control livers. (A) Representative gel image of Foxp3 and IL‐10 mRNA expression. Foxp3 and IL‐10 mRNA were detected in PBC and chronic viral hepatitis (CVH) livers, but were not detected in normal livers (NL). (B–D) Semi‐quantitative levels of Foxp3 (B), IL‐10 (C) and TGFβ (D) mRNA expression in PBC and control livers. The levels of Foxp3, IL‐10 and TGFβ mRNA were significantly increased in PBC livers, when compared with normal livers. Gel images were evaluated using NIH image and the expression was normalised as a ratio using human G3PDH as a housekeeping gene. Data expressed as mean (SD). *p<0.05 vs normal liver; **p<0.05 vs CVH liver.
Foxp3
The level of Foxp3 mRNA expression was high in PBC livers compared with normal livers (p<0.05) (fig 3B). Although Foxp3 mRNA expression tended to be higher in CVH livers compared to normal livers, there was no significant difference.
IL‐10
The level of Il‐10 mRNA expression was high in the early stage of PBC livers compared with normal livers and CVH livers (p<0.05) (fig 3C). In advanced stage PBC, the level of Il‐10 mRNA expression was high compared to normal livers (p<0.05).
TGFβ
The level of TGFβ mRNA expression was high in the early stage of PBC livers compared with normal livers (p<0.05) (fig 3D). Although TGFβ mRNA expression tended to be higher in advanced stage PBC and CVH livers compared to normal livers, there was no significant difference.
Increased mRNA expression of IFNγ and TNFα and the ratio of IFNγ/Foxp3 in PBC livers
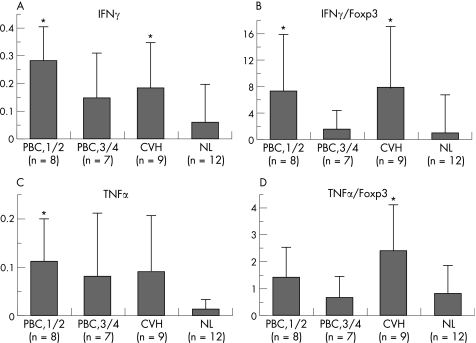
Figure 4 shows the semi‐quantitative level of IFNγ and TNFα mRNA expression and the ratios of IFNγ/Foxp3 and TNFα/Foxp3 in PBC and control livers.
Figure 4 Semi‐quantitative reverse transcriptase (RT)‐PCR for IFNγ and TNFα mRNA in primary biliary cirrhosis (PBC) and control livers. (A–B) Semi‐quantitative level of IFNγ mRNA expression (A) and the ratio of IFNγ/Foxp3 (B). The level of IFNγ mRNA and the ratio of IFNγ/Foxp3 were significantly increased in early stage of PBC and chronic viral hepatitis (CVH) livers, when compared with normal livers (NL). (C–D) Semi‐quantitative level of TNFα mRNA expression (C) and TNFα/Foxp3 (D). TNFα mRNA expression was rather high in early stage PBC, and the ratio of TNFα/Foxp3 was significantly high in CVH liver compared to normal livers. Gel images were evaluated using NIH image and the expression was normalised as a ratio using human G3PDH as a housekeeping gene. Data expressed as mean (SD). *p<0.05 vs normal liver.
IFNγ
The level of IFNγ mRNA expression was high in the early stage of PBC and CVH livers compared with normal livers (p<0.05) (fig 4A). The ratio of IFNγ/Foxp3 was significantly high in the early stage of PBC and CVH livers compared with normal livers (p<0.05) (fig 4B).
TNFα
The level of TNFα mRNA expression tends to be high in PBC and CVH livers and a significant difference was seen between the early stage of PBC and normal livers (p<0.05) (fig 4C). The ratio of TNFα/Foxp3 was significantly high in CVH livers compared with normal livers (p<0.05) (fig 4D).
Discussion
We have investigated the distribution of Foxp3+Tregs and the level of mRNA expression of Foxp3 and related cytokines in PBC and control livers to clarify the significance of Foxp3+Tregs in the pathogenesis of PBC. We thought that the loss of self‐tolerance in the small bile ducts might cause CNSDC in PBC, and that the number of Tregs in portal tracts, particularly those with CNSDC might be reduced. However, we failed to find a decrease of Foxp3+Tregs reflecting sustained tolerance to self‐antigen in the present study. Foxp3+Tregs infiltration was in parallel with the extent of chronic inflammation in portal tracts in both PBC and CVH livers. It was of interest that the extent of Foxp3+Tregs infiltration was rather high in portal tracts with CNSDC. There was a difference between early stage PBC and late stage PBC livers in the extent of Foxp3+Tregs infiltration in inflamed portal tract with CNSDC.
Furthermore, the level of Foxp3, IL‐10 and TGFβ mRNA expression was higher in PBC than normal livers and the level of IL‐10 mRNA expression was significantly higher in early stage PBC, when compared with CVH. The level of IFNγ and TNFα was also high in early stage PBC and CVH livers. These data also support the rather high extent of Foxp3+Tregs infiltration in parallel with the extent of chronic inflammation detected by immunohistochemical study. It is of interest that the ratio of IFNγ/Foxp3 tends to be high in the early stage of PBC compared to the advanced stage of PBC, although the ratio was also high in CVH as well as early PBC. The increased ratio of IFNγ/Foxp3 may be related to the active inflammation irrespective of aetiology. Taken together, results of the present study suggest that Foxp3+Tregs may be involved in the maintenance of chronic inflammation in PBC and CVH livers and that Foxp3+Tregs may not be related to the pathogenesis of PBC. In contrast to the present study, Lan et al recently reported the relative decrease of Foxp3+Tregs in PBC and suggested that Foxp3+Tregs may play role in the loss of tolerance in PBC.17 However, their functional studies did not reveal a global PBC Tregs defect.17 Further studies are needed to clarify the real function and target(s) of Tregs infiltrating to portal tracts in PBC.
Similar to our present study, several recent reports have demonstrated the increased infiltration of Foxp3+Tregs in damaged organs or target tissues in autoimmune diseases.32,33 These reports suggested that suppressor cells migrate to and/or multiply at the sites of inflammation as part of the immune response to combat injurious inflammation,33 and these Tregs may be unable to down‐modulate the autoimmune response.32 Recently, naturally‐occurring Tregs were reported not to contribute to the development of airway tolerance induced by orally administered antigen.34 Taken together, Foxp3+Tregs may not cover all types of immune tolerance and Foxp3+Tregs may not be related to the pathogenesis of some autoimmune diseases including PBC.
In conclusion, in PBC and CVH livers, the extent of Tregs infiltration in portal tracts was in parallel with the extent of chronic inflammation, irrespective of disease, and Tregs infiltration was extensive in portal tracts with CNSDC. This report examining naturally occurring Tregs failed to support the interesting idea that Tregs failure is related to the development of CNSDC via loss of self‐tolerance in PBC.
Take‐home messages
Whether naturally‐occurring regulatory T cells (Foxp3+Tregs) are involved in the pathogenesis of primary biliary cirrhosis (PBC) was investigated.
In PBC and chronic viral hepatitis (CVH) livers, the amounts of infiltrating Foxp3+Tregs in portal tracts were in parallel with the degree of portal inflammation, irrespective of disease.
The infiltration of Foxp3+Tregs into portal tracts with chronic non‐suppurative destructive cholangitis (CNSDC) in PBC was greatest in comparison with inflamed portal tracts in CVH or those without CNSDC in PBC.
The level of Foxp3, IL‐10 and TGFβ mRNA expression was high in PBC compared with normal livers.
The evaluation of Foxp3+Tregs did not support the hypothesis that the reduced regulatory function accounts for the development of CNSDC in PBC.
Abbreviations
CNSDC - chronic non‐suppurative destructive cholangitis
CVH - chronic viral hepatitis
Foxp3 - Forkhead box P3
IL - interleukin
IFN - interferon
PBC - primary biliary cirrhosis
RT - reverse transcriptase
TGF - transforming growth factor
Th - T helper
TNF - tumour necrosis factor
Tregs - regulatory T cells
Footnotes
Competing interests: None declared.
References
- 1.Gershwin M E, Mackay I R, Sturgess A.et al Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J Immunol 19871383525–3531. [PubMed] [Google Scholar]
- 2.Kaplan M. Primary biliary cirrhosis. N Engl J Med 19963351570–1580. [DOI] [PubMed] [Google Scholar]
- 3.Portmann B, Nakanuma Y. Diseases of the bile ducts. In: MacSween R, Burt A, Portmann BC, Ishak K, Scheuer P, Anthony P, eds. Pathology of the liver, 4th edn. London: Churchill Livingstone, 2001435–506.
- 4.Nakanuma Y, Ohta G. Histometric and serial section observations of the intrahepatic bile ducts in primary biliary cirrhosis. Gastroenterology 1979761326–1332. [PubMed] [Google Scholar]
- 5.Fussey S, Guest J, James O.et al Identification and analysis of the major M2 autoantigens in primary biliary cirrhosis. Proc Natl Acad Sci USA 1988858654–8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kita H, Matsumura S, He X S.et al Analysis of TCR antagonism and molecular mimicry of an HLA‐A0201‐restricted CTL epitope in primary biliary cirrhosis. Hepatology 200236(4 Pt 1)918–926. [DOI] [PubMed] [Google Scholar]
- 7.Shimoda S, Van de Water J, Ansari A.et al Identification and precursor frequency analysis of a common T cell epitope motif in mitochondrial autoantigens in primary biliary cirrhosis. J Clin Invest 19981021831–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kita H, Lian Z X, Van de Water J.et al Identification of HLA‐A2‐restricted CD8(+) cytotoxic T cell responses in primary biliary cirrhosis: T cell activation is augmented by immune complexes cross‐presented by dendritic cells. J Exp Med 2002195113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakaguchi S. Naturally arising Foxp3‐expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non‐self. Nat Immunol 20056345–352. [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi S, Setoguchi R, Yagi H.et al Naturally arising Foxp3‐expressing CD25+CD4+ regulatory T cells in self‐tolerance and autoimmune disease. Curr Top Microbiol Immunol 200630551–66. [DOI] [PubMed] [Google Scholar]
- 11.Fehervari Z, Sakaguchi S. CD4+ Tregs and immune control. J Clin Invest 20041141209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furuno K, Yuge T, Kusuhara K.et al CD25+CD4+ regulatory T cells in patients with Kawasaki disease. J Pediatr 2004145385–390. [DOI] [PubMed] [Google Scholar]
- 13.Sugiyama H, Gyulai R, Toichi E.et al Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol 2005174164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kukreja A, Cost G, Marker J.et al Multiple immuno‐regulatory defects in type‐1 diabetes. J Clin Invest 2002109131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crispin J C, Martinez A, Alcocer‐Varela J. Quantification of regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun 200321273–276. [DOI] [PubMed] [Google Scholar]
- 16.Bacchetta R, Passerini L, Gambineri E.et al Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest 20061161713–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lan R Y, Cheng C, Lian Z X.et al Liver‐targeted and peripheral blood alterations of regulatory T cells in primary biliary cirrhosis. Hepatology 200643729–737. [DOI] [PubMed] [Google Scholar]
- 18.Oertelt S, Kenny T P, Selmi C.et al SNP analysis of genes implicated in T cell proliferation in primary biliary cirrhosis. Clin Dev Immunol 200512259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oertelt S, Lian Z X, Cheng C M.et al Anti‐mitochondrial antibodies and primary biliary cirrhosis in TGF‐beta receptor II dominant‐negative mice. J Immunol 20061771655–1660. [DOI] [PubMed] [Google Scholar]
- 20.Honda M, Kawai H, Shirota Y.et al Differential gene expression profiles in stage I primary biliary cirrhosis. Am J Gastroenterol 20051002019–2030. [DOI] [PubMed] [Google Scholar]
- 21.Zachou K, Rigopoulou E I, Tsikrikoni A.et al Autoimmune hepatitis type 1 and primary biliary cirrhosis have distinct bone marrow cytokine production. J Autoimmun 200525283–288. [DOI] [PubMed] [Google Scholar]
- 22.Shindo M, Mullin G E, Braun‐Elwert L.et al Cytokine mRNA expression in the liver of patients with primary biliary cirrhosis (PBC) and chronic hepatitis B (CHB). Clin Exp Immunol 1996105254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neuman M, Angulo P, Malkiewicz I.et al Tumor necrosis factor‐alpha and transforming growth factor‐beta reflect severity of liver damage in primary biliary cirrhosis. J Gastroenterol Hepatol 200217196–202. [DOI] [PubMed] [Google Scholar]
- 24.Ludwig J. Small‐duct primary sclerosing cholangitis. Semin Liver Dis 19911111–17. [DOI] [PubMed] [Google Scholar]
- 25.Desmet V, Gerber M, Hoofnagle J.et al Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology 1994191513–1520. [PubMed] [Google Scholar]
- 26.Nakanuma Y, Sasaki M. Expression of blood‐group‐related antigens in the intrahepatic biliary tree and hepatocytes in normal livers and various hepatobiliary diseases. Hepatology 198910174–178. [DOI] [PubMed] [Google Scholar]
- 27.Sasaki M, Ikeda H, Sato Y.et al Decreased expression of Bmi1 is closely associated with cellular senescence in small bile ducts in primary biliary cirrhosis. Am J Pathol 2006169831–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allan S E, Passerini L, Bacchetta R.et al The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J Clin Invest 20051153276–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manavalan J S, Kim‐Schulze S, Scotto L.et al Alloantigen specific CD8+CD28− FOXP3+ T suppressor cells induce ILT3+ ILT4+ tolerogenic endothelial cells, inhibiting alloreactivity. Int Immunol 2004161055–1068. [DOI] [PubMed] [Google Scholar]
- 30.Yagi H, Nomura T, Nakamura K.et al Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol 2004161643–1656. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki M, Tsuneyama K, Nakanuma Y. Aberrant expression of trefoil factor family 1 in biliary epithelium in hepatolithiasis and cholangiocarcinoma. Lab Invest 2003831403–1413. [DOI] [PubMed] [Google Scholar]
- 32.Marazuela M, Garcia‐Lopez M A, Figueroa‐Vega N.et al Regulatory T cells in human autoimmune thyroid disease. J Clin Endocrinol Metab 2006913639–3646. [DOI] [PubMed] [Google Scholar]
- 33.Cao D, Borjesson O, Larsson P.et al FOXP3 identifies regulatory CD25bright CD4+ T cells in rheumatic joints. Scand J Immunol 200663444–452. [DOI] [PubMed] [Google Scholar]
- 34.Mucida D, Kutchukhidze N, Erazo A.et al Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest 20051151923–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]