Abstract
Aims
To assess the sensitivity of a combined selective broth enrichment technique plus selective plating for the detection of group B streptococcus (GBS) colonisation in a large cohort of pregnant women from North‐Eastern Italy.
Methods
During 2002–2005, 5020 pregnant women were screened between the 35th and the 37th week of gestation. A lower vaginal sample and a rectal sample were collected and inoculated onto LIM broth and a selective colistin aztreonam blood agar plate (CAP). Direct agar plates were examined after 18–24 hours and, if negative, after 48 hours. LIM broth was subcultured after 18–24 hours onto a Columbia blood agar plate. All colonies suggestive for GBS were submitted to phenotypic identification.
Results
901 Women (17.9%) were positive for GBS. On 728 positive samples, corresponding to patients enrolled between 2003 and 2005, the results of selective direct plating and selective broth enrichment were compared. A total of 561 (77.1% of positive samples, corresponding to 13.9% of patients) were positive on direct selective agar; an additional 167 isolates (22.9% of samples, 4.1% of patients) were recovered from the LIM broth subculture.
Conclusions
The prevalence of GBS carriage in this population‐based study is a reliable estimate considering the sensitivity of the microbiological methods used, the rate of attendance of pregnant women to clinical and laboratory settings and the compliance to the protocol. Results confirm that the combination of selective enrichment broth and selective direct plating is a time‐saving and sensitive method.
Keywords: GBS, pregnant women, prevalence, culture methods
Group B streptococcus (GBS) or Streptococcus agalactiae is a leading cause of severe invasive diseases, such as early‐onset (first week of life) sepsis and pneumonia, in newborn babies. Neonatal infection is the result of an ascending spread of GBS from the rectum and perineum throughout the vagina. Colonisation during pregnancy may be transient, chronic or intermittent, and is asymptomatic in the majority of cases. Urinary tract infection sustained by GBS, either symptomatic or asymptomatic, is considered a risk factor for neonatal infection. Nevertheless, the major risk factor for early‐onset disease is maternal colonisation at time of delivery. Perinatal transmission can be prevented by intrapartum antimicrobial prophylaxis. The Centers for Disease Control (CDC), the American Academy of Paediatrics and the American College of Obstetricians and Gynaecologists developed guidelines on prevention of early‐onset group B streptococcal disease in newborn babies.1,2,3 Identification of women at risk for GBS transmission can be made by a clinical risk‐based approach or by universal prenatal microbiological screening. Evidence of a larger protective effect of the screening‐based approach compared with the risk‐based one leads to recommendation of the former procedure.1,4
It has been calculated that the screening‐based strategy can reduce early‐onset neonatal GBS disease by as much as 78%. Its effectiveness depends on the specimen sampling site, the timing of collection and the sensitivity of the microbiological methods used. Screening accuracy in predicting GBS carriage at delivery is higher if the cultures are collected within 5 weeks before delivery, and when both vaginal and rectal samples are examined.1 Microbiological culture methods include direct plating on solid media (i.e. blood agar plates with or without selective agents) and the use of selective broth media. To improve the accuracy of prenatal screening cultures in identifying intrapartum colonisation, the CDC recommend the use of selective enrichment broth culture. However, this practice, although increasingly adopted, is not still universally widespread, at least in Italy.5,6 New techniques have been developed to increase sensitivity or to decrease the time for GBS detection: selective/differential agar media, direct latex agglutination testing of selective broth medium, RNA probe assays, and nucleic acid amplification techniques.7,8,9,10,11,12 Few of these methods have shown a higher sensitivity than selective broth, with the exception of combined methods (e.g. GBS antigen detection and PCR performed on LIM broth after 24 hours' enrichment).13,14 However, nucleic acid amplification techniques have higher costs and require skills and technologies not universally available for routine testing.15 Thus, the standard culture‐based method to detect the viable microorganisms sustaining maternal colonisation is the currently established technique for secondary prevention of neonatal infections.
This study aimed to assess the prevalence of GBS colonisation in a large cohort of pregnant women from North‐Eastern Italy. In addition, we compared the sensitivity of a combination of selective broth enrichment plus selective plating with direct plating alone for the detection of GBS carriage.
Materials and methods
Patients
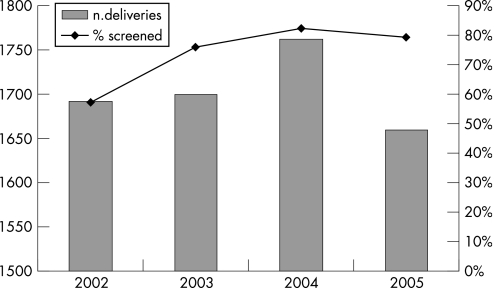
This survey studied pregnant women referred to the Burlo Garofolo Institute, the mother‐and‐child hospital of Trieste (North‐Eastern Italy), which serves a prevalently urban area with around 250 000 inhabitants. Almost all the pregnant women attend our hospital, which reports about 1800 deliveries/year (fig 1). In the period considered (2002–2005), the rate of babies born in our hospital ranged from 97% to 100% (mean rate 98.6%) of all newborn babies of this area. In this institute, a protocol for the prevention of neonatal GBS infections based on universal prenatal screening was launched in 2001. For this evaluation, all pregnant women screened from 1 January 2002 to 31 December 2005 were considered.
Figure 1 Rates of compliance with GBS screening.
Microbiology
Pregnant women were screened between the 35th and the 37th week of gestation, after obtaining their informed consent. A lower vaginal (introitus) sample and a rectal sample were taken by two separate swabs. Each pair of specimens, devoted exclusively to GBS culture, was sent to the microbiology laboratory and immediately processed.
The swabs were soaked with 0.5 ml of saline to be inoculated on selective broth medium (LIM broth) and on a selective (colistin aztreonam) blood agar plate (CAP agar). LIM broth (BD Diagnostics, Franklin Lakes, NJ, USA) is a Todd Hewitt base broth with 10 μg of colistin per ml and 15 μg of nalidixic acid per ml as inhibitory agents towards Gram‐negative microorganisms. CAP agar (Oxoid Ltd, Hampshire, UK) is a 5% sheep blood agar supplemented with colistin 10 μg per ml and aztreonam 2 μg per ml.
Direct CAP agar was incubated in 5% CO2 at 35°C and examined after 18–24 h. Typical colonies suggestive for GBS (with or without β‐haemolysis) were identified by Gram staining, negative catalase reaction, growth without aesculin hydrolysis on bile‐aesculin agar and detection of the Lancefield group B antigens by a rapid latex agglutination test (Phadebact Strep B, Boule Diagnostics, Stockholm, Sweden). When direct plating was positive, cultures were discarded; negative plates were re‐incubated and re‐inspected at 48 h.
LIM broth was incubated in 5% CO2 at 35°C; after 18–24 h, the broth was subcultured onto a Columbia blood agar plate (Oxoid Ltd), and incubated for 24 h in 5% CO2 at 35°C; colonies suggestive for GBS were identified as described above.
Statistical analysis
Prevalence rates are reported together with 95% CI. Comparison among proportions was made by the χ2 test.
Results
Overall, 5020 pregnant women were screened during the study period (2002–2005). Patient compliance, calculated on the basis of the deliveries that occurred in our hospital, increased sharply after the first year (fig 1). Since almost all pregnancies are covered by our hospital, the compliance rates could be extended to the pregnant population of the area.
Group B streptococcus was detected in 901 women, corresponding to a prevalence rate of 17.9% (table 1).
Table 1 Results of GBS screening at 35–37 weeks' gestation.
| Year | Samples (n) | GBS positive | |
|---|---|---|---|
| n (%) | 95% CI | ||
| 2002 | 970 | 173 (17.8) | 15.4 to 20.2 |
| 2003 | 1289 | 228 (17.7) | 15.6 to 19.8 |
| 2004 | 1448 | 246 (17.0) | 15.1 to 18.9 |
| 2005 | 1313 | 254 (19.2) | 17.2 to 21.5 |
| total | 5020 | 901 (17.9) | 16.9 to 19.0 |
The annual variation of prevalence was not statistically significant (χ2 test, p = 0.4), thus underlining the stability of GBS prevalence estimates.
The comparison between the selective broth enrichment technique and selective direct plating alone was performed on 728 samples which tested positive and were collected from January 2003 to December 2005. Overall, 561 pairs of swabs (77.1% of positive samples, corresponding to 13.9% of patients) were positive after 18–24 h on direct CAP agar. An additional 167 isolates (22.9% of samples, corresponding to 4.1% of patients) that failed to grow on CAP agar were recovered at 48 h from the LIM broth subcultured onto Columbia blood agar (table 2).
Table 2 GBS isolation and prevalence rates by direct plating on a selective blood agar plate (CAP agar) and by enrichment on selective broth medium (LIM broth).
| n (%) | 95% CI | |
|---|---|---|
| Number examined | 4050 | |
| GBS‐positive | 728 (18.0) | 16.8 to 19.2 |
| Positive by direct plating/total positive | 561/728 (77.1) | 74.0 to 80.1 |
| Positive by direct plating/total women | 561/4050 (13.9) | 12.8 to 14.9 |
| Additional positive by enrichment/total positive | 167/728 (22.9) | 19.9 to 26.0 |
| Additional positive by enrichment/total women | 167/4050 (4.1) | 3.5 to 4.7 |
Discussion
The strong evidence for the usefulness of the universal screening at 35–37 weeks of pregnancy to prevent GBS neonatal infection was mainly drawn from studies conducted in the United States.4 However, GBS is present worldwide and the prevalence of GBS carriers varies in relation to the geographical area and/or a number of demographic factors. Therefore, knowledge of the epidemiological situation of a defined area is crucial to decide the launch of a screening programme and to evaluate the cost‐effectiveness of such a strategy.
In this survey, the prevalence rate of GBS colonisation in late pregnancy was 18%. To our knowledge, this is one of the largest and most long‐lasting evaluations of GBS carriage recently conducted in a well‐defined area. Moreover, considering the rate of attendance of pregnant women to our clinical and laboratory settings and the compliance to the screening protocol, this study may be considered a population‐based one, where mechanisms of selection bias were probably absent.
A comparison of GBS colonisation prevalence among different studies is difficult to perform because of substantial methodological differences.16 Taking into account the similarity of two main methodological aspects (e.g. the use of selective broth media and the collection of both rectal and vaginal swabs), the prevalence of GBS carriers in this area is very similar to that reported by other studies in Italy, in some European countries, and in the USA.9,17,18,19,20,21,22 Outlier prevalence rates from other countries and also among different studies performed in the same country could reflect true geographical differences, but may be due either to selection biases or microbiological issues.23,24,25
Current microbiological methods include the use of selective broth media (i.e. Todd‐Hewitt broth supplemented with colistin or gentamicin plus nalidixic acid) and/or solid media (i.e. blood agar plates supplemented with colistin or neomycin plus nalidixic acid). Each detection method presents both advantages and limits. The CDC recommend the use of selective enrichment broth culture, which requires a subculture to a blood agar plate after overnight incubation: a minimum of 48 hours is therefore necessary for the identification of GBS positive samples. Furthermore, this method may give false negative results in the presence of a heavy growth of Enterococcus faecalis.26 To decrease result turnaround time, several authors suggest the use of direct plating onto a selective agar medium, which allows the identification of a great number of GBS carriers in 18–24 hours. Several formulations of selective agar have been proposed, i.e. colistin–nalidixic acid blood agar (CNA), neomycin–nalidixic acid agar, new‐GBS agar, Granada agar. There is still no consensus on which medium is preferable.27,28 In this study we used CAP blood agar, a modified CNA agar with aztreonam substituting nalidixic acid. Aztreonam is a monobactam with an activity spectrum limited to Gram‐negative aerobic bacilli, including Pseudomonas aeruginosa, and no activity against Gram‐positive bacteria, including GBS.29 In our experience, CAP agar allows a better detection of GBS colonies after 18–24 hours if compared to CNA medium (data not shown).
Direct plating alone is particularly useful in heavy colonisations, which are associated with higher risk for early‐onset disease. However, the sensitivity is poor in the presence of a light GBS colonisation, since it is likely to be masked by overgrowing bacteria. Hence, single methods alone may not be sufficient to detect GBS carriers. Our findings confirm that a combined strategy (direct plating on selective agar plus selective broth enrichment) is time‐saving (77.1% positive results were available within 24 hours) and at the same time has an increased sensitivity (22.9% of positive samples would have been missed without enrichment, meaning that 4.1% of women would not have been recognised as GBS carriers). Our results are in line with those reported by others.22,28
Take‐home messages
This is a large population‐based study where mechanisms of selection bias were probably absent.
During a four‐year evaluation, the prevalence of group B streptococcus (GBS) rectal–vaginal colonisation in pregnant women was established as 18%.
An enrichment broth culture in addition to direct agar plating detected a further 4% of GBS carriers.
In conclusion, the usefulness of a universal screening prevention strategy must be supported by local prevalence data obtained by sensitive microbiological methods. A reliable prevalence estimate for this area was established as 18%. For routine purposes, we recommend the use of a direct selective plate in addition to selective enrichment broth on vaginal and rectal swab specimens.
Acknowledgements
The excellent technical assistance of Mrs D Macorini, P Serra, and C Znidarcic is gratefully appreciated.
Abbreviations
CAP - colistin aztreonam blood agar plate
GBS - group B streptococcus
Footnotes
Funding: This survey was in part supported by a grant from IRCCS “Burlo Garofolo”.
Competing interests: None.
References
- 1.Schrag S, Gorwitz R, Fultz‐Butts K.et al Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep 2002511–22. [PubMed] [Google Scholar]
- 2.Committee on Infectious Diseases/Committee on Fetus and Newborn, American Academy of Pediatrics Revised guidelines for prevention of early‐onset group B streptococcal (GBS) disease. Pediatrics 199799489–496. [DOI] [PubMed] [Google Scholar]
- 3.ACOG Committee Opinion: number 279, December 2002. Prevention of early‐onset group B streptococcal disease in newborns. Obstet Gynecol 20021001405–1412. [DOI] [PubMed] [Google Scholar]
- 4.Schrag S J, Zell E R, Lynfield R.et al A population‐based comparison of strategies to prevent early‐onset group B streptococcal disease in neonates. N Engl J Med 2002347233–239. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Laboratory practices for prenatal group B streptococcal screening‐seven states, 2003. MMWR 200453506–509. [PubMed] [Google Scholar]
- 6.Berardi A, Lugli L, Rossi K.et al Prevention of group B streptococcal infection in a North‐Italian area. Pediatr Infect Dis J 200423691–692. [DOI] [PubMed] [Google Scholar]
- 7.Overman S B, Eley D D, Jacobs B E.et al Evaluation of methods to increase the sensitivity and timeliness of detection of Streptococcus agalactiae in pregnant women. J Clin Microbiol 2002404329–4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergeron M G, Ke D, Menard C.et al Rapid detection of group B streptococci in pregnant women at delivery. N Engl J Med 2000343175–179. [DOI] [PubMed] [Google Scholar]
- 9.Guerrero C, Martinez J, Menasalvas A.et al Use of direct latex agglutination testing of selective broth in the detection of group B streptococcal carriage in pregnant women. Eur J Clin Microbiol Infect Dis 20042361–62. [DOI] [PubMed] [Google Scholar]
- 10.Hansen S M, Sorensen U B. Method for quantitative detection and presumptive identification of group B streptococci on primary plating. J Clin Microbiol 2003411399–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park C J, Vandel N M, Ruprai D K.et al Detection of group B streptococcal colonization in pregnant women using direct latex agglutination testing of selective broth. J Clin Microbiol 200139408–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Picard F J, Bergeron M G. Laboratory detection of group B streptococcus for prevention of perinatal disease. Eur J Clin Microbiol Infect Dis 200423665–671. [DOI] [PubMed] [Google Scholar]
- 13.Rallu F, Barriga P, Scrivo C.et al Sensitivities of antigen detection and PCR assays greatly increased compared to that of the standard culture method for screening for group B streptococcus carriage in pregnant women. J Clin Microbiol 200644725–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies H D, Miller M A, Faro S.et al Multi‐center study of a rapid, molecular‐based assay for the diagnosis of group B streptococcal colonization in pregnant women. Clin Infect Dis 2004391129–1135. [DOI] [PubMed] [Google Scholar]
- 15.Haberland C A, Benitz W E, Sanders G D.et al Perinatal screening for group B streptococci: cost‐benefit analysis of rapid polymerase chain reaction. Pediatrics 2002110471–480. [DOI] [PubMed] [Google Scholar]
- 16.Trijbels‐Smeulders M A, Kollee L A, Adriaanse A H.et al Neonatal group B streptococcal infection: incidence and strategies for prevention in Europe. Pediatr Infect Dis J 200423172–173. [DOI] [PubMed] [Google Scholar]
- 17.Vergani P, Patanè L, Colombo C. Impact of different prevention strategies on neonatal group B streptococcal disease. Am J Perinatol 200219341–348. [DOI] [PubMed] [Google Scholar]
- 18.Bou G, Figueira M, Canle D.et al Evaluation of group B streptococcus differential agar for detection and isolation of Streptococcus agalactiae. Clin Microbiol Infect 200511676–678. [DOI] [PubMed] [Google Scholar]
- 19.Brimil N, Barthell E, Heindrichs U.et al Epidemiology of Streptococcus agalactiae colonization in Germany. Int J Med Microbiol 200629639–44. [DOI] [PubMed] [Google Scholar]
- 20.Kieran E, Matheson M, Mann A G.et al Group B streptococcus (GBS) colonisation among expectant Irish mothers. Ir Med J 19989121–22. [PubMed] [Google Scholar]
- 21.Regan J A, Klebanoff M A, Nugent R P. The epidemiology of group B streptococcal colonization in pregnancy. Vaginal Infections and Prematurity Study Group. Obstet Gynecol 199177604–610. [PubMed] [Google Scholar]
- 22.Jones N, Oliver K, Jones Y.et al Carriage of group B streptococcus in pregnant women from Oxford, UK. J Clin Pathol 200659363–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen S M, Uldbjerg N, Kilian M.et al Dynamics of Streptococcus agalactiae colonization in women during and after pregnancy and in their infants. J Clin Microbiol 20044283–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Votava M, Tejkalova M, Drabkova M.et al Use of GBS media for rapid detection of group B streptococci in vaginal and rectal swabs from women in labor. Eur J Clin Microbiol Infect Dis 200120120–122. [DOI] [PubMed] [Google Scholar]
- 25.Citernesi A, Formica G, Caruso S.et al Vaginal colonization of streptococcus B in pregnancy. Minerva Ginecol 199648227–233. [PubMed] [Google Scholar]
- 26.Dunne W M, Jr, Holland‐Staley C A. Comparison of NNA agar culture and selective broth culture for detection of group B streptococcal colonization in women. J Clin Microbiol 1998362298–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunne W M., Jr Comparison of selective broth medium plus neomycin‐nalidixic acid agar and selective broth medium plus Columbia colistin‐nalidixic acid agar for detection of group B streptococcal colonization in women. J Clin Microbiol 1999373705–3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elsayed S, Gregson D B, Church D L. Comparison of direct selective versus nonselective agar media plus LIM broth enrichment for determination of group B streptococcus colonization status in pregnant women. Arch Pathol Lab Med 2003127718–720. [DOI] [PubMed] [Google Scholar]
- 29.Wood W, Harvey G, Olson E S.et al Aztreonam selective agar for Gram positive bacteria. J Clin Pathol 199346769–771. [DOI] [PMC free article] [PubMed] [Google Scholar]