Abstract
Aims
To characterize the pharmacokinetics of amitriptyline and its metabolite nortriptyline following OROS® and IR treatments, and to correlate them with anticholinergic side-effects.
Methods
The pharmacokinetics and safety of amitriptyline following administration of an osmotic controlled release tablet (OROS® and an immediate release (IR) tablet were evaluated in 14 healthy subjects. In this randomized, open label, three-way crossover feasibility study, the subjects received a single 75 mg OROS® tablet, three 25 mg IR tablets administered every 8 h, or 3×25 mg IR tablets administered at nighttime. In each treatment arm serial blood samples were collected for a period of 84 h after dosing. The plasma samples were analysed by gas chromatography for amitriptyline and its metabolite nortriptyline. Anticholinergic effects such as saliva output, visual acuity, and subject-rated drowsiness and dry mouth were measured on a continuous scale during each treatment period.
Results
Following dosing with OROS® (amitriptyline hydrochloride), the mean maximal plasma amitriptyline concentration Cmax (15.3 ng ml−1) was lower and the mean tmax (25.7 h) was longer than that associated with the equivalent IR dose administered at nighttime (26.8 ng ml−1 and 6.3 h, respectively). The bioavailability of amitriptyline following OROS® dosing was 95% relative to IR every 8 h dosing, and 89% relative to IR nighttime dosing. The metabolite-to-drug ratios after the three treatment periods were similar, suggesting no change in metabolism between treatments. The relationships between plasma amitriptyline concentration and anticholinergic effects (e.g. reduced saliva weight, dry mouth, and drowsiness) were similar with all three treatments. Of the anticholinergic effects, only decreased saliva weight and dry mouth correlated well with plasma amitriptyline concentrations; drowsiness did not. There was no apparent correlation between anticholinergic effects and the plasma nortriptyline concentration.
Conclusions
The bioavailability of OROS® (amitriptyline hydrochloride) was similar to that of the IR treatments and the pharmacokinetics of amitriptyline after OROS® dosing may decrease the incidence of anticholinergic effects compared with that seen with nighttime dosing of the IR formulation. Therefore, this controlled-release formulation of amitriptyline may be appropriate for single daily administration.
Keywords: amitriptyline, anticholinergic effects, controlled-release, pharmacodynamics, pharmacokinetics
Introduction
A tricyclic agent with sedative effects, amitriptyline, is indicated for relief of the symptoms of depression. Although its mechanism of action in man is not well defined, amitriptyline is believed to be a potent inhibitor of noradrenaline re-uptake at the adrenergic nerve endings [1]. Amitriptyline is not a monoamine oxidase inhibitor, and its primary action does not involve stimulation of the central nervous system. Amitriptyline hydrochloride is currently available in an immediate-release tablet and an injectable formulation. The total daily adult dosage typically ranges from 75 to 150 mg day−1 for outpatient use, but doses of 200–300 mg day−1 are sometimes prescribed for hospitalized patients.
Amitriptyline is completely but slowly absorbed from the gastrointestinal tract after oral administration, and peak plasma concentrations are usually reached in 4–8 h. Amitriptyline undergoes extensive hepatic presystemic elimination, and its systemic bioavailability ranges from 33% to 62% after oral administration [2]. A highly lipophilic compound, amitriptyline is widely distributed throughout the body and extensively bound to tissue and plasma proteins [3]. About one-third to one-half of the drug is excreted within 24 h. The plasma half-life ranges from 10 to 28 h for amitriptyline and from 16 to 80 h for its active metabolite, nortriptyline [4, 5]. Amitriptyline’s most common side-effects, such as blurred vision, dry mouth, and constipation, are due to its anticholinergic effects. The sedative effect is thought to be mediated by blockade of histamine receptors [6]. Amitriptyline in high doses also has some cardiac effects, such as dysrhythmias, sinus tachycardia, and prolonged conduction time.
Schulz et al. [2] reported that a single dose (80–100 mg) of amitriptyline given to healthy volunteers was not well tolerated, suggesting that a gradual buildup in plasma amitriptyline concentration is preferable. The total daily dose may be given in divided doses, or once at nighttime to take advantage of the drug’s known sedative effect. When administered at nighttime amitriptyline has been well tolerated with few side-effects [7, 8].
An OROS® system has been developed to deliver 75 mg amitriptyline hydrochloride by a membrane-controlled, osmotic process for 22–24 h. The resultant slow increase in plasma amitriptyline concentration is intended to decrease the incidence of anticholinergic effects commonly experienced with peak plasma drug concentrations. The two-compartment core of each OROS® (amitriptyline hydrochloride) tablet contains the water-soluble drug amitriptyline hydrochloride in a drug compartment and inactive excipients in an osmotic push layer compartment. These components are enclosed within a cellulosic membrane that is permeable to water but impermeable to ions or to the drug.
The objectives of this feasibility study were to characterize the pharmacokinetics of amitriptyline and its metabolite nortriptyline following OROS® and IR treatments, and to correlate them with the anticholinergic effects.
Methods
Subjects
Fifteen healthy male subjects, 21–37 years (mean 29 years), with a mean weight of 77.4 kg (61–94 kg), were enrolled in the study.
This feasibility study protocol was reviewed and approved by the Institutional Review Board of Harris Laboratories (Lincoln, NE), where the clinical portion of the study was conducted. Written informed consent was obtained from each subject before entry. Inclusion criteria were as follows: body weight within ±15% of the ideal weight for the subject’s height, based on MetLife®; normal physical examination; and no clinically significant deviations in clinical and laboratory tests (vital signs, haematology, blood chemistry, and urinalysis). None of the subjects had smoked cigarettes for at least 3 months before study entry.
Subjects fasted for 10 h before and 4 h after the first dose of each treatment. All subjects refrained from consuming alcohol for 3 days before and during the study period. Caffeine-containing beverages were prohibited while subjects were at the study centre.
Study design
This was a randomized, open-label, three-way crossover study. The three treatments were as follows: 75 mg OROS® (amitriptyline hydrochloride) administered at 06.00 h; 25 mg Elavil® administered three times a day at 06.00 h, 14.00 h, and 22.00 h; and three 25 mg Elavil® administered at 22.00 h. The washout period between treatments was 14 days. Two days before receiving the first treatment, subjects were enrolled in the centre so that baseline measurements for anticholinergic effects (e.g. salivary flow rate, drowsiness and feeling of dry mouth) could be obtained over a period of 24 h. After the baseline measurements were taken, the subjects received their first treatment the following morning or at nighttime according to the randomization schedule. Each dose was taken with 240 ml of water. Subjects were instructed to swallow each tablet whole and not to chew, crush, or divide it, and to drink at least 8 cups of water per day. Standardized meals were served during the study period (breakfast at 07.00 h, lunch at 12.00 h, dinner at 18.00 h, and a snack at 21.00 h); for subjects receiving the IR treatment at nighttime, snack was not given. Subjects remained in the clinic 48–60 h after the initial dose, and they were instructed to return to the clinic for the remaining blood draws and measurements.
Assessments
Safety
Treatment safety assessments included blood pressure and heart rate measurements, which were taken before and at predetermined times throughout the study. In addition, laboratory parameters were assessed before and at the end of the study. Adverse events volunteered by subjects were also recorded.
Anticholinergic effects
Saliva output, dry mouth ratings, and visual acuity following each treatment were assessed at specific times during the study. The assessment times for saliva output and dry mouth were predose, 1, 2, 4, 6, 8, 12, 16, 24, 30, 36, 48, 60, 72 and 84 h post dosing. Likewise visual acuity test was done at predose, 8 and 16 h post dosing. Drowsiness ratings were obtained only for the OROS® (amitriptyline hydrochloride) and the IR every 8 h treatments. Because the IR nighttime treatment involved frequent awakenings for blood sample collection after dosing, drowsiness ratings were not solicited in this group.
At specific times during the study, the subjects rated their dry mouth sensation on a 100 mm Visual Analogue Scale (VAS) (0=normally moist; 100=extremely dry). Dry mouth assessments were done before the saliva output test.
The stimulated saliva output measurement was a modification of earlier published methods [9, 10]. The subject was asked to swallow his saliva just before the test. A drop of 1% citric acid was placed on his tongue; he was instructed not to swallow for 2 min and then to spit the saliva into a clean, dry, preweighed beaker.
The sedation ratings were done at specific times during the study, and the subjects gave their subjective assessments of drowsiness using a 100 mm VAS (0=extremely alert; 100=extremely drowsy). Subjects who fell asleep during the 06.00 h–22.00 h period were given the maximal score (100) for that period.
Visual function was assessed before dosing and at 8 and 16 h after the dose; near and far vision were evaluated using the near vision card and the Snellen eye chart, respectively.
Pharmacokinetics methods
Following dosing of each treatment, serial blood samples for pharmacokinetic evaluation of amitriptyline and nortriptyline were collected immediately before dosing and for a period of 84 h after administration of the dose.
The plasma samples were analysed for determination of amitriptyline and nortriptyline using a gas chromatography method. Amitriptyline, nortriptyline, and an internal standard, desipramine, were extracted from the alkaline plasma into a hexanol/butanol mixture. The hexanol/butanol phase was then back-extracted with hydrochloric acid. The acid phase was made alkaline, followed by extraction with butyl acetate. Analysis was done by gas chromatography with nitrogen phosphorous detection. This method was validated with a minimum quantifiable concentration of 0.5 ng ml−1 for amitriptyline and nortriptyline. The samples were kept frozen at −20° C before analysis, and a 1ml sample was used for analysis. The day to day variability in measurements of quality control samples for amitriptyline and nortriptyline was <7.0%.
Model independent methods were used to determine the following pharmacokinetic parameters for amitriptyline and nortriptyline: the maximum observed plasma concentrations (Cmax) and the corresponding sampling time (tmax), the terminal or disposition rate constant (λz), and the terminal or disposition half-life (t1/2). The area under the curve to the last sample collection point (AUC(0,t)) was computed using the linear trapezoidal rule, and the AUC value extrapolated to infinity (AUC(0,00)) was determined as the sum of AUC(0,t) plus the area extrapolated to infinity, calculated by the concentration at time t (Ct) divided by λz. In addition the relative bioavailability (F) of OROS (amitriptyline hydrochloride) was also computed using the IR every 8 h as the reference treatment.
Pharmacodynamics
For each treatment, plasma concentration and anticholinergic effects across all subjects were averaged at each sample collection time. The means of selected anticholinergic effects (decreased saliva production, drowsiness, and dry mouth) were correlated to plasma amitriptyline and nortriptyline concentrations. The data were fitted to the sigmoidal Hill equation using the nonlinear regression method in SAS:
 |
where:
Et=value (weight or rating) of the observed effect at time t
E0=baseline value (weight or rating)
EC50=plasma drug concentration required to produce 50% of the maximum effect
Emax=maximum effect (weight or rating) observed
Ct=plasma drug concentration at time t
γ=Hill coefficient
An alternative method for correlating drug concentration with anticholinergic effects was pursued by normalizing (linear transformation−z score) the anticholinergic effects toward the mean value and correlating this with plasma amitriptyline concentration. The advantage of transformation to z score is it enables comparison of plasma concentration and different measures of the anticholinergic effects in the same domain. The slopes of plots were compared to evaluate which anticholinergic effect correlated best to the plasma concentration. The effects were normalized as below:
where:
Enor=normalized (around mean) effect
Eobs=observed effect
Emean=mean of Eobs
Esd=standard deviation (s.d.) of Eobs
Statistics
Phamacokinetic parameters for the three treatments were compared using an analysis of variance model (anova) appropriate for a crossover study design. The statistical computer package PCSAS version 6.10 (SAS Institute, NC, USA) was used for statistical analysis. The variance model included terms for treatment, subject within sequence, treatment sequence, and period.
Results
Pharmacokinetics
Baseline measurements were completed for 15 subjects. One subject was withdrawn from the study before receiving any study medication because he admitted that he was a smoker. The baseline value for this subject was excluded from analysis. The remaining 14 subjects completed the study.
As presented in Table 1 and Figure 1, for the reference treatment i.e., IR every 8 h dosing, the plasma amitriptyline concentrations increased rapidly after the second hour of each of the first two doses and a mean maximum concentration of 19 ng ml−1 occurred about 6 h after dosing. The mean half-life value for amitriptyline following the every 8 h treatment was 19.4 h and the corresponding AUC(0, ∞) was 661 ng ml−1 h.
Table 1.
Pharmacokinetic parameters of amitriptyline following administration of amitriptyline: OROS® (amitriptyline hydrochloride), immediate release every 8 h (IR every 8 h) and immediate release (IR) at nighttime. Values are mean (s.d.).

Figure 1.
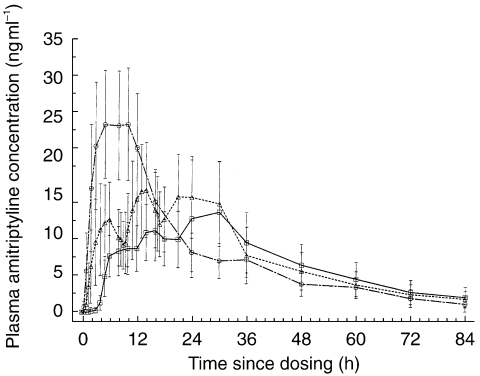
Mean (s.d.) observed plasma amitriptyline concentrations following administration of OROS® (□, amitriptyline hydrochloride), immediate release every 8 h (▵, IR every 8 h) and immediate release at nighttime (○) (n =14).
Following the OROS® (amitriptyline hydrochloride) administration, the plasma amitriptyline concentrations increased slowly, with a mean maximum concentration of 15.3 ng ml occurring at 25.7 h (tmax) and the half-life value of amitriptyline was reported to be 20.4 h. The mean AUC(0, ∞) was 593 ng ml−1 h. Similarly, following the nighttime dosing the mean maximum amitriptyline concentration was 26.8 ng ml−1, and the corresponding AUC(0, ∞) was 688 ng ml−1 h. The relative amitriptyline bioavailability with OROS® (amitriptyline hydrochloride) compared with IR nighttime and IR every 8 h treatments were 89% (90% CI 77%–102%) and 95% (90% CI 82%–108%), respectively.
The plasma nortriptyline concentrations exhibited a similar pattern to the parent drug amitriptyline after the three treatments, with maximal nortriptyline concentration of 12 ng ml−1 occurring after the IR nighttime treatment and the most delayed tmax of 34.6 h observed with OROS® (amitriptyline hydrochloride) treatment (Table 2 and Figure 2). The mean apparent elimination half-life values for nortriptyline were similar among all three treatments and ranged from 33 to 44 h. The mean nortriptyline AUC(0,t) was the highest following the nighttime treatment: 498 ng ml−1 h, as compared with 329 ng ml−1 h following OROS® (amitriptyline hydrochloride) treatment.
Table 2.
Pharmacokinetic parameters of nortriptyline following administration of amitriptyline: OROS®(amitriptyline hydrochloride), immediate release every 8 h (IR every 8 h) and immediate release (IR) at nighttime. Values are mean (s.d.).

Figure 2.

Mean (s.d.) observed plasma nortriptyline concentrations following administration of OROS® (□, amitriptyline hydrochloride), immediate release every 8 h (▵, IR every 8 h) and immediate release at nighttime (○) (n=14).
The mean metabolite-to-drug AUC(0, ∞) values for the OROS® (amitriptyline hydrochloride), IR every 8 h, and IR nighttime treatments were 0.91, 1.02, and 1.0, respectively, and were not significantly different from each other (P=0.37). Therefore, the extent of formation and elimination of nortriptyline was similar after each of the three treatments.
Anticholinergic effects
The mean ratings for dry mouth during the IR every 8 h and OROS® (amitriptyline hydrochloride) treatments were not significantly different from baseline or from each other. However, the mean ratings during the IR nighttime treatment were significantly higher than those associated with the other two treatments and the mean baseline values except 24 h after administration.
Mean weights of saliva produced after stimulation during OROS® (amitriptyline hydrochloride) and IR every 8 h treatments were not significantly different from each other or from the mean baseline values for most periods analysed. During IR nighttime treatment, the mean amount of saliva produced was significantly less than at baseline or during the other two treatments for most periods. The mean weight of saliva produced was observed to be the lowest following the IR nighttime dosing with a drop of 65% from baseline at 8 h after dosing, which was sustained for about 16 h after dosing when it was still only 50% of the baseline observation.
The mean drowsiness values for the OROS® (amitriptyline hydrochloride) and the IR every 8 h treatments were not significantly different from each other but were significantly increased from baseline at the 8 h measurement.
A minor, clinically insignificant decrease in visual acuity was observed after each of the three treatments as compared with baseline values. Seven subjects during OROS® (amitriptyline hydrochloride) and IR every 8 h treatments and five subjects during the IR nighttime treatments experienced this effect.
Concentration-effect relationship
After the IR nighttime dosing, the peripheral anticholinergic effect (saliva production) was most reduced (65%) from baseline at the 8 h measurement when the amitriptyline concentration was about 25 ng ml−1; this reduction was sustained until 16 h. In contrast, after OROS® (amitriptyline hydrochloride) and IR every 8 h dosing, the decrease in saliva production was less profound, and plasma drug concentrations were in the 7–15 ng ml−1 range. Similarly, highest dry mouth ratings were observed after nighttime dosing and were statistically different from baseline complementing the observation of decreased saliva production after the IR nighttime dosing of amitriptyline.
In summary, the subjective ratings for each of the anticholinergic effects such as dry mouth, drowsiness and saliva weights vs plasma amitriptyline concentration profile for each treatment exhibited similar pattern and were almost identical for most of the subjects. The mean weight of saliva produced decreased, and the mean of the subjective scores for dry mouth and drowsiness increased as plasma amitriptyline concentrations increased. None of the anticholinergic effects appeared to be correlated with the plasma nortriptyline concentrations.
The plasma amitriptyline concentrations and the corresponding anticholinergic effects of all treatments were pooled together to get a better estimate of the model parameters. The pooled data were fitted to the sigmoidal Emax model, and the parameters are listed in Table 3. As shown in Figure 3, saliva weight measurements and dry mouth assessments correlated well to plasma amitriptyline concentrations, whereas drowsiness assessments did not. The plasma drug concentration required for 50% of the maximal dry mouth effect was estimated to be 30 ng ml−1, which is very close to the mean Cmax value observed after the nighttime dose of 3×25 mg IR tablet (26.8 ng ml−1). This implies that the observed dry mouth effect is only half of the maximal effect. Based on the best-fit model, the maximum drop in saliva would be to 0.9 g, at a plasma drug concentration of about 30 ng ml−1.
Table 3.
Estimates of pharmacokinetic-pharmacodynamic parameters for the anticholinergic effects following the three study treatments (n=14). Data are presented as mean, where E0 is the baseline value, Emax is the maximal effect (weight or rating) observed, EC50 is the plasma drug concentration required to produce 50% of the maximal effect, and γ is the Hill coefficient.

Figure 3.

Predicted and mean observed plasma amitriptyline concentrations and anticholinergic effects following the three study treatments: OROS® (○, amitriptyline hydrochloride), immediate release every 8 h (▵, IR every 8 h) and immediate release at nighttime (•, IR HS).
Alternatively, comparison of the slopes of the z-score values and plasma amitriptyline concentrations for each of the anticholinergic effects, showed that although the slopes were similar for all three effects, the standard error of the slope for drowsiness vs concentration was the highest and least correlating compared with that of the other two effects (Figure 4). The range of amitriptyline concentration for drowsiness comparison was too narrow compared with that of other anticholinergic effects, to establish a reasonable concentration-effect relationship. In contrast, the dry mouth and saliva weight effects correlated well with plasma drug concentration and seemed to be similarly sensitive to changes in drug concentration.
Figure 4.

Relationship of plasma amitriptyline concentrations to the normalized anticholinergic effects following the three study treatments: OROS® (amitriptyline hydrochloride), immediate release every 8 h (IR every 8 h) and immediate release of night time (IR HS).
Discussion
The sustained-release characteristic of OROS® (amitriptyline hydrochloride) was confirmed by the relatively flat plasma concentration of amitriptyline for 36 h after dosing. The absorption of amitriptyline was not only sustained but also delayed, with undetectable amitriptyline concentrations for 4–6 h after dosing, which was attributed to the slow release characteristics of amitriptyline from OROS®. A similar pattern was also observed for nortriptyline. After IR nighttime dosing, the mean plasma amitriptyline concentration increased rapidly, peaked at 6 h (27 ng ml−1), and was maintained consistently higher compared with the other treatments for a period of 16–24 h. Minimal change in the drug to metabolite ratio was observed for the two formulations in the study. Amitriptyline undergoes extensive hepatic metabolism following IR administration, however, due to the extended release characteristics of OROS® it seems the extent of metabolism is independent of the site of release of the drug from the formulation, thereby resulting in similar drug to metabolite ratio. However, the three treatments results in very different concentration effect time profiles, this could be due to the sensitivity of these anticholinergic effects to the drug concentration in the body. The peripheral anticholinergic effect (decreased saliva production) was greatest after the IR nighttime dosing, with a 65% drop from baseline in mean saliva production at 8 h after dosing, when the plasma amitriptyline concentration was about 25 ng ml−1. This effect was sustained until 16 h after dosing, when the drop in saliva production was still at 50% of the baseline observation; this is consistent with reports by Schulz et al. [2] after a single 80 to 100 mg dose of amitriptyline to normal healthy subjects. In contrast, after the OROS® (amitriptyline hydrochloride) and IR every 8 h dosing, the decrease in saliva production was not as profound and statistically not different from baseline after 8 h following dosing, when the plasma amitriptyline concentrations were maintained in the 7–15 ng ml−1 range.
The overall concentration-effect relationship (using EC50 and (values) is shown in Figure 3, the mean observed data are superimposed on the predicted concentration-effect curve. This demonstrates that the prediction of the mean parameter estimates is reasonably accurate. The minimum saliva rate that could be reached is 0.9 g at a plasma amitriptyline concentration of about 30 ng ml−1. Similarly, the plasma drug concentration required for 50% of the maximum dry mouth effect was estimated to be 30 ng ml−1, which correlates well with the higher incidence of dry mouth ratings for the IR nighttime treatment, because the plasma concentration after this treatment is maintained at a relatively high level for a longer period.
OROS® (amitriptyline hydrochloride) administration resulted in lower and relatively well sustained plasma amitriptyline concentrations compared with the other two treatments in this study. Based on the concentration effect analysis, it would be reasonable to anticipate a lower incidence of unwanted anticholinergic effects after OROS® administration compared with IR nighttime treatment. This finding was also confirmed following multiple dosing of OROS® and IR [11]. Better patient compliance and greater tolerability because of a lower incidence of adverse effects are possible benefits of OROS® formulation.
In conclusion, based on this feasibility study the relative bioavailability of OROS® (amitriptyline hydrochloride) was similar to the two IR treatments and nortriptyline formation also appeared to be similar amongst the treatments. Some of the anticholinergic side-effects seem to be concentration-related so those side-effects may be reduced with a controlled release formulation. However, these conclusions are based on a study in a small group of healthy volunteers; the true clinical benefits of a controlled release dosage formulation need to be demonstrated in patients.
References
- 1.Dollery Sir Colin., editor. Therapeutic Drugs. Edinburgh: Churchill Livingstone; 1991. pp. A95–A100. [Google Scholar]
- 2.Schulz P, Turner-Tamiyasu K, Smith G, et al. Amitriptyline disposition in young and elderly men. Clin Pharmacol Ther. 1983b;33:360–366. doi: 10.1038/clpt.1983.46. [DOI] [PubMed] [Google Scholar]
- 3.Cassano GB, Sjostrand SE, Hansson E. Distribution of 14C-labelled amitriptyline in the rat brain. Psychopharmacologia. 1965;8:12–22. doi: 10.1007/BF00405357. [DOI] [PubMed] [Google Scholar]
- 4.Bowden CL, Koslow SH, Hanin I, Mass JW, Davis JM, Robins E. Effects of amitriptyline and imipramine on brain amine neurotransmitter metabolites in cerebrospinal fluids. Clin Pharmacol Ther. 1985;37:316–324. doi: 10.1038/clpt.1985.46. [DOI] [PubMed] [Google Scholar]
- 5.U’Prichard DC, Greenberg DA, Heninger GR. Tricyclic antidepressants: therapeutic properties and affinity for (-monoadrenergic receptor binding sites in the brain. Science. 1978;199:197–198. doi: 10.1126/science.202024. [DOI] [PubMed] [Google Scholar]
- 6.Richelson E. Tricyclic antidepressants and H1 receptors. Mayo Clin Proc. 1979;54:669–674. [PubMed] [Google Scholar]
- 7.Nakano S, Hollister LE. Chronopharmacology of amitriptyline. Clin Pharmacol Ther. 1983;33:453–459. doi: 10.1038/clpt.1983.61. [DOI] [PubMed] [Google Scholar]
- 8.Weise CC, Stein MK, Pereira-Ogan J, Csenalosi I, Rickels K. Amitriptyline once-daily vs. three times daily in depressed outpatients. Arch Gen Psychiatry. 1980;37:556–560. doi: 10.1001/archpsyc.1980.01780180069007. [DOI] [PubMed] [Google Scholar]
- 9.Ghose K, Coppen A, Turner P. Autonomic actions and interactions of mianserin hydrochloride (Org. GB 94) and amitriptyline in patients with depressive illness. Psychopharmacol. 1976;49:201–204. doi: 10.1007/BF00427291. [DOI] [PubMed] [Google Scholar]
- 10.Knorring L, Mornstad H, Forsgren L, Holmgren S. Acute effects of different antidepressant drugs on saliva secretion and accommodation range. Pharmacopsychiatry. 1986;19:106–110. doi: 10.1055/s-2007-1017165. [DOI] [PubMed] [Google Scholar]
- 11.Gupta S, Shah J, Guinta D, Hwang S. Multiple-dose pharmacokinetics and pharmacodynamics of OROS and immediate-release amitriptyline hydrochloride formulations. J Clin Pharmacol. 1998;38:60–67. doi: 10.1002/j.1552-4604.1998.tb04378.x. [DOI] [PubMed] [Google Scholar]


