Abstract
Aims
To evaluate the prevalence of transaminase elevation on placebo during Phase I trials.
Methods
Retrospective review of pooled transaminase data collected on placebo during 13 Phase I trials in 93 healthy volunteers hospitalized for 14 days, with determination of the prevalence of abnormally high values.
Results
20.4% of the 93 subjects showed at least one ALT value above the upper limit of the normal range (ULN), and 7.5% had at least one value twice ULN.
Conclusions
Laboratory safety results of Phase I trials should be interpreted with caution, in the light of data on placebo, to avoid premature discontinuation of the development of safe drugs wrongly believed to be hepatotoxic.
Keywords: healthy volunteers, Phase I, placebo, transaminases
Introduction
Hepatotoxicity is the leading reason for removal of new chemical entities (NCEs) from the market. A recent review of 29 drugs that were first approved and subsequently banned because of safety concerns showed that in eight cases (28%) liver damage was either the only reason (4 cases) or one of the reasons (4 cases) for discontinuation [1]. Of 130 drugs withdrawn from the market for safety reasons between 1964 and 1992, adverse effects on the liver were responsible in 18% of cases [2]. During drug development 29 of 320 NCEs were terminated due to clinical toxicity, including nine (31%) due to effects on the liver [3]. Information from preclinical studies were predicting possible liver effects in 4 out of these 9 drugs.
These data illustrate both the importance of detecting potential hepatotoxic effects of NCEs and the possibility of false-negative results during preclinical and clinical tests for hepatotoxicity. One of the key objectives of Phase I trials is to assess the safety of NCEs in humans, and in particular to document the absence of hepatotoxicity. Any significant increase in Alanine Amino Transferase (ALT) during this early phase, most notably in repeated-dose studies in healthy volunteers may lead to discontinuation of development of the drug. However, ALT elevation to levels above the upper limit of normal (ULN) can occur in subjects treated only with placebo. To assess the prevalence of this phenomenon and try to understand its significance, we established a database including ALT data obtained on placebo during Phase 1 trials sponsored by Synthélabo Recherche.
Methods
All the studies included in the database fullfilled the following criteria: tolerability trials of NCEs, placebo arm, repeated dosing for at least 14 days, strictly screened healthy male volunteers hospitalized throughout the study, and repeated transaminase determinations including baseline measurements.
The ULN for ALT varied from 30 IU l−1 to 50 IU l−1 across studies performed in different Phase I units according to the different methods in ALT assay used. To pool the data from different studies, transaminase levels were converted to multiples of the ULN. For example, if the ULN was 50 IU/l, an ALT value of 60 IU l−1 was converted to 1.2 ULN.
The healthy volunteers were all males aged 18–40 years, with no history of significant disease and normal findings from physical examination. All smoked less than 10 cigarettes per day, and smoking was prohibited during all the trials. All the volunteers were screened for morphine, amphetamines, cocaine, cannabis, benzodiazepines, and barbiturates prior to inclusion. Serologic tests for the HIV and hepatitis B virus were performed. Negative serologic tests for the hepatitis C virus was a criterion for half the study sample.
Transaminase assays were performed at preinclusion (less than 2 weeks before the start of the study), at inclusion (usually on the morning prior to the first administration) and the end-of-study evaluation.
The number of transaminase determinations done during the 14-day treatment period varied across studies, from two (usually days 7 and 14) to seven or more (in subjects with suspected transaminase elevation).
Prevalences were determined by counting each subject once, at the time point with the highest ALT level under treatment.
Results
Thirteen studies fullfilled our inclusion criteria. The prevalence of ALT elevation on placebo is shown in Table 1. Overall, 79.6% of the subjects receiving a placebo for 14 days during a Phase 1 trial had all transaminase levels below the ULN. Of the 20.4% of subjects who had at least one value above ULN, about two third (12.9% of the total) had their highest value between ULN and twice ULN, and only one third (7.5% of the total) had at least one value greater than twice ULN.
Table 1.
Prevalence of highest ALT values during 14-day tolerability Phase I studies (n = 13).

In one study (Figure 1), two subjects had values greater than three times ULN during treatment, which was predefined as the criterion for withdrawing subjects on a blind basis. During screening, these subjects had values slightly above the ULN, and should have been excluded, although baseline values were within the normal range.
Figure 1.

ALT (IU l−1) on placebo during study 9 (14 day tolerability study of an NCE). *end of treatment, **drop out.
In all the studies, subjects with abnormal ALT values underwent extensive investigation of a potential cause of ALT elevation. In particular, all subjects with ALT levels greater than twice ULN underwent an extensive viral and parasites screen covering most of the common causes of transaminase elevation, such as cytomegalovirus, Epstein-Barr virus, toxoplasmosis and Coxsackie viruses. Although this search was negative in most cases, seroconversion (IgM) was demonstrated in two subjects, providing an explanation for the transaminase elevation; for instance, subject 44 in study 8 (Figure 2) showed immunological evidence of recent mononucleosis.
Figure 2.

ALT (IU l−1) on placebo during study 8 (14 day tolerability study of an NCE). *end of treatment.
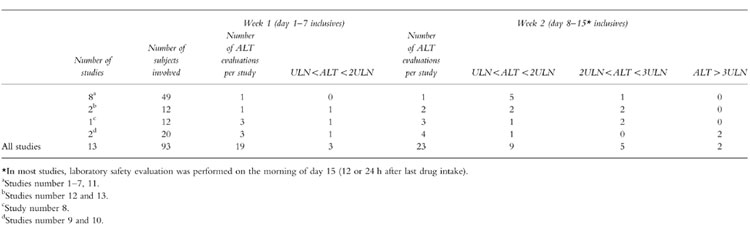
Most of the abnormal values were found well after the first week of hospitalization, as illustrated by the examples in Figures 1 and 2; cases during the first week of hospitalization were exceedingly rare (see Table 2). Some individuals did not reach the ULN but had ALT levels that tended to increase over time (see subjects 6 and 8 in study 9, Figure 1). ALT levels returned to normal within a few days of hospital discharge and discontinuation of placebo in the overwhelming majority of cases.
Table 2.
Time of appearance of highest ALT abnormal values.
Discussion
Although most investigators are aware that transaminase elevation can occur during placebo treatment very few publications on this issue are available. Our study of the Synthélabo Recherche database provides an estimate of the prevalence of transaminase elevation on placebo during Phase I trials in healthy subjects hospitalized for a fixed period of 14 days. Kobayashi and coworkers [4] found a lower prevalence of 12.5%, but their review included studies with treatment duration of only 7 days. Merz et al. [5] found a prevalence of over 20% in a review including studies with placebo treatment durations of 7 to 31 days (mean: 13.8 days).
The duration of hospitalization may have a major impact on the prevalence of transaminase elevation on placebo, since most cases occur during the second week of hospitalization. One factor may be an imbalance between reduced physical activity and maintained calorie intake. Kanamaru et al. [6] reported ALT elevation in a group of subjects who rested for 7 days, contrasting with an absence of significant changes in a control group of subjects who engaged in daily physical exercise. However, other studies involving bed rest for a week or more (spatial medecine, metabolic studies) did not report ALT elevation [7].
The composition of the diet may play a role. Porikos [8] showed that a combination of excess calories and a high sucrose intake was associated with enzyme elevation. The role of carbohydrates was further confirmed by an 8-day three-way cross-over study in 12 healthy subjects comparing a high fat diet (58% fat) providing 4500 kcal day−1, a high carbohydrate diet (32% sugar, 27% CHO) providing 4400 kcal day−1, and a ‘healthy’ diet providing 1900 kcal day−1 [9]. Whereas liver function tests remained normal in all the subjects on the healthy and high fat diets, significant increases in ALT levels were observed in five of the 12 subjects on the high carbohydrate diet. In all our Phase I trials, the diet provided less than 2500 kcal day−1 with a reasonable proportion of carbohydrates, but the carbohydrate intake was not closely maintained within predefined limits.
Kobayashi and colleagues [4] concluded that factors associated with an increased risk of transaminase elevation were a baseline ALT level lower than the AST level and/or a high body mass index. When applied to our pooled studies, these factors failed to predict transaminase elevation on placebo. More recently, Merz et al. [5] performed a meta-analysis of 13 placebo-controlled multiple-dose Phase I studies in which subjects with ALT elevation on placebo were compared with those with no clinically significant changes in ALT levels. Baseline ALT values greater than 10 IU l−1 and a baseline AST value lower than the baseline ALT value had a sensitivity of 73% and a specificity of 74% for predicting ALT level elevation during the trial. In another study [10], the same group used a probabilistic neural network to predict ALT elevation. Using baseline values of eight routine laboratory safety parameters (platelet count, total bilirubin, ALT, AST, potassium, creatinine, urea, and uric acid), the network allowed general prediction of increasing ALT levels during the trial: sensitivity was 100% and specificity was 85%.
Little is known about intrasubject variability of liver enzyme levels [11]. In 8 out of 13 studies presented here, only two measurements of ALTs (day 7 and 14 or 15) were performed under treatment while in studies 3, 4, 5 and up to 7 serial measurements were made in order to monitor the abnormal changes. Therefore, the findings may be largely if not completely explained by the multiplicity of the sampling. In order to weigh the potential role of the multiple measurements in the observed phenomenon, several considerations need to be taken into account. Firstly, these serial measurements are not independent because they are performed in the same individuals and this lack of independence is illustrated by Figure 1. Although the probability of obtaining at least one abnormal value after a series of samples cannot be made by multiplying the probability of each sampling, the repeated measurements increase the probability of observing an ‘abnormal’ value: 11 subjects with abnormal data out of 61 (18%) were obtained from the studies where 2 or 3 ALT evaluations were performed while 8 subjects with abnormal data out of 32 (25%) came from studies with 6–7 ALT evaluations. Secondly, the time of appearance of the abnormal values may play a much more important role than the multiplicity of sampling. As illustrated in Table 2, abnormal values occurred more frequently during the second week of treatment despite of a similar overall number of ALT evaluations in the first (n = 16) and the second week (n = 23). This was still the case when considering only the mild increases (<2ULN) which are more likely to be found ‘by chance’: 3 cases during the first week vs 9 cases during the second week. Moreover, when considering only the 8 studies with two assessments (one on day 7 and one on day 14, with a theoretically equal chance of an abnormal result on each week) 100% of the abnormal values (6 cases) were found during the second week of treatment. Thirdly and probably most importantly, it is clear that the patterns of abnormal tests in some subjects seem to indicate a study related role and not a chance observation due to the repetition of the tests in the same subject.
In summary, it is now recognized that transaminase elevation in placebo-treated subjects during Phase I trials lasting longer than seven days are not an artefact due to ‘bad luck’, ‘‘unhealthy’’ volunteers, or ‘poor Phase I unit organization’, but a real phenomenon that deserves further study. Its prevalence needs to be determined and its potential causes elucidated via prospective studies investigating characteristics of healthy volunteers at baseline, diet during the trial, physical activity vs. inactivity, and other potential factors. The potential role of the multiplicity of the sampling must be considered.
In conclusion, the laboratory safety results of Phase I trials should be interpreted with caution, in the light of data in the placebo arm, to avoid premature discontinuation of the development of NCEs wrongly believed to be hepatotoxic based on transaminase elevations due to nondrug-related causes, such as experimental conditions.
References
- 1.Bakke OM, Manocchia M, De F, Kaitin KI, Lasagna L. Drug safety discontinuations in the United Kingdom. United States, Spain from 1974 through 1993: a regulatory perspective. Clin Pharmacol Ther. 1995;58:108–17. doi: 10.1016/0009-9236(95)90078-0. [DOI] [PubMed] [Google Scholar]
- 2.Spriet-Pourra C, Auriche M. Scrip Reports/ Richmond, Surrey, United Kingdom: PJB Publications; 1988. Drug withdrawal from sale: an analysis of the phenomenon and its implications. [Google Scholar]
- 3.Lymley C. Clinical toxicity: could it have been predicted? Pre-marketing experience. In: Lumley CE, Walker SR, editors. Animal Toxicity Studies: Their Relevance for Man. Lancaster, UK: Quay; 1990. pp. 49–56. [Google Scholar]
- 4.Kobayashi M, Yamada N, Shibata H, Nishikawa T. Elevated serum transaminase values in volunteers after administration of placebo in a phase I study. Jpn J Clin Pharmacol Ther. 1993;24:493–6. [Google Scholar]
- 5.Merz M, Seiberling M, Höxter G, Hölting M, Wortha HP. Elevation of liver enzymes in multiple dose trials during placebo treatment: are they predictable? J Clin Pharmacol. 1997;37:791–8. doi: 10.1002/j.1552-4604.1997.tb05626.x. [DOI] [PubMed] [Google Scholar]
- 6.Kanamaru M, Nagashima S, Uematsu T, Nakashima M. Influence of 7-day hospitalization for phase I study on the biochemical laboratory tests of healthy volunteers. Jpn J Clin Pharmacol Ther. 1989;20:493–4. [Google Scholar]
- 7.Mikines KJ, Dela F, Tronier B, Galbo H. Effect of 7 days of bed rest on dose–response relation between plasma glucose and insulin secretion. Am J Physiol. 1989;257:E43–E48. doi: 10.1152/ajpendo.1989.257.1.E43. (Endocrinol Metab; 20): [DOI] [PubMed] [Google Scholar]
- 8.Porikos KP, Van TB. Diet-induced changes in serum transaminase and triglyceride levels in healthy adult men—Role of sucrose and excess calories. Am J Med. 1983;75:624–30. doi: 10.1016/0002-9343(83)90444-8. [DOI] [PubMed] [Google Scholar]
- 9.Purkins L, Eve MD, Love ER, Smith C, Cowan C. The effect of diet on selected liver function tests. Eur J Clin Pharmacol. 1997;52(Suppl.):A115. [Google Scholar]
- 10.Merz M, Seiberling M. Increasing ALT levels in clinical trials: prediction by means of artificial neural networks. Eur J Clin Pharmacol. 1997;52(Suppl.):A97. [Google Scholar]
- 11.Arnold J, Berger A, Leese P, Hayden D. Normal intra-subject variation in repeated measurement of liver enzymes and other serum chemistries. Clin Pharmacol Ther. 1987;41:207. [Google Scholar]


