Abstract
Aims
To measure morphine and morphine-6-glucuronide in the plasma and cerebrospinal fluid of children following a single intravenous dose of morphine.
Methods
Twenty-nine paired samples of cerebrospinal fluid and plasma were collected from children with leukaemia undergoing therapeutic lumbar puncture. An intravenous dose of morphine was administered at selected intervals before the procedure. Concentrations of morphine and morphine-6-glucuronide (M6G) were measured in each sample. Morphine was measured using a specific radioimmunoassay (r.i.a.) and M6G was measured using a novel enzyme-linked immunosorbent assay (ELISA).
Results
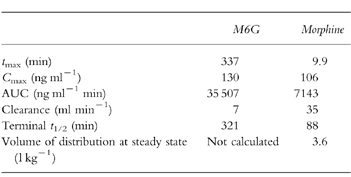
The ELISA for measuring M6G was highly sensitive. The intra-and interassay variations were less than 15%. Using a two-compartment model for plasma morphine, the area under the curve to infinity (AUC, 7143 ng ml−1 min), volume of distribution (3.6 l kg−1) and elimination half-life (88 min) were comparable with those reported in adults. Clearance (35 ml min−1) was higher than that in adults. Morphine-6-glucuronide was readily synthesized by the children in this study. The elimination half-life (321 min) and AUC (35507 ng ml−1 min) of plasma M6G were much greater than those of morphine.
Conclusions
Extensive metabolism of morphine to M6G in children with cancer has been demonstrated. These data provide further evidence to support the importance of M6G accumulation after multiple doses. There was no evidence that morphine passed more easily into the CSF of children than adults.
Keywords: cerebrospinal fluid, children, morphine-6-glucuronide, morphine, plasma
Introduction
It is now clear that both morphine and its metabolite morphine-6-glucuronide are as pharmacologically active in the child as in the adult. Though exact relative potency is not certain, M6G is thought to be very much more potent than morphine as an analgesic[1–8], and because of differences in the receptor interactions of the two drugs, may have fewer adverse effects[8–12].
The opioid receptors through which the two compounds act are found primarily in the central nervous system (CNS)[4, 13, 14]. A more precise estimate of their pharmacological influence might therefore be gained by measuring concentrations in the cerebrospinal fluid (CSF), a more accurate reflection of access of the ligands to CNS opioid receptors, rather than by simply measuring plasma concentrations. It has been assumed that the blood–brain barrier of children is more easily permeable to opioids than that of adults[15], but this has not been proven.
One reason for the lack of data in children is that it is difficult to obtain appropriate samples of CSF. Children receiving treatment for leukaemia and who require repeated intrathecal chemotherapy undergo frequent lumbar puncture (LP), and morphine is often prescribed. These patients also have indwelling central venous access. In this patient group, it was possible to measure morphine and M6G in both plasma and CSF with minimal intervention.
Methods
Study subjects
The study was reviewed by the Ethics Committee of the Royal Marsden Hospital and The Institute of Cancer Research. The parents of 27 patients were approached. Of these, 10 declined to participate. In all, 17 patients were studied on a total of 29 occasions. Ten samples were obtained from boys and 19 from girls. The median age was 5.5 years (range 1.4–15.9 years) and the median weight was 20.0 kg (range 9.3 kg to 54.5 kg).
Study protocol
Patients between the ages of 1 and 18 years who were scheduled to undergo routine lumbar puncture (LP) as part of treatment for acute leukaemia were eligible. They were excluded if there was a contraindication to opioid therapy or LP. All patients had central venous access. Morphine at a dose of 250 μg kg−1, 125 μg kg−1 or 60 μg kg−1 was given by intravenous infusion over 15 min at selected intervals before LP was carried out under general anaesthesia. At the time of the LP a sample of CSF not exceeding 0.5 ml in volume was set aside for analysis, and a simultaneous sample of blood (not exceeding 0.5 ml kg−1 in volume) was removed from the central venous line. Plasma samples were immediately centrifuged and plasma and CSF were stored at −20° C.
Assay methods
Morphine
Samples were assayed for morphine using a specific r.i.a.[16] with tritiated morphine radiolabel. The lower limit of detection for the assay is 0.28 ng ml−1 (0.88 nmol l−1).
Morphine-6-glucuronide
Samples were assayed for M6G using an antigen coated competitive enzyme-linked immunosorbent assay (ELISA) which was an adaptation of the r.i.a. previously described[17]. Ninety-six well microtitre plates (Dynatech Immulon 2) were coated with aminobutylnormorphine-6 glucuronide ovalbumin conjugate (0.05 μg ml−1, 150 μ l/well in 70 mm sodium barbitone) for 150 min at 37° C. After washing three times with 0.01% Tween20/water, 150 μl 5% lactose was added to each well. The plates were dried amd stored at 4° C with desiccant. Under these conditions the plates were stable for eight weeks.
The plates were washed before use. Antiserum (diluted 1:30 000), standard M6G (0.01–500 ng ml−1), quality controls and samples were diluted in PBS buffer with gelatin and Tween 20 (37 mm NaCl, 8 mm Na2HPO4, 2.7 mm KCl, 1 g l−1 gelatin, 0.1 g l−1 thiomersal and 0.1% Tween 20). Non-specific binding and zero standard controls (B0) were included on each plate and all standards, samples and controls were assayed in duplicate. Plates were incubated for 1 h at 37° C. Antibodies bound to the plate were visualized by incubation with goat antirabbit IgG-horseradish peroxidase conjugate (Sigma) for 1 h and subsequent reaction with tetramethyl benzidine/hydrogen peroxide substrate. After acidification the colour was read at 450 nm. Concentration of M6G was determined from standard curves using a smoothed spline data reduction program (RIASMART, Canberra-Packard).
Specificity of the antisera was as previously described[17]. Additionally, morphine-3,6, diglucuronide was tested and showed no significant cross-reaction (0.008%).
Theoretical assay sensitivity, defined as a two-standard deviation fall from B0, was 0.04 ng ml−1. Plasma samples were diluted at least 20 times to eliminate matrix effects (detection limit 0.8 ng ml−1). There was no matrix effect with CSF (detection limit 0.04 ng ml−1). Interassay variation of the standards was 7.5%. Sample interassay variation was 14.5% (12.4±1.8 ng ml−1). Mean recovery of 50 ng ml−1 M6G added to plasma was 102.5% (s.d. 8.6, n =7). As reported previously for the r.i.a.[17], addition of increasing concentrations of morphine and M3G had no effect on the recovery of M6G.
Comparative analysis of the same samples using h.p.l.c.[18] showed that there was a linear relationship across the range of results ( y =1.74x−3.3, r =0.93, n =49). The relationship of h.p.l.c. data with ELISA data was thus very similar to the relationship of h.p.l.c. data with r.i.a.[17].
Pharmacokinetic analysis
Morphine data were standardized to a dose of 250 μg kg−1 and analysed as though all came from a single patient using WinNonLin, pharmacokinetic model 10. This was a two-compartment, i.v. infusion model (over 15 min), with macro-constants, no lag time and 1/y^ error weighting. The equation of the curve was in the form ct =A(e−αt−e−αt*)+B(e−βt−e−βt*) where t* =infusion time (min) Initial estimates were A =1000, α =0.03, B =0.07, β =0.01.
A noncompartment model was used to estimate AUC and terminal elimination half-life (λz) for M6G in plasma. A two-compartment model (Figure 1) fitted the data for plasma morphine. Correlation between observed and predicted concentrations was 0.9213 (Akaike and Schwartz Criteria 105 and 103, respectively).
Figure 1.
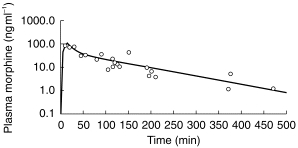
Concentration of morphine in plasma (ng ml−1) following administration of morphine to children with leukaemia. —predicted concentrations, ○ =observed concentrations.
Results
Pharmacokinetic parameters for plasma and CSF morphine and CSF are shown in Table 1. The maximum CSF concentration, and time to maximum concentration were, respectively, 16 ng ml−1 and 125 min for morphine, and 34 ng ml−1 and 470 min for M6G.
Table 1.
Comparison of pharmacokinetic parameters of plasma M6G and morphine (all data standardised to 250 μg kg−1).
In the first 30 min after intravenous morphine, plasma concentrations of morphine were more than 10 times those in CSF. After 2 h, plasma and CSF concentrations were approximately equal. The plasma concentration of M6G generally exceeded that of CSF (figure 2b). Initially the ratio was high, reaching a maximum of nearly 500:1 after 105 min, but then declined until four hours, after which plasma and CSF concentrations declined in parallel such that plasma concentrations remained approximately 10 times those in the CSF.
Figure 2.
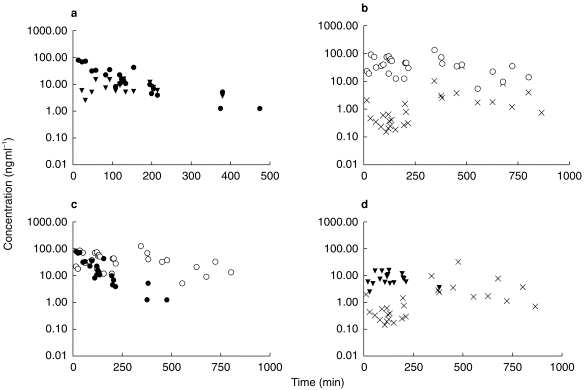
Comparison of (a) morphine concentrations in plasma and CSF. (b) morphine-6-glucuronide concentrations in plasma and CSF. (c) morphine and M6G concentrations in plasma. (d) morphine and M6G concentrations in CSF. • =plasma morphine, ▾ =CSF morphine, ○ =plasma M6G, x =CSF M6G.
In the plasma, morphine concentrations initially exceeded those of the metabolite. The ratio fell over the course of the first 1–2 h, whereafter plasma M6G exceeded morphine. In the CSF, concentrations of morphine exceeded those of the metabolite at all points where measurements of both were available. Morphine appeared to be eliminated more quickly from CSF than M6G (figure 2d).
Discussion
The ELISA used in this study was a nonradioactive adaptation of a previously described immunoassay[17]. It was particularly suited to this paediatric study, where sample volumes were low, by virtue of its exquisite sensitivity. Specificity and correlation with h.p.l.c. were similar to those described for the r.i.a.
Morphine in plasma and CSF
This study suggests that the plasma kinetics of morphine (Table 1, Figure 1) in children over 1 year old are little different from those reported in adults. As in other studies in children[19–28], the mean clearance of morphine was rather higher than that in adult studies[29–32], but half-life, volume of distribution and AUC were all similar to those of adults. Since it was possible to standardize the data by weight and dose and obtain a credible pharmacokinetic profile, the volume of distribution does not appear to alter significantly through childhood after a year old. This supports evidence from other paediatric studies[27], and suggests that in calculating a dose for children, the current extrapolation on which paediatric doses are based (the same dose per kg weight as that of an adult) is appropriate. The elimination half-life, from this study, is also similar to that of adults, suggesting that the dosage interval used in healthy adults also remains appropriate for children over a year old. However, these data do not exclude the possibility that a child’s response to a given plasma concentration of morphine is different from that of an adult.
Previous studies of morphine kinetics in children[19, 22, 24, 25, 27, 33–35] have used one-, two- or three-compartment models. Our data were better fitted by a two-compartment model than a single-compartment model (data not shown).
Modelling based on population pharmacokinetics might have been considered preferable to standardization of the morphine data and analysis as though from a single patient. However, such analysis requires prior knowledge of the study population that is not available for children, and although numbers of patients in this study compared favourably with other paediatric studies, they are still smaller than those ideally required for population analyses. Furthermore, because the timing of sample collection was constrained by clinical expediency, the number of points for each patient was small and there were few data at each individual time point. There was also insufficient prior knowledge for detailed modelling of plasma metabolite data. A noncompartment approach was therefore used to estimate AUC and elimination half-life.
This study provided no evidence that the blood–brain barrier of children over 1 year old is more permeable to morphine than that of an adult. The ratio of plasma:CSF concentrations of morphine at equilibrium after 2 h was 1:0.9, similar to that of adults[32, 36–38]. The time taken to reach maximum concentration in the CSF was slightly longer in one adult study[32], but this is probably the result of using intramuscular rather than intravenous morphine in that study. An equilibrium was reached rapidly, at which CSF and plasma concentrations of morphine were approximately equal.
Morphine-6-glucuronide in plasma and CSF
The capacity to glucuronidate morphine has been shown in the 15-week fetus[39] and in preterm and full-term neonates[26, 33, 34, 40–43]. In this study, M6G was detectable in the earliest plasma and CSF samples, analysed 11 min after morphine.
The time taken for the plasma concentration of M6G to exceed that of morphine in this study (2 h) was similar to that in an adult study[11]. However, maximum plasma concentration of M6G, AUC for M6G in plasma and the AUC ratio of plasma M6G:morphine (molar ratio 3:1) were all significantly greater in this study than in comparable adult studies[11, 44]. This is probably due to methodological differences since both adult studies were over a shorter period and used h.p.l.c., which is less sensitive than immunoassay but also less subject to variability. Another factor may have been that all the children in this study were anaesthetized. It has been shown in adults[45] that peak concentrations and AUC of M6G in plasma are greater in anaesthetized patients. An alternative explanation is that there is a real difference between adults and children in the capacity to generate or to eliminate M6G; this possibility should be addressed in further paediatric studies.
Despite being less lipophilic than morphine, M6G was detected at 11 min, in the first CSF sample examined. The maximum concentration of M6G occurred much later than that of morphine (470 min, compared with 125 min), but was of comparable magnitude. Clinical experience suggests that the maximum benefit, as well as the most severe adverse effects, of an intravenous dose of morphine are seen well before the time to peak CSF concentration of M6G in this study. Such effects coincide rather with the time taken to peak concentration of morphine in CSF (125 min). This suggests that the effectiveness of a single intravenous dose of morphine is mediated by morphine itself, rather than by M6G. It has been argued[46–49] that after multiple doses the clinical effects are those of accumulating M6G. If adverse effects are more related to morphine than to M6G[8–12], these findings would explain the observation that adverse effects are most severe during the first 2 days of ongoing opioid therapy[50].
Conclusions
The data from this study suggest that the plasma kinetics of morphine in paediatric cancer patients over 1 year old are little different from those of healthy adults. The study is the first to measure morphine and M6G simultaneously in the CSF of children; the data indicate that the access of morphine and its active metabolite M6G into the CSF of children is similar to that seen in adults. There is no evidence to support the belief that the child’s blood–brain barrier offers less resistance to the passage of opioids than that of an adult.
Acknowledgments
The authors are grateful to Mr Simon Joel at the Barry Reed Oncology Laboratories at St Bartholomew’s Hospital, London for his help in providing comparative h.p.l.c. data, and to Mrs Gina Dick, research sister at the Royal Marsden Hospital for her invaluable help in the project.
References
- 1.Shimomura K, Kamata O, Ueki S, Ida S, Oguri K. Analgesic effect of morphine glucuronides. Tohoku J Exp Med. 1971;105:45–52. doi: 10.1620/tjem.105.45. [DOI] [PubMed] [Google Scholar]
- 2.Pasternak GW, Bodnar RJ, Clark JA, Inturrisi CE. Morphine-6-glucuronide, a potent mu agonist. Life Sci. 1987;41:2845–2849. doi: 10.1016/0024-3205(87)90431-0. [DOI] [PubMed] [Google Scholar]
- 3.Abbott FV, Palmour RM. Morphine-6-glucuronide: analgesic effects and receptor binding profile in rats. Life Sci. 1988;43:1685–1695. doi: 10.1016/0024-3205(88)90479-1. [DOI] [PubMed] [Google Scholar]
- 4.Paul D, Standifer KM, Inturrisi CE, Pasternak GW. Pharmacological characterization of morphine-6-beta glucuronide, a very potent morphine metabolite. J Pharmacol Exp Ther. 1989;251:477–483. [PubMed] [Google Scholar]
- 5.Gong QL, Hedner T, Hedner J, Bjorkman R, Nordberg G. Antinociceptive and ventilatory effects of the morphine metabolites: morphine-6-glucuronide and morphine-3-glucuronide. Eur J Pharmacol. 1991;193:47–56. doi: 10.1016/0014-2999(91)90199-z. [DOI] [PubMed] [Google Scholar]
- 6.Osborne R, Joel S, Trew D, Slevin M. Analgesic activity of morphine-6-glucuronide. Lancet. 1988;i:828. doi: 10.1016/s0140-6736(88)91691-1. [DOI] [PubMed] [Google Scholar]
- 7.Foley KM, Portenoy RK, Thaler HT, Friedlander-Klar H, Inturrisi CE. The metabolite, morphine-6-glucuronide, contributes to the analgesic effects of morphine. Proc Am Soc Clin Oncol. 1991;10:97. doi: 10.1038/clpt.1992.42. [DOI] [PubMed] [Google Scholar]
- 8.Osborne R, Thompson P, Joel S, Trew D, Patel N, Slevin M. The analgesic activity of morphine-6-glucuronide. Br J Clin Pharmacol. 1992;34:130–138. doi: 10.1111/j.1365-2125.1992.tb04121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis CL. Toxicity and pharmacokinetics of morphine and morphine-6-glucuronide. Br J Anaesthesia. 1991;67:362–363. doi: 10.1093/bja/67.3.362-b. [DOI] [PubMed] [Google Scholar]
- 10.Peat SJ, Hanna MH, Knibb AA, Ponte J. Morphine-6-glucuronide: effect on ventilation in normal volunteers. Pain. 1991;45:101–104. doi: 10.1016/0304-3959(91)90170-3. [DOI] [PubMed] [Google Scholar]
- 11.Hanna MH, Peat SJ, Knibb A, Fung C. Disposition of morphine-6-glucuronide and morphine in healthy volunteers. Br J Anaesth. 1991;64:547–550. doi: 10.1093/bja/66.1.103. [DOI] [PubMed] [Google Scholar]
- 12.Thompson PI, John L, Wedzicha JA, Slevin ML. Comparison of the respiratory depression induced by morphine and its active metabolite morphine-6-glucuronide. Br J Cancer. 1990;62:484. [Google Scholar]
- 13.Hucks D, Thompson PI, McLoughlin L, et al. Explanation at the opioid receptor level for differing toxicity of morphine and morphine-6-glucuronide. Br J Cancer. 1992;65:122–126. doi: 10.1038/bjc.1992.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasternak GW, Wood PJ. Minireview: multipe mu opiate receptors. Life Sci. 1986;38:1889. doi: 10.1016/0024-3205(86)90217-1. [DOI] [PubMed] [Google Scholar]
- 15.Yaster M, Deshpande JK, Maxwell LG. The pharmacologic management of pain in children. Comp Ther. 1989;15:14–26. [PubMed] [Google Scholar]
- 16.Chapman DJ, Joel SP, Aherne GW. Evaluation of a differential radioimmunoassay technique for the determination of morphine and morphine-6-glucuronide in human plasma. J Pharmaceut Biomed Anal. 1994;12:353–360. doi: 10.1016/0731-7085(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 17.Chapman DJ, Cross MJ, Joel SP, Aherne GW. A specific radioimmunoassay for the determination of morphine-6-glucuronide in human plasma. Ann Clin Biochem. 1995;32:297–302. doi: 10.1177/000456329503200306. [DOI] [PubMed] [Google Scholar]
- 18.Joel SP, Osborne RJ, Slevin ML. An improved method for the simultaneous determination of morphine and its principal glucuronide metabolites. J Chromatogr. 1988;430:394–399. doi: 10.1016/s0378-4347(00)83176-x. [DOI] [PubMed] [Google Scholar]
- 19.Dahlström B, Bolme P, Feychting H, Noack G, Paalzow L. Morphine pharmacokinetics in children. Clin Pharmacol Ther. 1979;26:354–365. doi: 10.1002/cpt1979263354. [DOI] [PubMed] [Google Scholar]
- 20.Chinyanga HM, Vandenberge H, Bohn D, Macleod S, Soldin S. Pharmacokinetics of morphine in young children following cardiac surgery. Anesthesiol. 1983;59:447. [Google Scholar]
- 21.Vanderberghe H, MacLeod S, Chinyanga M, Endrenyi L, Soldin S. Pharmacokinetics of intravenous morphine in balanced anesthesia: studies on children. Drug Metab Rev. 1983;14:887–903. doi: 10.3109/03602538308991414. [DOI] [PubMed] [Google Scholar]
- 22.Nahata MC, Miser AW, Reuning RH. Variation in morphine pharmacokinetics in children with cancer. Dev Pharmacol Therapeutics. 1985;8:182–188. doi: 10.1159/000457035. [DOI] [PubMed] [Google Scholar]
- 23.Greene RF, Miser AW, Lester CM, Balis FM, Poplack DG. Cerebrospinal fluid and plasma pharmacokinetics of morphine infusions in pediatric cancer patients and rhesus monkeys. Pain. 1987;30:339–348. doi: 10.1016/0304-3959(87)90022-4. [DOI] [PubMed] [Google Scholar]
- 24.Lynn AM, Slattery JT. Morphine pharmacokinetics in early infancy. Anesthesiol. 1987;66:136–139. doi: 10.1097/00000542-198702000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Olkkola KT, Maunskela E-L, Korpela R, Rosenberg PH. Kinetics and dynamics of postoperative intravenous morphine in children. Clin Pharmacol Ther. 1988;44:128–136. doi: 10.1038/clpt.1988.127. [DOI] [PubMed] [Google Scholar]
- 26.Choonara IA, McKay P, Hain R, Rane A. Morphine metabolism in children. Br J Clin Pharmacol. 1989;28:599–604. doi: 10.1111/j.1365-2125.1989.tb03548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McRorie TI, Lynn AM, Nespeca MK, Opheim KE, Slattery JT. The maturation of morphine clearance and metabolism. Am J Dis Childhood. 1992;146:972–976. doi: 10.1001/archpedi.1992.02160200094036. [DOI] [PubMed] [Google Scholar]
- 28.Robieux IC, Kellner JD, Coppes MJ, et al. Analgesia in children with sickle cell crisis: comparison of intermittent opioids versus continuous infusion of morphine and placebo-controlled study of oxygen inhalation. Pediatric Hematolgy Oncol. 1992;9:317–326. doi: 10.3109/08880019209016603. [DOI] [PubMed] [Google Scholar]
- 29.Stanski DR, Greenblatt DJ, Lowenstein E. Kinetics of intravenous and instramuscular morphine. Clin Pharmacol Ther. 1978;24:52–59. doi: 10.1002/cpt197824152. [DOI] [PubMed] [Google Scholar]
- 30.Murphy MR, Hug CC. Pharmacokinetics of intravenous morphine in patients anaesthetised with enflurane-nitrous oxide. Anesthesiol. 1981;54:187. doi: 10.1097/00000542-198103000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Aitkenhead AR, Vater M, Achola K, Cooper CMS, Smith G. Pharmacokinetics of single-dose iv morphine in normal volunteers and patients with end-stage renal failure. Br J Anaesth. 1984;56:813–819. doi: 10.1093/bja/56.8.813. [DOI] [PubMed] [Google Scholar]
- 32.Nordberg G, Borg L, Hedner T, Mellstrand T. CSF and plasma pharmacokinetics of intramuscular morphine. Eur J Clin Pharmacol. 1985;27:677–681. doi: 10.1007/BF00547048. [DOI] [PubMed] [Google Scholar]
- 33.Bhat R, Chari G, Gulati A, Aldana O, Velamati R, Bhargava H. Pharmacokinetics of a single dose of morphine in preterm infants during the first week of life. J Pediatrics. 1990;117:477–481. doi: 10.1016/s0022-3476(05)81102-3. [DOI] [PubMed] [Google Scholar]
- 34.Barrett DA, Elias-Jones AC, Rutter N, Shaw PN, Davis SS. Morphine kinetics after diamorphine infusion in premature neonates. Br J Clin Pharmacol. 1991;32:31–37. doi: 10.1111/j.1365-2125.1991.tb05609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pokela M-L, Olkkola KT, Seppälä T, Koivisto M. Age-related morphine kinetics in infants. Dev Pharmacol Ther. 1993;20:26–34. doi: 10.1159/000457538. [DOI] [PubMed] [Google Scholar]
- 36.Venn R, Michalkiewicz A, Hardy P, Wells C. Concentrations of morphine, morphine metabolites and peptides in human CSF and plasma. Pain. 1990;5:188. [Google Scholar]
- 37.Hand CW, Blunnie WP, Claffey LP, McShane AJ, McQuay HJ, Moore RA. Potential analgesic contribution from morphine-6-glucuronide in CSF. Lancet. 1987;2:1207–8. doi: 10.1016/s0140-6736(87)91341-9. [DOI] [PubMed] [Google Scholar]
- 38.Hoskin PJ, Hanks GW, Aherne GW, Chapman D, Littleton P, Filshie J. The bioavailability and pharmacokinetics of morphine after intravenous, oral and buccal administration in healthy volunteers. Br J Clin Pharmacol. 1989;27:499–505. doi: 10.1111/j.1365-2125.1989.tb05399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pacifici GM, Säwe J, Kager L, Rane A. Morphine glucuronidation in human fetal and adult liver. Eur J Clin Pharmacol. 1982;22:553–558. doi: 10.1007/BF00609630. [DOI] [PubMed] [Google Scholar]
- 40.Carrupt P-A, Testa B, Bechalany A, El N, Descas P, Perrissoud D. Morphine-6-glucuronide and morphine-3-glucuronide as molecular chameleons with unexpected lipophilicity. J Med Chem. 1991;34:1272–1275. doi: 10.1021/jm00108a005. [DOI] [PubMed] [Google Scholar]
- 41.Choonara I, Lawrence A, Michalkiewicz A, Bowhay A, Ratcliffe J. Morphine metabolism in neonates and infants. Br J Clin Pharmacol. 1992;34:434–437. doi: 10.1111/j.1365-2125.1992.tb05652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hartley R, Green M, Quinn M, Levene MI. Pharmacokinetics of morphine infusion in premature neonates. Arch Dis Childhood. 1993;69:55–58. doi: 10.1136/adc.69.1_spec_no.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hartley R, Quinn M, Green M, Levene MI. Morphine glucuronidation in premature neonates. Br J Clin Pharmacol. 1993;35:314–317. [PMC free article] [PubMed] [Google Scholar]
- 44.Osborne R, Joel S, Trew D, Slevin M. Morphine and metabolite behavior after different routes of morphine administration: demonstration of the importance of the active metabolite morphine-6-glucuronide. Clin Pharmacol Ther. 1990;47:12–19. doi: 10.1038/clpt.1990.2. [DOI] [PubMed] [Google Scholar]
- 45.Sear JW, Hand CW, Moore RA, McQuay HJ. Studies on morphine disposition: influence of general anaesthesia on plasma concentrations of morphine and its metabolites. Br J Anaesth. 1989;62:22–27. doi: 10.1093/bja/62.1.22. [DOI] [PubMed] [Google Scholar]
- 46.Hanks GW, Hoskin PJ, Aherne GW, Turner P, Poulain P. Explanation for potency of repeated oral doses of morphine. Lancet. 1987;ii:723–726. doi: 10.1016/s0140-6736(87)91083-x. [DOI] [PubMed] [Google Scholar]
- 47.Portenoy RK, Khan E, Layman M, et al. Chronic morphine therapy for cancer pain: plasma and cerebrospinal fluid morphine and morphine-6-glucuronide concentrations. Neurol. 1991;41:1457–1461. doi: 10.1212/wnl.41.9.1457. [DOI] [PubMed] [Google Scholar]
- 48.Portenoy RK, Foley KM, Stulman J, et al. Plasma morphine and morphine-6-glucuronide during chronic morphine therapy for cancer pain: plasma profiles, steady-state concentrations and the consequences of renal failure. Pain. 1991;47:13–19. doi: 10.1016/0304-3959(91)90005-I. [DOI] [PubMed] [Google Scholar]
- 49.Hoskin PJ, Hanks GW, Heron CW, Aherne GW, Chapman D. M6G and its analgesic action in chronic use. Clin J Pain. 1989;5:199–200. [PubMed] [Google Scholar]
- 50.Portenoy RK. Management of common opioid side effects during long-term therapy of cancer pain. Ann Acad Med. 1994;23:160–170. [PubMed] [Google Scholar]


