Abstract
Aims
Oral activated charcoal is used to treat drug overdose and is effective at reducing drug absorption when administered within 1 h of drug ingestion. There are fewer data on efficacy when the delay is longer, as is the case in most drug overdoses. This study investigated the efficacy of activated charcoal at preventing paracetamol (acetaminophen) absorption after simulated overdose when administration was delayed between 1 and 4 h.
Methods
An open randomized-order four-way crossover study was performed in healthy volunteers comparing the effect of activated charcoal 50 g on the absorption of 3 g paracetamol tablets when administered after an interval of 1, 2 or 4 h or not at all. Plasma paracetamol concentrations were measured over 9 h after paracetamol ingestion using h.p.l.c. and areas under the curve between 4 and 9 h (AUC(4,9 h)) calculated as a measure of paracetamol absorption.
Results
Activated charcoal significantly reduced paracetamol AUC(4,9 h) when administered after 1 h (mean reduction 56%; 95% Confidence intervals 34, 78; P < 0.002) or 2 h (22%; 6, 39; P < 0.03) but not after 4 h (8%; −8, 24). When administered after 1 h activated charcoal reduced individual plasma paracetamol concentrations significantly at all times between 4 and 9 h after paracetamol administration. Administration at 2 or 4 h had no significant effect.
Conclusions
These results in healthy volunteers cannot be extrapolated directly to poisoned patients. However, they provide no evidence of efficacy for activated charcoal when administered after an interval of more than 2 h.
Keywords: activated charcoal, paracetamol overdose
Introduction
Oral activated charcoal is often used to treat drug overdose. Compared with other gastrointestinal decontamination methods, such as gastric lavage, induction of emesis or whole bowel irrigation, it is simple to administer and relatively safe. Its efficacy in preventing drug absorption has been studied in vivo for a wide variety of agents; these studies have recently been subject to detailed review [1]. Large reductions in drug absorption occur when activated charcoal is administered soon after drug ingestion. For example, paracetamol absorption can be reduced by 83% by activated charcoal administered within five minutes [2]. Activated charcoal appears at least as effective as other gastrointestinal decontamination methods when used one hour after simulated drug overdose [3].
Activated charcoal's ability to reduce drug absorption decreases as the interval between drug ingestion and activated charcoal administration is increased [1]. A relatively small number of studies have examined the efficacy of activated charcoal administration when delayed by more than 1 h. This is an important question since the proportion of patients presenting more than 1, 2 or 4 h after drug overdose was 81%, 54% and 27% in one recent study [4]. Clinical trials in poisoned patients would be most useful but there are major practical difficulties in performing these and the numbers of patients required would be very large. Most of the information available has been obtained from volunteer studies, although these have limitations.
These studies were performed in healthy volunteers to measure the efficacy of activated charcoal in reducing the systemic absorption of paracetamol (acetaminophen) when delayed 1, 2 or 4 h after paracetamol administration. Paracetamol was chosen as the study drug because it is the most common drug taken in overdose in the United Kingdom [4–6].
Methods
Ethical approval for the study was obtained from the Local Research Ethics Committee. An open, randomized order crossover trial was performed in healthy volunteer subjects, each of whom, after giving informed written consent, was studied on four occasions separated by at least 1 week. On each occasion paracetamol was ingested. On three occasions this was followed by activated charcoal after an interval of 1, 2 or 4 h. On the other occasion no activated charcoal was given and this experiment acted as a control.
Experimental procedure
Subjects fasted overnight and consumed a standard breakfast of toast 1 h before ingesting 3 g paracetamol in tablet form. Activated charcoal (Carbomix) was ingested as slurry with 400 ml water according to the manufacturers instructions. Lunch was taken 5 h after paracetamol ingestion. Blood samples were collected via a cannula before and over 9 h following paracetamol ingestion. Subjects remained ambulant during the experiments.
Plasma from blood samples was separated by centrifugation and frozen until analysis. Plasma paracetamol concentrations were measured by h.p.l.c. with u.v. spectroscopy. This method has a limit of detection of <1 mg l−1. Coefficients of variation of repeated measurements were 6.9% at 5.8 mg l−1 and 4.3% at 27.4 mg l−1.
Data processing
Plasma paracetamol concentrations were plotted against the time of sample collection for each experimental phase, for each subject. Pharmacokinetic variables for paracetamol were calculated using a nonlinear curve-fitting computer program (Model-PK, Version 1.0, McPherson Scientific, Rosanna, Australia) employing compartment independent analysis. The primary outcome measurement used to estimate paracetamol absorption and the effect of activated charcoal was the area under the curve between 4 and 9 h (AUC(4,9 h)). This was calculated for each experiment using the trapezoidal method. Relative reductions in AUC between the activated charcoal and control experiments were calculated and mean reductions with 95% confidence intervals calculated. A similar analysis was done for the total area under the curve between paracetamol administration and infinity (AUC(0,∞). Additionally, assessment was made for the occurrence of statistically significant differences in individual plasma paracetamol concentrations between experimental phases.
Statistical analysis
For each measured variable, and for differences between the control and activated charcoal phases, mean values were calculated with 95% confidence intervals. Generalized Linear Model Analysis of Variance was used to establish whether differences existed between AUC values for experimental groups, and between AUCs for groups formed on the basis of experimental order. Additionally, t-tests were used to compare AUCs for each of the activated charcoal phases with the control phase AUC. Repeated measures anova tests were performed to establish the existence of differences between the concentrations in each experimental group at individual time points.
Results
Ten healthy volunteers (six males) took part. Their mean age (range), height and weight were 23 years (20–41), 177 cm (160–193) and 70 kg (51–85). One subject withdrew after two experimental limbs (control limb and activated charcoal after 1 h) for reasons unconnected to the study and was not replaced. The data for this subject were used in the calculation of mean values and 95% confidence intervals for the control and 1 h limbs, but not in comparisons of data from different limbs by analysis of variance.
Mean paracetamol concentrations under control conditions and following activated charcoal administration at 1, 2 or 4 h after paracetamol are shown in Figure 1. Compared with the control phase, statistically significant reductions in plasma concentrations occurred at all time points between 4 and 9 h when activated charcoal was administered after an interval of 1 h. Later administration of activated charcoal did not significantly affect individual paracetamol concentrations.
Figure 1.
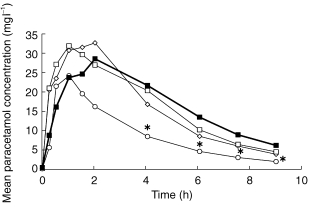
Effects of oral activated charcoal when given at 1 (○), 2 (◊) or 4 h (□) or not at all (control, ▪) after a 3 g oral paracetamol dose. *P < 0.05 vs control.
The effects of activated charcoal on areas under the plasma concentration—time curve are shown in Table 1. AUC(4,9 h), the primary outcome measure, was significantly reduced by activated charcoal given at 1 or 2 h. Administration after 4 h had no significant effect, although the 95% confidence intervals indicate that a reduction of less than 24% cannot be excluded. Significant reductions in AUC(0,∞) were observed when activated charcoal was administered after 1 h, but not after 2 or 4 h. Differences did not occur between either AUC measurement when groups were formed on the basis of experimental order.
Table 1.
Effect of activated charcoal (50 g) on paracetamol (3 emsp14;g) absorption when administered after 1, 2 or 4 h, compared with control (no activated charcoal). Mean values (95% confidence intervals). Statistical significance from control calculated using anova (n = 9).
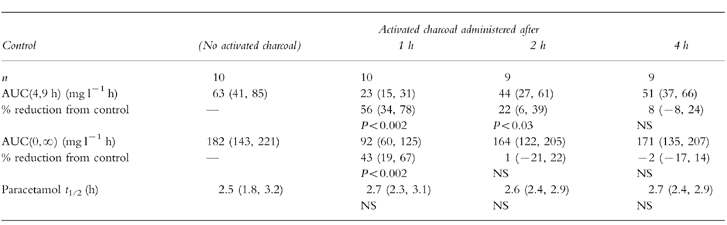
Since AUC may be affected by changes in elimination of drugs as well as differences in absorption, and repeated doses of activated charcoal affect the elimination of some drugs, the effects of these single doses of activated charcoal on paracetamol elimination half-life were assessed (Table 1). No significant differences were observed.
Discussion
The results of this study indicate that, in healthy volunteers, activated charcoal given after an interval of 1 h reduces paracetamol absorption. The size of this effect is likely to be of clinical relevance. Administration after 2 h produces a smaller but statistically significant reduction in AUC(4,9 h), although the clinical relevance of a reduction averaging 22% is questionable. For this interval no significant reduction in AUC(0,∞) was detected. This is a less sensitive measurement since the majority of the area is contributed to by plasma concentrations taken before charcoal administration or extrapolated after the final 9 h sample. Activated charcoal administered at 4 h did not have a detectable effect. Although the power of the study was such that a reduction in AUC(4,9 h) of less than 24% cannot be excluded, such a reduction is unlikely to be of clinical relevance.
These results with paracetamol are broadly consistent with results using paracetamol and other drugs. Using urinary excretion as a measure of absorption, 30 g activated charcoal reduced absorption of 5 g paracetamol elixir by 48, 44 and 33% when administered after 15, 30 and 120 min [7]. For nortriptyline 75 mg, activated charcoal 10 g reduced AUC by 74%, 37% and 13% when administered at 30 min, 2 h and 4 h [8]. Activated charcoal 25 g reduced absorption of 1 g salicylate by 41, 21, and 9% when given after 0, 1 or 3 h [9]. It also reduced absorption of pholcodeine by 26% and 17% after 2 and 5 h [10] and had no significant effect on the absorption of fluoxetine when administered after 2 or 4 h [11]. Activated charcoal 15 g reduced absorption of doxepin when given after 30 min but not after 3 h [12].
For slow release drugs or those with delayed absorption, the efficacy of delayed activated charcoal may be greater: activated charcoal 25 g reduced absorption of amlodipine by 99%, 49% and 15% when administered immediately or after intervals of 2 or 6 h [13]. Similarly, the same dose reduced absorption of a slow release verapamil preparation by 35% and 32% when given after 2 or 4 h, but did not significantly affect absorption of a standard release verapamil preparation after these intervals [14].
Care must be exercised in extrapolating data from healthy volunteers to the clinical situation. Several potential differences exist between the conditions of this study and the clinical situation. The dose of paracetamol used in this study was necessarily lower. However, a full dose of activated charcoal was administered. The ratio of charcoal to paracetamol (50:3) is much higher than that usually obtained in clinical use, particularly for paracetamol overdose. This increased ratio will enhance the efficacy of the charcoal. On the other hand, it takes longer for peak plasma concentrations to occur after a toxic dose than a lower dose [15] and thus the efficacy of delayed activated charcoal may be increased in the clinical situation. Stomach conditions and contents may vary considerably between the healthy subjects in this study and patients taking drug overdoses; the presence of food in the stomach may reduce [16, 17] or enhance [17] the efficacy of delayed activated charcoal. This is partly dependent on the interval between overdose and charcoal administration. Alcohol ingestion is common in overdose patients. Although alcohol reduces drug binding to charcoal in vitro, effects in vivo appear small [18]. Co-administration of other drugs may delay gastric emptying and thus paracetamol absorption. The effect of this on the efficacy of delayed activated charcoal is currently unknown and further studies would be helpful. In our study subjects remained ambulant and gastric emptying and drug absorption characteristics may be different under these conditions compared to patients remaining in the supine position.
The recent joint position statement from the American Academy of Clinical Toxicology and European Association of Poisons Control Centres and Clinical Toxicologists concludes that there are insufficient data to support or exclude the use of activated charcoal more than 1 h after drug ingestion [1]. These current data for paracetamol are broadly consistent with this position and are likely to apply to other drugs with similar absorption and binding characteristics. They justify the use of activated charcoal up to 1 and possibly 2 h after drug ingestion, but they do not provide evidence to support use if the interval is longer.
If these results from volunteers are an accurate reflection of the efficacy of activated charcoal in the clinical setting, early administration of activated charcoal is expected to reduce plasma paracetamol concentration at 4 h after overdose. There is no evidence that the use of activated charcoal affects the relationship between the 4 h paracetamol concentration and the risk of liver injury. Thus, the administration of activated charcoal should not affect the interpretation of the treatment nomogram and early use of activated charcoal may therefore reduce the need for antidotal treatment.
Acknowledgments
We are grateful to the volunteers who took part in this research. This study did not receive external financial support.
References
- 1.American Academy of Clinical Toxicology and European Association of Poisons Control Centres and Clinical Toxicologists. Position Statement: Single dose activated charcoal. J Toxicol Clin Toxicol. 1997;35:721–741. doi: 10.3109/15563659709162569. [DOI] [PubMed] [Google Scholar]
- 2.Levy B, Houston B. Effect of activated charcoal on acetaminophen absorption. Pediatrics. 1976;58:432–435. [PubMed] [Google Scholar]
- 3.Tenenbein M, Cohen S, Sitar DS. Efficacy of ipecac-induced emesis, orogastric lavage, and activated charcoal for acute drug overdose. Ann Emergency Medicine. 1987;10:838–841. doi: 10.1016/s0196-0644(87)80518-8. [DOI] [PubMed] [Google Scholar]
- 4.Thomas SHL, Bevan L, Bhattacharyya S, et al. Presentation of poisoned patients to accident and emergency departments in the north of England. Human Exp Toxicology. 1996;15:466–470. doi: 10.1177/096032719601500602. [DOI] [PubMed] [Google Scholar]
- 5.Bialas MC, Reid PG, Beck P, Lazarus JH, Smith PM, Scorer RC, Routledge PA. Changing patterns of self-poisoning in a UK health district. Q J Med. 1996;89:893–901. doi: 10.1093/qjmed/89.12.893. [DOI] [PubMed] [Google Scholar]
- 6.Hawton K, Fagg J. Trends in deliberate self poisoning and self injury in Oxford. 1976–90. Br Med J. 1992;304:1409–1411. doi: 10.1136/bmj.304.6839.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rose SR, Gorman RL, Oderda GM, Klein-Schwartz W, Watson WA. Simulated acetaminophen overdose: pharmacokinetics and effectiveness of activated charcoal. Ann Emergency Med. 1991;20:1064–1068. doi: 10.1016/s0196-0644(05)81353-8. [DOI] [PubMed] [Google Scholar]
- 8.Dawling S, Crome P, Braithwaite R. Effect of delayed administration of activated charcoal on nortriptyline absorption. Eur J Clin Pharmacol. 1978;14:445–447. doi: 10.1007/BF00716388. [DOI] [PubMed] [Google Scholar]
- 9.Levy G, Tsuchiya T. Effects of activated charcoal on aspirin absorption in man. Clin Pharmacol Ther. 1972;13:317–322. doi: 10.1002/cpt1972133317. [DOI] [PubMed] [Google Scholar]
- 10.Laine K, Kivisto KT, Ojala-Karlsson P, Neuvonen PJ. Effect of activated charcoal on the pharmacokinetics of pholcodine, with special refernce to delayed charcoal ingestion. Ther Drug Monit. 1997;19:46–50. doi: 10.1097/00007691-199702000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Laine K, Kivisto KT, Pelttari S, Neuvonen PJ. The effect of activated charcoal on the absorption of fluoxetine, with special reference to delayed charcoal administration. Pharmacol Toxicol. 1996;79:270–273. doi: 10.1111/j.1600-0773.1996.tb00272.x. [DOI] [PubMed] [Google Scholar]
- 12.Scheinin M, Virtanen R, Iisalo E. Effect of single and repeated doses of activated charcoal on the pharmacokinetics of doxepin. Int J Clin Pharmacol Ther Toxicol. 1985;23:38–42. [PubMed] [Google Scholar]
- 13.Laine K, Kivisto KT, Laakso I, Neuvonen PJ. Prevention of amlodipine absorption by activated charcoal: effect of delay in charcoal administration. Br J Clin Pharmacol. 1997;43:29–33. doi: 10.1111/j.1365-2125.1997.tb00029.x. [DOI] [PubMed] [Google Scholar]
- 14.Laine K, Kivisto KT, Neuvonen PJ. Effect of delayed administration of activated charcoal on the absorption of conventional and slow-release verapamil. J Toxicol—Clin Toxicol. 1997;35:263–268. doi: 10.3109/15563659709001210. [DOI] [PubMed] [Google Scholar]
- 15.Thomas SHL. Paracetamol (acetaminophen) poisoning. Pharmacol Ther. 1993;60:91–120. doi: 10.1016/0163-7258(93)90023-7. [DOI] [PubMed] [Google Scholar]
- 16.Levy G, Soda DM, Lampman TA. Inhibition by ice cream of the antidotal efficacy of activated charcoal. Am J Hosp Pharm. 1975;32:289–291. [PubMed] [Google Scholar]
- 17.Olkkola KT, Neuvonen PJ. Do gastric contents modify antidotal efficacy of oral activated charcoal? Br J Clin Pharmacol. 1984;18:663–669. doi: 10.1111/j.1365-2125.1984.tb02527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neuvonen PJ, Olkkola KT, Alenen T. Effect of ethanol and pH on the adsorption of drugs to activated charcoal: studies in vitro and in man. Acta Pharmacol Toxicol. 1984;54:1–7. doi: 10.1111/j.1600-0773.1984.tb01888.x. [DOI] [PubMed] [Google Scholar]


