Abstract
Aims
To compare the serum pharmacokinetics of fosinoprilat with enalaprilat and lisinopril after 1 and 10 days of dosing with fosinopril, enalapril and lisinopril.
Methods
Patients with congestive heart failure (CHF, NYHA Class II–IV) and chronic renal insufficiency (creatinine clearance ≤30 ml min−1) were randomized to receive fosinopril, enalapril or lisinopril in two parallel-group studies. In the first study 24 patients were treated with 10 mg fosinopril (n = 12 patients) or 2.5 mg enalapril (n = 12) every morning for 10 consecutive days. In the second study 31 patients were treated with 10 mg fosinopril (n = 16 patients) or 5 mg lisinopril (n = 15) every morning for 10 consecutive days. Samples of blood were collected for determination of pharmacokinetic parameters. The area under the curve (AUC) between the first and last days of treatment and the accumulation index (AI) were the primary outcome measures.
Results
All three angiotensin converting enzyme (ACE) inhibitors exhibited a significant increase in AUC between the first and last days of treatment in both studies. The difference between the AI for fosinoprilat (1.41) and enalaprilat (1.96) was statistically significant (95% CI: 1.05, 1.84). Similarly, the difference between the AI for fosinoprilat (1.21) and lisinopril (2.76) was statistically significant (95% CI: 1.85, 2.69). All three ACE inhibitors completely inhibited serum ACE for 24 h. All treatments were well tolerated.
Conclusions
Fosinoprilat exhibits significantly less accumulation than enalaprilat or lisinopril in patients with CHF and renal insufficiency, most probably because fosinoprilat is eliminated by both the kidney and liver, and increased hepatic elimination can compensate for reduced renal clearance in patients with kidney dysfunction.
Keywords: angiotensin-converting enzyme, congestive heart failure, enalapril, enalaprilat, fosinopril, fosinoprilat, lisinopril, pharmacokinetics, renal insufficiency
Introduction
Medical treatment of patients with congestive heart failure (CHF) is complicated, because this condition is associated with reduced perfusion of end organs involved in drug clearance, including the liver and kidney [1]. As a result, CHF often entails reduced clearance, longer elimination half-life, and accumulation of chronically administered drugs [1]. Kidney dysfunction [2], a possible consequence of CHF, may further alter the pharmacokinetic parameters of drugs eliminated by renal excretion.
Although a number of angiotensin converting enzyme (ACE) inhibitors are effective in CHF [3–5], their utility may differ, especially in the face of concomitant renal impairment. For example, the elimination of lisinopril and enalapril is significantly decreased in patients with either CHF or renal impairment [6–8], and reduced doses for both drugs have been advocated to avoid adverse events [6, 7, 9].
Fosinopril is a phosphinic acid ACE inhibitor [10], and, like enalapril, is a pro-drug that undergoes conversion to an active di-acid in the gastrointestinal mucosa and the liver [10]. Unlike most other ACE inhibitors, however, fosinopril is eliminated by both hepatic metabolism and renal excretion [11]. A recent study has demonstrated that the hepatic clearance of fosinoprilat, the active metabolite of fosinopril, is increased in patients with kidney dysfunction due in part to an increase in biliary secretion [12]. This property may make fosinopril particularly useful in patients with CHF and renal impairment.
The present studies were undertaken to compare the single and multiple-dose pharmacokinetic parameters of fosinoprilat with enalaprilat and lisinopril in patients with both CHF and renal insufficiency.
Portions of the results discussed herein were presented at the Third International Congress on Heart Failure, Geneva, Switzerland, April 21–25, 1995.
Methods
Patients
Patients of both sexes, ranging in age from 18 to 85 years, were enrolled in these multicentre, open-label, randomized, parallel-group studies. Both studies were approved by an Ethics Committee and all subjects gave written informed consent. Twenty-eight patients entered the fosinoprilat-enalaprilat study in three study centres and 31 entered the fosinoprilat-lisinopril study in nine study centres. All patients underwent a screening process consisting of a complete medical history and physical examination, including a 12-lead electrocardiogram and a two-dimensional echocardiograph. Samples were also collected for haematology, blood chemistry, and urinalysis. All evaluations, except for the echocardiograph, were repeated within 48 h after the final drug dose on day 10.
Eligible patients met the following criteria: New York Heart Association Class II–IV CHF, left ventricular ejection fraction ≤40%, creatinine clearance ≤30 ml min−1 and cardiomegaly as evidenced by either chest X-ray indings or echocardiography. The origin of cardiac failure, relevant concomitant diagnosis and concomitant drug treatments were recorded.
The following exclusion criteria were applied: history of allergy or sensitivity to ACE inhibitors; myocardial infarction or cerebrovascular accident within the past 6 months; evidence of rapidly deteriorating renal function; respiratory function <55% of normal; liver function values >50% above the upper limit of normal or gamma-glutamyl transpeptidase levels > 100 U l−1; treatment with either cytotoxic or immunosuppressant drugs; need for dialysis; white blood cell counts <3500 mm−3 or neutrophil counts <1500 mm−3; use of potassium-sparing diuretics (other diuretics were permitted provided a constant dose was used and treatment was initiated for at least 2 weeks before day 1 of study drug administration); serum potassium levels exceeding the upper limit of normal; suspicion or documentation of bilateral artery stenosis. Non-steroidal anti-inflammatory medications other than aspirin (<175 mg day−1 or 350 mg every other day) were prohibited within 7 days of ingestion of each study drug.
Medication
In the fosinopril-enalapril study, patients were randomized to receive either 10 mg fosinopril or 2.5 mg enalapril every morning for 10 consecutive days. In the fosinopril-lisinopril study patients were randomized to receive either 10 mg fosinopril or 5 mg lisinopril every morning for 10 consecutive days. A 2.5 mg dose of enalapril and a 5 mg dose of lisinopril were chosen to limit the risk of drug accumulation [13]. On days 1 and 10, patients fasted 8 h before and 4 h after dosing.
Assessment of vital signs
Sitting blood pressure was measured before, and 0.5, 1, 1.5, 2, 2.5, 3, and 3.5 h after study drug administration on day 1 and 4, 6, 8, 12, and 24 h after administration on days 1 and 10.
Blood sampling for serum fosinoprilat, enalaprilat and lisinopril concentrations and ACE activity
On days 1 and 10, 5 ml of blood was obtained in a Vacutainer™ tube without anticoagulant prior to and 0.5, 1, 2, 3, 4, 6, 8, 12, and 24 h after drug administration. Blood samples were also collected before drug administration on days 3 or 4 and 7 or 8. After collection the blood samples were left at room temperature for approximately 30 min to allow clotting to occur. The samples were then centrifuged for 15 min, the serum divided and transferred to two plastic tubes, and stored immediately at −20° C.
Analytical methods
Concentrations of serum fosinoprilat, enalaprilat and lisinopril were assayed by a modified radio-enzymatic method with a lower limit of quantification of 2.0 ng ml−1 [14]. The assay detects formation of [3H]-hippuric acid formed when serum samples are incubated in the presence of [3H]-hippuryl-glycyl-glycine and purified ACE from rabbit lung. The amount of hippuric acid generated in the assay incubation mixture is inversely proportional to the concentration of fosinoprilat, enalaprilat or lisinopril. Coefficients of variation ranged from 2.0% to 8.8% and from 6.3% to 14.2% in the fosinoprilat-enalaprilat and the fosinoprilat-lisinopril studies, respectively, for replicate measurements (n = 6). Interassay coefficients of variation for 10 ng ml−1 and 60 ng ml−1 standards were <15% for all assays. Serum ACE activity was determined by modification of a commercial radioassay (Ventrex ACE Microvial™ Radioassay System, Ventrex Laboratories Inc., Portland, Maine) and expressed as activity units (% substrate hydrolysed min−1). The assay is based on conversion of [3H]-hippuryl-glycyl-glycine to [3H]-hippuric acid by active ACE in the serum sample.
Pharmacokinetic analysis
Non-compartmental pharmacokinetic parameters including maximum serum concentration (Cmax), time to maximum serum concentration (tmax), and area under the time vs serum concentration curve (AUCτ) were calculated from the serum concentrations of fosinoprilat, enalaprilat and lisinopril on days 1 and 10. In addition, an accumulation index (AI) was calculated as the ratios of the AUCτs on days 1 and 10. Minimum serum concentration (Cmin) was determined from blood samples drawn prior to dosing on days 2, 3 or 4, 7 or 8, and 10, as well as from a sample taken 24 h after the final dose on day 10.
Statistical analysis
Homogeneity of treatment groups was compared for quantitative variables by two-sample t-tests and for qualitative variables by Fisher's exact tests. Two-sided 95% confidence intervals (CI) for the ratio of day 10 (steady state) to day 1 (first dose) geometric means (Cmax and AUCτ) or median difference between day 10 and day 1 (tmax) were determined for fosinoprilat, enalaprilat and lisinopril. A two-way analysis of variance or Friedman's test was used to assess Cmin for the attainment of steady state.
Between group comparisons of quantitative variables were made by means of two-sample t-tests or Wilcoxon rank-sum tests. Qualitative variables were analysed with Fisher's exact tests.
Between-group comparison of the AI was determined with a two-sided 95% CI for the ratio of the geometric mean of the enalaprilat or lisinopril AI to the fosinoprilat AI (adjusted for baseline creatinine clearance and centre). All statistical tests were two-sided, with P <0.05 as the level accepted for significance.
Results
Demographics
Of the 28 patients who entered the fosinoprilat-enalaprilat study, 24 (10 women and 14 men) were considered evaluable. Four patients from the fosinopril group withdrew from the study: two because of adverse events (diarrhoea possibly related to drug in one patient, ventricular fibrillation unrelated to drug in the second), one at his own request and one on the advice of his physician. No significant differences in the demographic data of evaluable patients were noted between treatment groups (Table 1).
Table 1.
Demographic characteristics of evaluable patients.
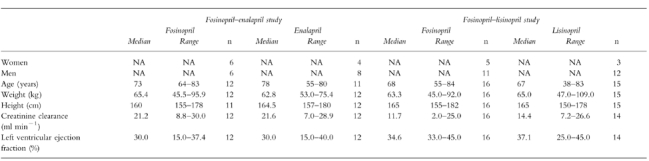
A total of 31 patients entered and completed the fosinoprilat-lisinopril study: five women and 11 men received fosinopril; three women and 12 men were treated with lisinopril. A re-calculation of the screening, prestudy creatinine clearance of one male patient in the lisinopril group who completed the study gave a result much greater than 30 ml min−1. Since this patient should not have entered the study, he was excluded from the pharmacokinetic analyses but not from the safety analysis. No significant differences in the demographic data of evaluable patients were noted between treatment groups (Table 1).
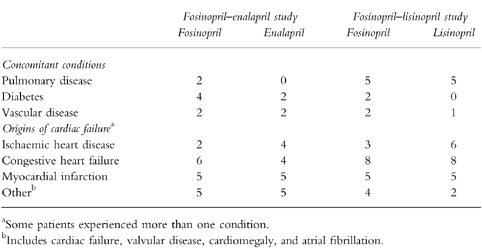
All patients in both groups received concomitant medication during the trial: medication required to maintain a patient's health remained unaltered during the course of the studies. Concomitant medications consisted of long-term treatments for pre-existing diseases, most often CHF; but also included pulmonary disease (asthma, COPD), diabetes and vascular disease (Table 2). The origins of cardiac failure in both studies included ischaemic heart disease, CHF, and myocardial infarct (Table 2).
Table 2.
Concomitant conditions and origins of cardiac failure.
Pharmacokinetic evaluation: I. Fosinopril–enalapril study
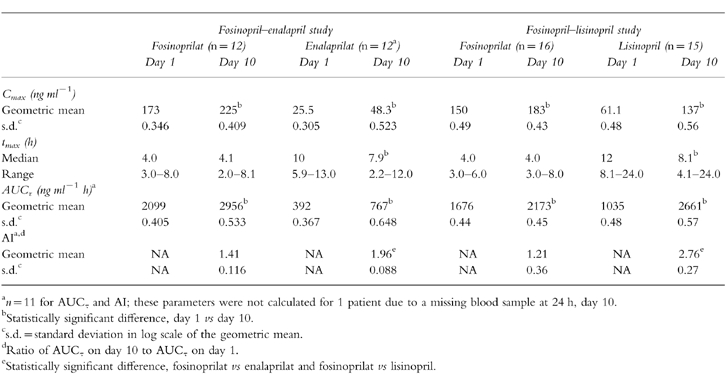
Mean serum concentrations of fosinoprilat and enalaprilat over 24 h after dosing on day 1 and day 10 are illustrated in Figure 1. On day 1 the Cmax for fosinoprilat was 173.0 ng ml−1, tmax was 4.0 h, and AUCτ was 2099.0 ng ml−1 h (Table 3). The respective values for enalaprilat were 25.5 ng ml−1, 10.0 h, and 392.0 ng ml−1 h (Table 3).
Figure 1.
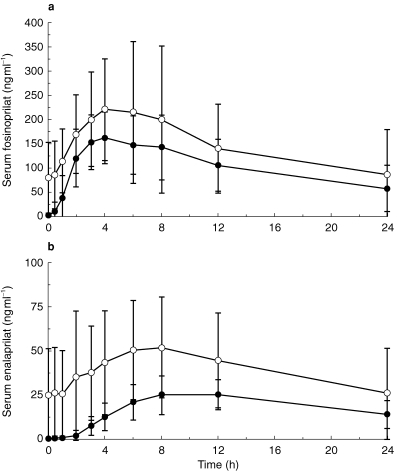
Mean serum concentration vs time curves for a) fosinoprilat and b) enalaprilat on days 1 and 10. Values are presented as the arithmetic mean±s.d. • single dose, ○ multiple dose.
Table 3.
Summary of pharmacokinetic parameters for fosinoprilat and enalaprilat following single and multiple doses.
Steady state was rapidly achieved in the patients who received fosinopril. No significant differences in Cmin values (mean±s.d.) of fosinoprilat were noted 24 h after dosing on day 1 (55.9±48.2 ng ml−1), prior to drug administration on days 3 or 4 (75.5±59.7 ng ml−1), 7 or 8 (66.0±53.6 ng ml−1), and 10 (78.9±73.9 ng ml−1), or 24 h after the final dose (84.0±94.1 ng ml−1). Steady state also occurred rapidly in enalapril-treated patients. Cmin values of enalaprilat did not differ significantly 24 h after dosing on day 1 (13.5±7.6 ng ml−1), before administration on days 3 or 4 (25.0±24.8 ng ml−1), 7 or 8 (23.1±25.1 ng ml−1), and 10 (24.6±26.3 ng ml−1), or 24 h after the last dose on day 10 (25.6± 25.8 ng ml−1).
Pharmacokinetic evaluation on the tenth day of dosing at steady state revealed accumulation of both ACE inhibitors, with significantly greater levels for enalaprilat than for fosinoprilat. On day 10, the Cmax for fosinoprilat was 225.0 ng ml−1 (95% CI for ratio of day 10 to day 1 geometric mean: 1.04, 1.62), tmax was 4.1 h, and AUCτ was 2956.0 ng ml−1 h (95% CI for ratio of day 10 to day 1 geometric mean: 1.09, 1.82) (Table 3). Corresponding values for enalaprilat on day 10 were 48.3 ng ml−1 (95% CI: 1.56, 2.30) 7.9 h (95% CI for median difference between day 10 and day 1: −4.76, −0.03), and 767.0 ng ml−1 h (95% CI: 1.61, 2.38) (Table 3). The AI was 1.41 for fosinoprilat and 1.96 for enalaprilat. The difference between the treatment groups was statistically significant (point estimate 1.391; 95% CI: 1.05, 1.84).
Pharmacokinetic evaluation: II. Fosinopril–lisinopril study
Mean serum concentrations of fosinoprilat and lisinopril over 24 h after dosing on day 1 and day 10 are illustrated in Figure 2. On day 1, the Cmax for fosinoprilat was 150.0 ng ml−1, tmax was 4.0 h and AUCτ was 1676 ng ml−1 h (Table 3). The respective values for lisinopril were 61.1 ng ml−1, 12.0 h, and 1035 ng ml−1 h (Table 3).
Figure 2.
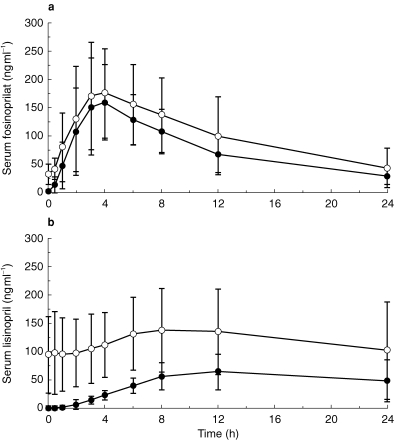
Mean serum concentration vs time curves for a) fosinoprilat and b) lisinopril on days 1 and 10. Values are presented as the arithmetic mean±s.d. • single dose, ○ multiple dose.
Steady state was rapidly achieved in the patients who received fosinopril. No significant differences in Cmin values (arithmetic mean±s.d.) of fosinoprilat were noted 24 h after dosing on day 1 (33.1±14.6 ng ml−1), prior to drug administration on days 3 or 4 (39.1±20.2 ng ml−1), 7 or 8 (43.9±29.5 ng ml−1), and 10 (36.1±24.6 ng ml−1), or 24 h after the final dose (50.6±38.1 ng ml−1). The Cmin for lisinopril was approaching steady state by the last day of dosing. The Cmin on day 10 or 24 h after the final dose (87.2±69.0 and 105.4±85.6 ng ml−1, respectively) were significantly higher for lisinopril than those recorded 24 h after drug administration on days 3 or 4 (62.5±46.2 ng ml−1) and 7 or 8 (69.0±59.7 ng ml−1).
Pharmacokinetic evaluation on the tenth day of dosing revealed accumulation of both ACE inhibitors, with significantly greater levels for lisinopril than for fosinoprilat. On day 10, the Cmax for fosinoprilat was 183.0 ng ml−1 (95% CI for ratio of day 10 to day 1 geometric mean: 0.98, 1.51), tmax was 4.0 h and AUCτ was 2173 ng ml−1 h (95% CI for ratio of day 10 to day 1 geometric mean: 1.05, 1.60) (Table 3). Corresponding values for lisinopril on day 10 were 137.0 ng ml−1 (95% CI: 1.85, 2.73), 8.1 h (95% CI: 5.76, −0.04) and 2661 ng ml−1 h (95% CI: 2.16, 3.07). The AI was 1.21 for fosinoprilat and 2.76 for lisinopril. The difference between the treatment groups was statistically significant (point estimate 2.23; 95% CI: 1.85, 2.69).
Inhibition of serum ACE activity
Fosinopril in both studies and enalapril and lisinopril had almost identical effects on serum ACE activity. Maximum inhibition was achieved within 2 h of the first dose and maintained for at least 24 h. Maximum inhibition of serum ACE activity was also noted for both drugs before dosing on days 3 or 4, 7 or 8, and 10. Serum ACE activity in both groups remained fully inhibited 24 h after dosing on day 10.
Effects on blood pressure
As expected, sitting systolic and diastolic blood pressure were reduced during the 24 h after treatment with fosinopril in both studies and with enalapril or lisinopril. The reductions were maintained throughout the drug administration period.
Tolerability
All three ACE inhibitors were well tolerated. All clinical and laboratory adverse events, regardless of their attribution are presented in Tables 4 and 5, respectively.
Table 4.
Clinical adverse events recorded during treatmenta.
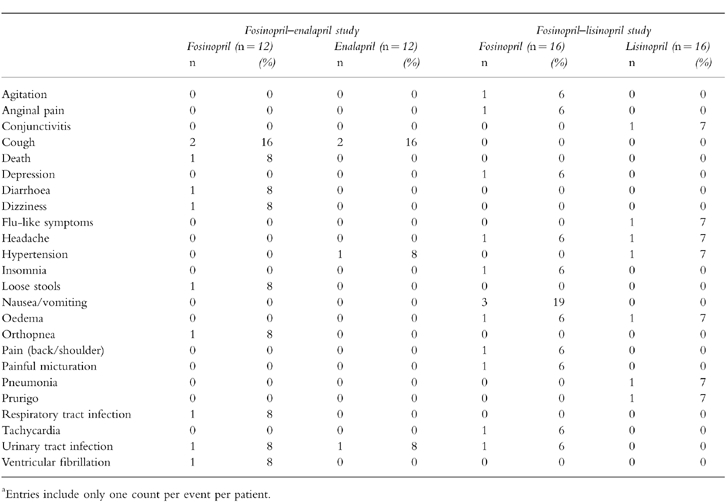
Table 5.
Laboratory adverse events recorded during treatmenta.
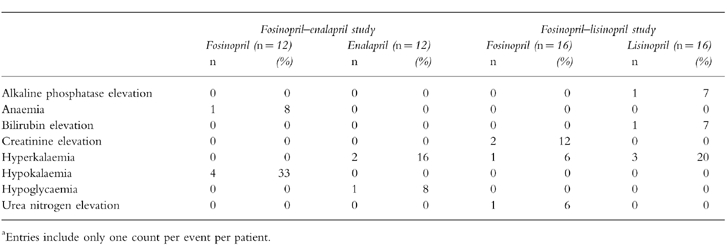
Among the patients who received fosinopril in the fosinopril-enalapril study, the most common adverse events were hypokalaemia (four patients) and cough (two patients). Two of the four hypokalaemic patients receiving fosinopril were also receiving a non-potassium-sparing diuretic before and during this study. All hypokalaemic patients received potassium chloride before and/or during the study. Hyperkalaemia and cough (two patients each) were the most common adverse events in the enalapril group. Only cough in two patients receiving fosinopril and in two receiving enalapril and hyperkalaemia in one patient receiving enalapril were considered related or possibly related to the study drug.
Although one fosinopril-treated patient died during the trial as a result of ventricular fibrillation, the death was not attributed to the study drug. Two additional patients (one who received fosinopril and one treated with enalapril) died after the trial was completed. Neither death was attributed to ACE-inhibitor therapy.
The most common adverse event among fosinopril-treated patients in the fosinopril-lisinopril study was nausea and/or vomiting (three patients). Hyperkalaemia was the most common adverse event in the lisinopril group (three patients).
Discussion
In the fosinopril-enalapril study accumulation of enalaprilat was significantly greater than that of fosinoprilat after repeated administration to patients with both CHF and renal insufficiency. The AUCτ for fosinoprilat increased slightly over the study period (AI = 1.41), compared with an almost two-fold increase (AI = 1.96) with enalapril. The failure to detect differences in the Cmin values of enalaprilat may have been a function of the power of the study.
In the fosinopril-lisinopril study, accumulation of lisinopril was significantly greater than that of fosinoprilat. The AUCτ for fosinoprilat increased slightly over the study period (AI = 1.21) while the increase was almost three-fold (AI = 2.76) with lisinopril. With long-term treatment, the accumulation of lisinopril may be even greater than the present data indicate, since lisinopril-treated patients in the present study were only approaching steady state on the last day of dosing.
The greater accumulation of enalaprilat and lisinopril in CHF patients with renal insufficiency, reflected by their greater AI values, may suggest the need for dose adjustment in this population. The dual elimination of fosinoprilat, reflected by the lower AI values, avoids the need for dose adjustment.
The pharmacokinetic differences between fosinoprilat and enalaprilat or lisinopril reported here are consistent with results obtained in other studies. Sica et al. [13] compared the multiple-dose pharmacokinetics of fosinoprilat with enalaprilat and lisinopril in patients with chronic renal insufficiency (creatinine clearance <30 ml min−1). After 10 days of dosing with the parent drugs, the AUC increased by only 26.8% for fosinoprilat and by 76.6% and 161.7% for enalaprilat and lisinopril, respectively. The AIs for fosinoprilat (1.27), enalaprilat (1.77) and lisinopril (2.62) were similar to those reported here. In dialysis patients, the pharmacokinetics of fosinoprilat were similar to those in patients with renal impairment [15].
The significant accumulation of enalaprilat in patients with CHF and renal insufficiency reported here is also consistent with results from several prior pharmacokinetic evaluations in patients with renal insufficiency alone. Kelly et al. [8] reported that the mean Cmax for enalaprilat after 10 days of dosing was nearly 2.5 times higher in patients with renal insufficiency than in normal volunteers. Fruncillo et al. [16] demonstrated that both the single-and multiple-dose pharmacokinetics of enalaprilat were significantly altered in patients with renal dysfunction. The AUC after the first dose was 186 ng ml−1 h in healthy volunteers, compared with 884 ng ml−1 h in patients whose creatinine clearance ranged from 10 to 39 ml min−1. After 10 days of dosing, the AI was 1.2 in normal subjects and 1.6 in patients with renal dysfunction. Lowenthal et al. [17] also reported elevated serum concentrations and reduced urinary excretion of enalaprilat in patients with impaired renal function. Similarly, Dickstein [18] reported a 32% increase in Cmax in patients with CHF.
Other studies have also demonstrated significant accumulation of lisinopril in patients with either CHF or chronic renal insufficiency. Both Gautam et al. [6] and Till et al. [19] reported higher serum levels of lisinopril in patients with CHF than in healthy individuals. In contrast, Johnston & Duffin [20] documented no alterations in lisinopril pharmacokinetics in patients with CHF. Shionoiri et al. [7], Kelly et al. [21] and van Schaik et al. [22] noted reduced clearance and increased plasma concentrations of lisinopril in patients with renal dysfunction. In one study, the effective half-life of lisinopril doubled and tripled in patients with mild and severe renal impairment, respectively [22]. These results are not surprising in view of the elimination of lisinopril solely by renal excretion [19, 23].
In patients with chronic renal insufficiency, including those with CHF in our study, the greater stability of fosinoprilat pharmacokinetics is almost certainly a function of dual routes of elimination with 44% of an intravenously administered dose excreted in urine and 46% in faeces [11]. Singhvi et al. [11] have suggested that biliary excretion of fosinoprilat might provide an alternate route of elimination in patients with renal dysfunction. The compensatory increase in hepatic elimination of fosinoprilat was demonstrated most clearly by Hui et al. [12], who showed that more than 80% of the radioactivity associated with an oral dose of fosinopril was recovered in the faeces of patients with severe renal dysfunction. This characteristic distinguishes fosinoprilat from enalaprilat and lisinopril, which are eliminated primarily by the kidneys [11].
The bioavailability of lisinopril also may be altered in patients with CHF by as much as 45% following oral administration [11]. In contrast, Liao et al. [24] reported that the oral bioavailability of fosinopril was 29.3% in patients with CHF and 28.6% in normal individuals.
In the present study, there was no difference between enalapril or lisinopril and fosinopril in the inhibition of ACE activity in patients with CHF and renal insufficiency. However, enalaprilat and lisinopril accumulated to a significantly greater extent than fosinoprilat in these patients. The dual renal and hepatic elimination of fosinoprilat may account for this difference in accumulation. Because fosinoprilat does not accumulate to a great degree, dose adjustment of either single-or multiple-dose oral regimens of fosinopril is not indicated in patients with CHF and renal insufficiency.
References
- 1.Shammas FV, Dickstein K. Clinical pharmacokinetics in heart failure. An updated review. Clin Pharmacokinet. 1988;15:94–113. doi: 10.2165/00003088-198815020-00002. [DOI] [PubMed] [Google Scholar]
- 2.Ljungman S, Kjekshus J, Swedberg K. Renal function in severe congestive heart failure during treatment with enalapril (the Cooperative North Scandinavian Enalapril Survival Study [CONSENSUS] Trial) Am J Cardiol. 1992;70:479–487. doi: 10.1016/0002-9149(92)91194-9. [DOI] [PubMed] [Google Scholar]
- 3.Massie BM. Angiotensin-converting enzyme inhibition in mild to moderate congestive heart failure: an evolving approach. Am Heart J. 1988;1:402S–409S. doi: 10.1093/ajh/1.4.402s. [DOI] [PubMed] [Google Scholar]
- 4.Nicholls MG, Ikram H, Fitzpatrick MA, Crozier IG. Converting enzyme inhibitors in heart failure. Eur Heart J. 1988;9(Suppl H):77–83. doi: 10.1093/eurheartj/9.suppl_h.77. [DOI] [PubMed] [Google Scholar]
- 5.Zannad F, van den Broek SAJ, Bory M. Comparison of treatment with lisinopril versus enalapril for congestive heart failure. Am J Cardiol. 1992;70:78C–83C. doi: 10.1016/0002-9149(92)91362-8. [DOI] [PubMed] [Google Scholar]
- 6.Gautam PC, Vargas E, Lye M. Pharmacokinetics of lisinopril (MK521) in healthy young and elderly subjects and in elderly patients with cardiac failure. J Pharm Pharmacol. 1987;39:929–931. doi: 10.1111/j.2042-7158.1987.tb03130.x. [DOI] [PubMed] [Google Scholar]
- 7.Shionoiri H, Minamisawa K, Ueda S, et al. Pharmacokinetics and anti-hypertensive effects of lisinopril in hypertensive patients with normal and impaired renal function. J Cardiovasc Pharmacol. 1990;16:594–600. doi: 10.1097/00005344-199010000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Kelly JG, Doyle G, Donohue J, et al. Pharmacokinetics of enalapril in normal subjects and patients with renal impairment. Br J Clin Pharmacol. 1986;21:63–69. doi: 10.1111/j.1365-2125.1986.tb02823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz D, Averbuch M, Pines A, Kornowski R, Levo Y. Renal toxicity of enalapril in very elderly patients with progressive, severe congestive heart failure. Chest. 1991;100:1558–1561. doi: 10.1378/chest.100.6.1558. [DOI] [PubMed] [Google Scholar]
- 10.Guthrie R. Fosinopril: an overview. Am J Cardiol. 1993;72:22H–24H. doi: 10.1016/0002-9149(93)91051-i. [DOI] [PubMed] [Google Scholar]
- 11.Singhvi SM, Duchin KL, Morrison RA, Willard DA, Everett DW, Frantz M. Disposition of fosinopril sodium in healthy subjects. Br J Clin Pharmacol. 1988;25:9–15. doi: 10.1111/j.1365-2125.1988.tb03275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hui KK, Duchin KL, Kripalani KJ, Chan D, Kramer PK, Yanagawa N. Pharmacokinetics of fosinopril in patients with various degrees of renal function. Clin Pharmacol Ther. 1991;49:457–467. doi: 10.1038/clpt.1991.54. [DOI] [PubMed] [Google Scholar]
- 13.Sica DA, Cutler RE, Parmer RJ, Ford NF. Comparison of the steady-state pharmacokinetics of fosinopril, lisinopril and enalapril in patients with chronic renal insufficiency. Clin Pharmacokinet. 1991;20:420–427. doi: 10.2165/00003088-199120050-00006. [DOI] [PubMed] [Google Scholar]
- 14.Swanson BN, Stauber KL, Alpaugh WC, Weinstein SH. Radioenzymatic assay of angiotensin converting enzyme inhibitors in plasma and urine. Anal Biochem. 1985;148:401–407. doi: 10.1016/0003-2697(85)90245-3. [DOI] [PubMed] [Google Scholar]
- 15.Gehr TWB, Sica DA, Grasela DM, Duchin KL. The pharmacokinetics and pharmacodynamics of fosinopril in haemodialysis patients. Eur J Clin Pharmacol. 1993;45:431–436. doi: 10.1007/BF00315514. [DOI] [PubMed] [Google Scholar]
- 16.Fruncillo RJ, Rocci ML, Jr, Vlasses PH, et al. Disposition of enalapril and enalaprilat in renal insufficiency. Kidney Int. 1987;31(Suppl 20):S-117–S-122. [PubMed] [Google Scholar]
- 17.Lowenthal DT, Irvin JD, Merrill D, et al. The effect of renal function on enalapril kinetics. Clin Pharmacol Ther. 1985;38:661–666. doi: 10.1038/clpt.1985.242. [DOI] [PubMed] [Google Scholar]
- 18.Dickstein K. Pharmacokinetics of enalapril in congestive heart failure. Drugs. 1986;32(Suppl 5):40–44. doi: 10.2165/00003495-198600325-00006. [DOI] [PubMed] [Google Scholar]
- 19.Till AE, Dickstein K, Aarsland T, Gomez HJ, Gregg H, Hichens M. The pharmacokinetics of lisinopril in hospitalised patients with congestive heart failure. Br J Clin Pharmacol. 1989;27:199–204. doi: 10.1111/j.1365-2125.1989.tb05351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston D, Duffin D. Pharmacokinetic profiles of single and repeat doses of lisinopril and enalapril in congestive heart failure. Am J Cardiol. 1992;70:151C–153C. doi: 10.1016/0002-9149(92)91378-h. [DOI] [PubMed] [Google Scholar]
- 21.Kelly JG, Doyle GD, Carmody M, Glover DR, Cooper WD. Pharmacokinetics of lisinopril, enalapril and enalaprilat in renal failure: effects of haemodialysis. Br J Clin Pharmacol. 1988;26:781–786. doi: 10.1111/j.1365-2125.1988.tb05319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Schaik BAM, Geyskes GG, van der Wouw PA, van Rooij HH, Porsius AJ. Pharmacokinetics of lisinopril in hypertensive patients with normal and impaired renal function. Eur J Clin Pharmacol. 1988;34:61–65. doi: 10.1007/BF01061419. [DOI] [PubMed] [Google Scholar]
- 23.Ulm EH, Hichens M, Gomez HJ, et al. Enalapril maleate and a lysine analogue (MK-521): disposition in man. Br J Clin Pharmacol. 1982;14:357–362. doi: 10.1111/j.1365-2125.1982.tb01991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao W, Delany C, Garland T, et al. Single dose oral and intravenous pharmacokinetics and pharmacodynamics of fosinopril in patients and matching controls. J Clin Pharmacol. 1994;34:1025–1028. [Google Scholar]


