Abstract
Aims
To assess the effects of caffeine on the pharmacokinetics of clozapine in healthy volunteers.
Methods
This was an open label randomized crossover study in 12 nonsmoking healthy male volunteers. The subjects received a single oral dose of 12.5 mg clozapine in each phase with or without concomitant intake of caffeine (mean dose: 550 mg day−1, range: 400–1000 mg day−1). Serum concentrations of clozapine and its metabolites desmethyl-clozapine and clozapine-N-oxide were measured during a 48 h period in each phase. In addition, serum concentrations of caffeine and the metabolite paraxanthine were monitored.
Results
A 19% increase in mean clozapine AUC(0,∞) (P = 0.05) and a 14% decrease of mean oral clearance of clozapine were observed during concomitant intake of caffeine (P = 0.05) compared with intake of only clozapine. Statistically significant decreases of mean ratios between AUC(0,12h) for desmethyl-clozapine and AUC(0,12h) for clozapine (−18%), and between AUC(0,12h) for clozapine-N-oxide and AUC(0,12h) for clozapine (−23%) were observed during the caffeine phase (P = 0.03 and 0.02, respectively). Oral clearance of clozapine and the ratio AUC(0,12h) for desmethyl-clozapine/AUC(0,12h) for clozapine were correlated with the paraxanthine/caffeine ratio in serum after intake of caffeine (rs = 0.62; P = 0.03 and rs = 0.77; P = 0.003, respectively).
Conclusions
These results suggest that caffeine in daily doses of 400–1000 mg inhibits the metabolism of clozapine to an extent that might be clinically significant in certain individuals.
Keywords: caffeine, clozapine, CYP1A2, drug interaction, pharmacokinetics
Introduction
The atypical antipsychotic agent clozapine is metabolized principally by the enzyme CYP1A2 [1, 2]. Patients with psychosis have been reported to consume high quantities of caffeine [3, 4], another CYP1A2 substrate. In a case report [5], caffeine was suggested to increase the plasma concentration of clozapine, hereby causing toxic symptoms. Moreover, it has been reported that temporal withdrawal of habitual caffeine intake increases clozapine metabolism [6]. On the other hand, caffeine seems to induce the CYP1A2 activity in rats [7, 8]. The present study was undertaken to investigate systematically whether caffeine affects the pharmacokinetics of clozapine in humans.
Methods
Subjects
Twelve nonsmoking male volunteers between 20 and 39 years old (mean 29 years) took part in the investigation. They were healthy, as assessed by medical history, physical examination and routine blood chemistry tests. Their body weight ranged between 72 and 105 kg (mean 85 kg). They were all characterized as extensive metabolisers of drugs catalysed by CYP2D6 and CYP2C19 by means of the test drugs dextromethorphan and mephenytoin, respectively [9]. Except for the study medications, the study subjects were drug free for at least 2 weeks before start of the study and during the entire sampling period. The study protocol was approved of by the regional Ethics Committee at Umeå University Hospital and each subject gave informed consent before entering the study.
Protocol
The study was performed using a randomized cross over design. Each phase was separated by an interval of at least 2 weeks. In both phases a single dose of 12.5 mg clozapine (Leponex, Sandoz, Basel, Switzerland) was given at 08.00 h after an overnight fast. No food intake except standardized meals at noon and at 17.00 h was allowed during the first 12 h after intake of clozapine. During the control phase intake of food or beverages containing caffeine or other methylxanthines was not allowed from 18 h before clozapine intake until the end of the sampling period. During the caffeine phase the subjects received oral doses of 100 mg caffeine (1 tablet of 100 mg Koffein ACO; ACO, Helsingborg, Sweden) −2, 2, 6, 10, 15, 22, 28, 32 and 39 h after clozapine intake. In addition, dietary caffeine intake (food and beverages) was allowed and was registered and estimated according to data from the Swedish National Food Administration.
Sample collection
For the analysis of clozapine and its metabolites 10 ml venous blood were collected immediately before intake of clozapine and 1, 2, 3, 4, 5, 6, 8, 10, 12, 24, 32 and 48 h later. For the analysis of caffeine and its major metabolite paraxanthine 10 ml venous blood were collected 0 and 24 h after clozapine intake in the control phase and 6, 10 and 24 h after clozapine intake in the caffeine phase Serum was separated within 45 min and stored at −70° C for the clozapine assay and at −20° C for the caffeine assay.
Analytical methods
Serum levels of clozapine and its metabolites desmethyl-clozapine and clozapine-N-oxide were determined by a previously described reversed phase high performance liquid chromatography (h.p.l.c.) method [10, 11]. The limit of quantification was 2 nmol l−1 for clozapine as well as for its metabolites. The method was linear for all analytes up to at least 3000 nmol l−1. The intra-assay coefficients of variation ranged between 1.8% and 7.8% and the interassay coefficients of variation ranged between 1.9% and 14.8% for clozapine and its metabolites. The recoveries varied between 81% and 90%. Caffeine did not interfere with the clozapine assay.
Concentrations of caffeine and paraxanthine in serum were analysed by a h.p.l.c. method described in detail elsewhere [12]. The recoveries for caffeine and paraxanthine were 112% and 102%, respectively. The intra-assay and interassay coefficient of variations were 1.9% and 10.9%, respectively, for caffeine and 5.1% and 12.1%, respectively, for paraxanthine. The method was linear at least to 250 μmol l−1 and the limit of quantification was 0.5 μmol l−1.
Pharmacokinetic assessments
Peak concentrations (Cmax) and peak concentration times (tmax) were taken directly from the original data. Other pharmacokinetic parameters were calculated using the pharmacokinetic program packages Siphar/Win, version 1.13 (Simed SA, Créteil, France). AUC for clozapine was calculated using a two-compartment model. Mean residual areas for clozapine made up 21 and 19% of the total areas for the caffeine phase and the control phase, respectively. For the clozapine metabolites, a noncompartment model was used. Later than 12 h after clozapine intake the concentrations of the clozapine metabolites were below the limit of quantification in several subjects. The extrapolated AUC from 12 h to infinity made up more than 50% of the total AUC for desmethylclozapine in three and four subjects in the control and caffeine phase, respectively, and for clozapine-N-oxide in one and six subjects in the control and caffeine phase, respectively. Therefore, AUC values based upon concentrations from 0 to 12 h (AUC(0,12h)) were calculated for desmethylclozapine and clozapine-N-oxide. The ratio paraxanthine/caffeine was calculated as the mean of the three samples.
Statistical analysis
Statistical calculations were performed with the statistical program package Statistica (version 5.0; Statsoft, Ok, USA). Spearman's rank correlation test and Wilcoxon's test for paired data were used for statistical analysis. Statistical level of significance was set at P = 0.05. It was calculated that 12 subjects were required to reveal a 20% change of clozapine AUC between the two phases with α = 0.05 and β = 0.20.
Results
Clozapine and desmethyl-clozapine could be detected in serum of all subjects for up to 36 h. The concentrations of clozapine-N-oxide were above the limit of quantification for at least 12 h in all but three subjects. A value of 1 nmol l−1, i.e. half of the limit of quantification, was assigned in these cases to be able to calculate AUC(0,12h).
In the caffeine phase the trough mean serum concentrations of caffeine and paraxanthine (range 6.6 μmol l−1–39.1 μmol l−1) and 14.4 μmol l−1 (range 5.0 μmol l−1–28.1 μmol l−1), respectively. In the control phase, the serum concentrations of caffeine and paraxanthine were below the limit of quantification in nine and seven subjects, respectively. The other subjects had low concentrations. During the caffeine phase, the total caffeine intake ranged between 500 mg and 700 mg day 1 and between 400 mg and 1000 mg day 2, with a mean of 550 mg day−1.
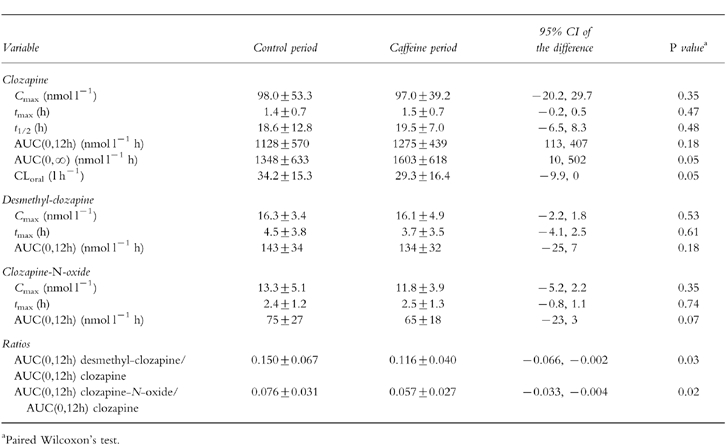
Pharmacokinetic parameters are shown in Table 1. A 19% increase (range: −14%–+97%) (P = 0.05) in mean clozapine AUC(0,∞) and a 14% decrease (range: −49% to +7%) (P = 0.05) of mean oral clearance of clozapine was observed during concomitant intake of caffeine as compared with the control phase. There were statistically significant decreases in mean ratios between AUC(0,12h) for desmethyl-clozapine and AUC(0,12h) for clozapine (−18%), and between AUC(0,12h) for clozapine-N-oxide and AUC(0,12h) for clozapine (−23%) when caffeine was added (Table 1). The individual differences in these ratios ranged between −51% and +6%, and −52% and +11%, respectively. There were no relationships between trough values of caffeine and changes in clozapine AUC between the two phases (rs −0.31, P = 0.33) or between the caffeine doses and changes in clozapine AUC (rs −0.07, P = 0.83).
Table 1.
Pharmacokinetic parameters after an oral dose of 12.5 mg clozapine with or without intake of caffeine (n = 12). Results are expressed as means±s.d.
Oral clearance of clozapine in the caffeine phase correlated significantly with the paraxanthine/caffeine ratio of the three measurements in the caffeine phase (rs = 0.62, P = 0.03) (Figure 1a). Moreover, there was also a significant correlation between the desmethyl-clozapine AUC(0,12h)/clozapine AUC(0,12h) ratio and the paraxanthine/caffeine ratio (rs = 0.77, P = 0.003) (Figure 1b), in contrast, no significant relationship between the clozapine-N-oxide AUC(0,12h)/clozapine AUC ratio and the mean paraxanthine/caffeine ratio was revealed (rs = 0.47, P = 0.12).
Figure 1.
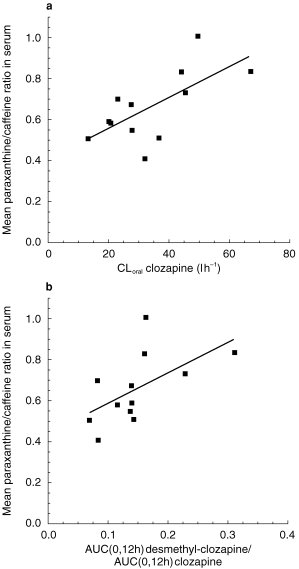
a) Relationship between oral clearance of clozapine in the control phase and mean value of three paraxanthine/caffeine ratios in the caffeine phase in 12 non-smoking healthy volunteers who were given a single dose of 12.5 mg clozapine in each phase. A significant positive correlation was revealed (rs = 0.62;/P = 0.03). b) Relationship between the desmethyl-clozapine AUC(0,12h)/clozapine AUC(0,12h) ratio in the control phase and mean value of three paraxanthine/caffeine ratios in the caffeine phase. A significant positive correlation was revealed (rs = 0.77, P = 0.003).
All study subjects suffered from sedation after intake of clozapine. Other reported side-effects were dry mouth, nausea, dizziness, headache, myalgia, restless legs and unclear speech. One subject reported recurrent episodes of nausea related to the intake of caffeine.
Discussion
The principal finding in the present study is that caffeine inhibits clozapine clearance. Previously published reports indicate that clozapine is metabolized mainly by CYP1A2 which is responsible for the N-demethylation to desmethyl-clozapine [1, 2] and can alter the metabolism of clozapine [5, 6]. In seven schizophrenic patients average clozapine concentrations decreased 37% and the desmethyl-clozapine/clozapine ratio increased 120% 5 days after caffeine withdrawal compared with baseline values [6]. The estimated daily caffeine intake was 1100 mg in one subject and 150–200 mg in the remaining subjects. In a case report, caffeine intake (1200 mg day−1) was suspected to increase the plasma concentration of clozapine resulting in symptoms indicative of clozapine toxicity [5]. After caffeine withdrawal plasma concentration of clozapine fell with 58% and the desmethyl-clozapine/clozapine ratio, which has been suggested to be a estimate of CYP1A2 activity, increased from 0.42 to 0.52.
Our results are consistent with these previous observations although the decrease in clozapine clearance when clozapine was administered with caffeine was moderate. However, in one of the 12 subjects, clozapine AUC was nearly doubled indicating that certain individuals may be more predisposed to this effect. The decrease in the ratio AUC(0,12h) for desmethyl-clozapine and AUC(0,12h) for clozapine indicates that the underlying mechanism is reduced CYP1A2 activity caused by caffeine intake. The role of CYP1A2 is further supported by the finding that the above mentioned ratios were significantly correlated to the paraxanthine/caffeine ratio, an index used to measure CYP1A2 activity [13].
Dose-dependent elimination of caffeine in humans has been reported in several studies [14–17]. It has been suggested that the elimination of caffeine is linear for doses up to 5 mg kg−1 and becomes nonlinear somewhere between 5 and 7.5 mg kg−1 [14]. Due to the dose-dependent kinetics of caffeine, a pronounced and disproportional reduction of clearance for clozapine and other CYP1A2 substrates might be the result when caffeine is ingested in higher quantities than those used in this study, which was 4–12 mg kg−1 day−1. Moreover, the capacity-limited metabolism of caffeine could be a result of the saturation of CYP1A2 by caffeine itself or a metabolite of caffeine.
In conclusion, the present study indicates that caffeine reduces clozapine clearance most likely by inhibiting CYP1A2. Changes in habitual caffeine intake can therefore explain some of the large kinetic variability for clozapine [18] and may have clinical consequences in certain individuals. Hence, we recommend that variations in the intake of caffeine should be taken into consideration when clozapine is used.
Acknowledgments
We acknowledge Kerstin Granström, Gunnel Persbo-Lundqvist and Emma Söderström for excellent clinical and technical assistance. Staffan Hägg has a fellowship in clinical pharmacology, set up by Merck, Sharp and Dohme.
References
- 1.Bertilsson L, Carrillo JA, Dahl ML, et al. Clozapine disposition covaries with CYP1A2 activity determined by a caffeine test. Br J Clin Pharmacol. 1994;38:471–473. doi: 10.1111/j.1365-2125.1994.tb04385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jerling M, Lindstrom L, Bondesson U, Bertilsson L. Fluvoxamine inhibition and carbamazepine induction of the metabolism of clozapine: evidence from a therapeutic drug monitoring service. Ther Drug Monit. 1994;16:368–374. doi: 10.1097/00007691-199408000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Hughes JR, McHugh P, Holtzman S. Caffeine and schizophrenia. Psychiatr Serv. 1998;49:1415–1417. doi: 10.1176/ps.49.11.1415. [DOI] [PubMed] [Google Scholar]
- 4.Winstead DK. Coffee consumption among psychiatric inpatients. Am J Psychiatry. 1976;133:1447–1450. doi: 10.1176/ajp.133.12.1447. [DOI] [PubMed] [Google Scholar]
- 5.Odom White A, Leon J. Clozapine levels and caffeine. J Clin Psychiatry. 1996;57:175–176. [PubMed] [Google Scholar]
- 6.Carrillo JA, Herraiz AG, Ramos SI, Benitez J. Effects of caffeine withdrawal from the diet on the metabolism of clozapine in schizophrenic patients. J Clin Psychopharmacol. 1998;18:311–316. doi: 10.1097/00004714-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Chen L, Bondoc FY, Lee MJ, Hussin AH, Thomas PE, Yang CS. Caffeine induces cytochrome P4501A2: induction of CYP1A2 by tea in rats. Drug Metab Dispos. 1996;24:529–533. [PubMed] [Google Scholar]
- 8.Ayalogu EO, Snelling J, Lewis DF, Talwar S, Clifford MN, Ioannides C. Induction of hepatic CYP1A2 by the oral administration of caffeine to rats: lack of association with the Ah locus. Biochim Biophys Acta. 1995;17:89–94. doi: 10.1016/0925-4439(95)00071-b. [DOI] [PubMed] [Google Scholar]
- 9.Spigset O, Granberg K, Hägg S, Söderström E, Dahlqvist R. Non-linear fluvoxamine disposition. Br J Clin Pharmacol. 1998;45:257–263. doi: 10.1046/j.1365-2125.1998.00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weigmann H, Hiemke C. Determination of clozapine and its major metabolites in human serum using automated solid-phase extraction and subsequent isocratic high-performance liquid chromatography with ultraviolet detection. J Chromatogr. 1992;583:209–216. doi: 10.1016/0378-4347(92)80554-4. [DOI] [PubMed] [Google Scholar]
- 11.Hägg S, Granberg K, Spigset O, Persbo Lundqvist G, Mjörndal T, Dahlqvist R. Absence of interaction between erythromycin and a single dose of clozapine. Eur J Clin Pharmacol. 1999;55:221–226. doi: 10.1007/s002280050621. [DOI] [PubMed] [Google Scholar]
- 12.Spigset O, Hägg S, Söderström E, Dahlqvist R. Lack of correlation between fluvoxamine clearance and CYP1A2 activity as measured by systemic caffeine clearance. Eur J Clin Pharmacol. 1999;54:943–946. doi: 10.1007/s002280050579. [DOI] [PubMed] [Google Scholar]
- 13.Spigset O, Hägg S, Söderström E, Dahlqvist R. The paraxanthine/caffeine ratio in serum or saliva as a measure of CYP1A2 activity: When should the sample be obtained? Pharmacogenetics. 1999;9:409–412. [PubMed] [Google Scholar]
- 14.Kamimori GH, Lugo SI, Penetar DM, et al. Dose-dependent caffeine pharmacokinetics during severe sleep deprivation in humans. Int J Clin Pharmacol Ther. 1995;33:182–186. [PubMed] [Google Scholar]
- 15.Denaro CP, Brown CR, Wilson M, Jacob P 3rd, Benowitz NL. Dose-dependency of caffeine metabolism with repeated dosing. Clin Pharmacol Ther. 1990;48:277–285. doi: 10.1038/clpt.1990.150. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan GB, Greenblatt DJ, Ehrenberg BL, et al. Dose-dependent pharmacokinetics and psychomotor effects of caffeine in humans. J Clin Pharmacol. 1997;37:693–703. doi: 10.1002/j.1552-4604.1997.tb04356.x. [DOI] [PubMed] [Google Scholar]
- 17.Tang Liu DD, Williams RL, Riegelman S. Disposition of caffeine and its metabolites in man. J Pharmacol Exp Ther. 1983;224:180–185. [PubMed] [Google Scholar]
- 18.Jerling M, Merle Y, Mentre F, Mallet A. Population pharmacokinetics of clozapine evaluated with the nonparametric maximum likelihood method. Br J Clin Pharmacol. 1997;44:447–453. doi: 10.1046/j.1365-2125.1997.t01-1-00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


