Abstract
Aims
Formoterol is a β2-adrenoceptor agonist marketed as a racemic mixture of the active (R; R)- and inactive (S; S)-enantiomers (rac-formoterol). The drug produces prolonged bronchodilation by inhalation but there is significant interpatient variability in duration of effect. Previous work has shown that in humans formoterol is metabolized by conjugation with glucuronic acid but little is known about the stereoselectivity of this reaction. The aim of the present study was to investigate the glucuronidation of formoterol enantiomers in vitro by human liver microsomes.
Methods
The kinetics of formation of formoterol glucuronides during incubation of racemate and of single formoterol enantiomers with human liver microsomes (n = 9) was characterized by chiral h.p.l.c. assay.
Results
The kinetics of glucuronidation of the two formoterol enantiomers obeyed the Michaelis-Menten equation. Glucuronidation of formoterol was stereoselective and occurred more than two times faster for (S; S)-formoterol than for (R; R)-formoterol. In incubations with single formoterol enantiomers, the median (n = 9) Km values for (R; R)-glucuronide and (S; S)-glucuronide were 827.6 and 840.4 μm, respectively, and the median Vmax values were 2625 and 4304 pmol min−1 mg−1, respectively. Corresponding values determined in incubations with rac-formoterol were 357.2 and 312.1 μm and 1435 and 2086 pmol min−1 mg−1 for (R; R)- and (S; S)-glucuronide, respectively. Interindividual variation was large with the ratio of Vmax/Km (S; S/R; R) ranging from 0.57 to 6.90 for incubations with rac-formoterol.
Conclusions
Our study demonstrates that glucuronidation of formoterol by human liver microsomes is stereoselective and subject to high interindividual variability. These findings suggest that clearance of formoterol in humans is subject to variable stereoselectivity which could explain the variation in duration of bronchodilation produced by inhaled formoterol in patients with asthma.
Keywords: formoterol, human liver microsomes, stereoselective glucuronidation
Introduction
β2-Adrenoceptor agonists are used in the treatment of asthma and are generally administered via the lung [1]. The drugs are marketed as racemic mixtures although only the R-enantiomers are pharmacologically active [2]. A number of in vivo and in vitro studies has shown that the metabolism of β2-adrenoceptor agonists is stereoselective [3–7]. This has important consequences in the oral delivery of these drugs since they are subject to extensive first pass metabolism [3, 8]. It may also be important in the action of inhaled β2-adrenoceptor agonists since the majority of an inhaled dose is swallowed [9, 10].
Formoterol is a potent long-acting β2-adrenoceptor agonist administered via inhalation. It has two asymmetric centres but is used clinically as the racemic mixture of the active (R; R)-and inactive (S; S)-formoterol (rac-formoterol) [11]. A nonchiral study of urinary excretion of formoterol enantiomers after oral administration of rac-formoterol showed that the drug mainly undergoes glucuronidation in humans [12]. In addition, a chiral study of urinary excretion of formoterol enantiomers after inhalation of dry powder suggested that metabolism of formoterol is stereoselective [13]. The aim of the present study was to investigate the glucuronidation of formoterol enantiomers by human liver microsomes.
Methods
Chemicals and reagents
rac-formoterol fumarate (R; R)- and (S; S)-formoterol fumarate were kindly donated by Ciba-Geigy (Basle, Switzerland). H.p.l.c. grade 2-propanol, AR grade ethyl acetate, sodium dihydrogen orthophosphate, disodium hydrogen orthophosphate and sodium hydrogen carbonate were from BDH (Poole, UK). Uridine 5′-diphosphoglucuronic acid (UDPGA) and β-glucuronidase (EC 3.2.1.31, Type H-1 from Helix pomatia ) were purchased from Sigma Chemical Company (St Louis, MO, USA). Brij-58 was purchased from Aldrich Chemical Company (St Louis, MO, USA). EDTA-Na2 was from May & Baker (Dagenham, UK). Distilled, deionized water was produced by a Milli-Q Reagent Water System (Millipore, MA, USA).
Chromatography
The h.p.l.c. system consisted of a Shimadzu LC-10AD pump (Shimadzu Corporation, Kyoto, Japan), a manual injector fitted with a 50 μl loop (Rheodyne 7125, Cotati, CA, USA), a chiral-CBH (Cellobiohydrolase) 10×3.0 mm guard column and a chiral-CBH 100×4.0 mm analytical column (ChromTech AB, Hagersten, Sweden). Detection was via an ESA coulometric electrochemical detector with a Model 5020 guard cell operated at 1000 mV and a Model 5011 dual-electrode analytical cell (ESA, Inc., Bedford, MA, USA) with detectors 1 and 2 set at 300 and 700 mV, respectively. The signal from detector 2 was processed by a Hitachi D-2500 Chromato-Integrator (Hitachi Ltd, Tokyo, Japan) to obtain peak heights. The mobile phase of 0.025 m sodium phosphate buffer containing 10% 2-propanol and 50 μm EDTA-Na2 pH 6.3 was filtered through a 0.45 μm filter and degassed by sonication under vacuum before use. The flow rate was 0.9 ml min−1 and the system was operated at ambient temperature. Under these conditions (R; R)- and (S; S)-formoterol eluted at 9.0 and 15.0 min, respectively (α=3.4). The glucuronides were not detected and metabolite concentrations were determined as formoterol enantiomers after enzymatic cleavage of metabolites with β-glucuronidase.
Human liver microsomes
Nine human liver samples were included in the study. The mean age of the liver donors was 63 years (47–71 years). Livers 1 and 2 were from registered organ donors (Caucasian males) who were healthy at the time of death. Livers 3–9 were surgical waste from patients (Caucasian males) undergoing removal of liver tumours secondary to colorectal cancer. Histologically normal liver tissue was separated from diseased tissue by a pathologist. The use of the tissue was approved by the Central Regional Health Authority Ethics Committee (Wellington). The liver samples were stored at −84° C until used. Microsomes were prepared by differential centrifugation as previously described by Robson et al. [14]. The protein content of the microsomal preparation was determined by the Lowry method [15].
Incubation
The microsomal incubation mixture (final volume 250 μl) consisted of 50 mm Tris buffer pH 7.4, 10 mm MgCl2, an aliquot of microsomal preparation and varying final concentrations of either (R; R)-formoterol fumarate (S; S)-formoterol fumarate (100–1000 μm ) or rac-formoterol fumarate (200–2000 μm ). Mixtures were preincubated with 0.1 mg Brij-58/mg protein for 30 min at room temperature. Reactions were initiated by the addition of 3 mm UDPGA. Incubation without the substrate served as blank and incubation without UDPGA served as control. Incubations were carried out in a shaking waterbath at 37° C and were terminated by the addition of 750 μl of 50 mm Tris buffer pH 8.5 and vortexing with 4 ml of ethyl acetate. All incubations were done in duplicate.
Analytical methods
Incubations were extracted with a further 2×4 ml ethyl acetate to remove all unmetabolized formoterol. Aliquots (200 μl) of the remaining aqueous phase were transferred to clean glass tubes and evaporated to dryness in a Speed Vac Concentrator with a −106° C trap (SVC 200H, Savant Instruments, Farmingdale, NY, USA) under reduced pressure and 400 g. Residues were treated with 1 ml aliquots of 0.1 m acetate buffer pH 5.0 containing β-glucuronidase (2000 units) in a shaking waterbath at 37° C for 20 h. Formoterol enantiomers were shown to be stable under these conditions. After treatment, 2 ml of water was added and the pH adjusted to 8.5 by addition of 0.5 g sodium hydrogen carbonate. Formoterol enantiomers were then extracted with 5 ml ethyl acetate. Portions of the organic phase (3.5 ml) were transferred to clean glass tubes and evaporated to dryness. Residues were dissolved in 100 μl mobile phase and 20 μl aliquots analysed by chiral h.p.l.c.
Standard curves for formoterol enantiomers were determined by treating formoterol (prepared by evaporation of formoterol standards 200 μl of 1.25–20.0 μm rac-formoterol in methanol) in the same way as residues of incubations but without adding β-glucuronidase and without incubation at 37° C. The standard curves were linear (r > 0.99) and the recoveries for formoterol enantiomers were >80%. Intra-and interday coefficients of variation of the assay were <10%.
Data analysis
Linear regression was carried out using a validated computer program (Pharmaceutical Statistical Regression, School of Pharmacy, University of Otago, Dunedin, New Zealand). Enzyme kinetic parameters [maximum velocity of reaction (Vmax) and the Michaelis-Menten constant (Km)] were determined using a least squares nonlinear modelling program (MINIM, Dr R. Purves, Department of Pharmacology, University of Otago). Enzyme kinetic parameters are given as median and range. Differences in the enzyme kinetic parameters between (R; R)- and (S; S)-formoterol were evaluated by a paired nonparametric test. The 95% confidence intervals for differences between medians (CI) are given along with two-tailed P-values determined using the Wilcoxon paired signed rank test with a significance level of P < 0.05.
Results
Incubation of single enantiomers of formoterol produced only one metabolite and there was no reaction in the absence of UDPGA (Figure 1). During incubation of rac-formoterol and the individual enantiomers, the rate of glucuronidation was linear for both enantiomers over 120 min at microsomal protein concentrations up to 3.0 mg ml−1. A microsomal protein concentration of 600 μg ml−1 and incubation time of 60 min were used to determine enzyme kinetic parameters.
Figure 1.
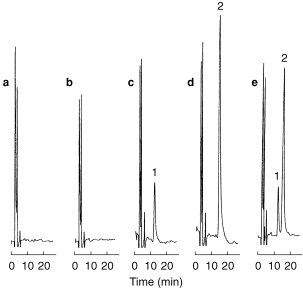
H.p.l.c. chromatograms obtained for (a) blank incubation sample (without formoterol), (b) incubation sample spiked with 2000 μm rac-formoterol (without UDPGA), (c) incubation sample spiked with 200 μm (R; R)-formoterol (with UDPGA), (d) incubation sample spiked with 200 μm (S; S)-formoterol (with UDPGA), and (e) incubation sample spiked with 400 μm rac-formoterol (with UDPGA). Microsomal protein concentration 600 μg ml−1, incubation time 60 min. Peaks: 1=(R; R)-formoterol, 2=(S; S)-formoterol.
The rate of formation of glucuronides in incubations of (R; R)-, (S; S)- and rac-formoterol and Eadie-Hofstee plots of transformed data are illustrated in Figure 2. The formation of glucuronides was described by Michaelis-Menten kinetics and kinetic parameters for the nine liver samples are given in Table 1. In incubations with pure enantiomers, the Km values were not significantly different [P = 0.95, 95% CI (−212, 187)]. The Vmaxvalues were also not significantly different [P = 0.07, 95% CI (−3239, −119)] although in every case except liver 8, the Vmax of (S; S)-formoterol was greater than that of its antipode. Accordingly the data were reanalysed excluding liver 8. In this case, the Km values were again not significantly different [P = 0.91, 95% CI (−249, 215)] but the Vmax for (S; S)-formoterol was now significantly greater than that for (R; R)-formoterol [P = 0.008, 95% CI (1124, 3313)]. In incubations with racemate, the metabolic profile of the two enantiomers was similar to that in incubations with single enantiomers but the median values of kinetic parameters were significantly smaller for both (R; R)-formoterol [Km, P = 0.004, 95% CI (−724, −217); Vmax, P = 0.004, 95% CI (−2315, −63)] and (S; S)-formoterol [Km, P = 0.004, 95% CI (−666, −391); Vmax, P = 0.004, 95% CI (−3071, −1365)]. The interindividual variation of the efficiencies (Vmax/Km ) was large for both enantiomers (Figure 3) and the stereoselectivity of glucuronidation, as indicated by the ratio of Vmax/Km for S; S/R; R, also varied widely.
Figure 2.
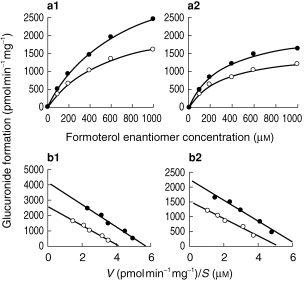
Enantioselective glucuronidation of formoterol by human liver microsomes. (a) Velocity vs substrate concentration and (b) corresponding Eadie-Hofstee plots for incubation of (a1) and (b1) single enantiomers and (a2) and (b2) rac-formoterol with human liver microsomes. Microsomal protein concentration 600 μg ml−1, incubation time 60 min. (S; S)-formoterol; o (R; R)-formoterol. Data points represent the median values for nine human livers.
(S; S)-formoterol; o (R; R)-formoterol. Data points represent the median values for nine human livers.
Table 1.
Enzyme kinetic parameters for the glucuronidation of (R; R)- and (S; S)-formoterol during incubation of single enantiomers and rac-formoterol with human liver microsomes (Data are median and range for nine human livers).

Figure 3.
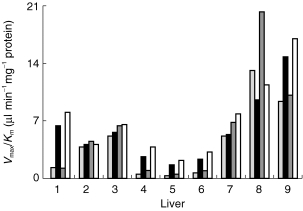
Variation in the efficiencies (Vmax/Km ) for glucuronidation of formoterol during incubation of single enantiomers and rac-formoterol with human liver microsomes prepared from nine human livers. (R; R)-formoterol (incubation with (R; R)-formoterol); ▪ (S; S-formoterol (incubation with (S; S)-formoterol; (R; R)-formoterol (incubation with racemate); □ (S; S)-formoterol (incubation with racemate).
Discussion
This study provides the first kinetic characterization of the glucuronidation of formoterol enantiomers by human liver microsomes. To obtain reasonably precise values of Km and Vmax, the initial substrate concentration in incubations should cover the range 0.5 Kmto 5 Km [16] and the rate at the highest concentration should approximate the Vmax value. The range of formoterol enantiomer concentrations used was satisfactory in incubations of racemate but limited in incubations of single enantiomers by the availability of pure enantiomers. However, visual inspection of Eadie-Hofstee plots (Figure 2) showed that the linearity and scatter of the data collected were satisfactory.
The fact that Km and Vmax values determined in incubations of rac-formoterol were smaller than corresponding values determined in incubations of single enantiomers indicates some enantiomer–enantiomer interaction during rac-formoterol glucuronidation. The Km values for the two enantiomers were similar and well above the usual therapeutic range (low pg ml−1 range) suggesting that saturation of metabolism is unlikely in clinical practice. The glucuronidation of formoterol showed a more than two-fold preference for (S; S)-formoterol, although in one case the order was reversed (liver 8), The data are consistent with in vivo results from our laboratory showing that urinary and faecal excretion of (S; S)-formoterol glucuronide exceeds that of (R; R)-formoterol glucuronide by a factor greater than two after oral dosing.
The stereoselectivity of glucuronidation of formoterol was mainly due to an enantiomeric difference in Vmax. In comparison with other β2-adrenoceptor agonists, the stereochemical pattern of formoterol metabolism is similar to that for terbutaline [5] but opposite to that for salbutamol [6] both of which occur by sulphation in humans. Like formoterol, terbutaline stereoselectivity mainly results from a difference in Vmax but for salbutamol, where metabolism involves a preference for the (R)-enantiomer, stereoselectivity mainly arises from an enantiomeric difference in Km.
Human response to therapeutic doses of inhaled formoterol has been shown to vary considerably in duration of effect [17]. Our study shows that stereoselectivity in both the rate (Vmax ) and extent (the ratio of Vmax/Km ) of formoterol glucuronidation varies widely between subjects. This variability in vivo could give rise to different plasma levels of formoterol enantiomers which in turn could explain the variability in clinical response. Large interindividual variation in the rate of human hepatic microsomal glucuronidation in vitro has been reported previously for other drugs [18, 19].
The interindividual variation in uridine 5′-diphospho- glucuronosyltransferase (UDPGT) activity may be related to differences in age, sex, diet, disease state, exposure to xenobiotics or pharmacogenetics [20, 21]. In addition, it has been shown that some human UDPGT isoenzymes are stereoselective [22, 23] and that some can be selectively induced by pretreatment with different agents [21, 24]. Since most of the liver samples in our study were surgical waste from patients undergoing removal of liver tumours, the large variation in UDPGT activity and stereoselectivity is more likely to reflect the influence of disease state and/or previous drug treatment.
Our study demonstrates that glucuronide conjugation of formoterol by human liver microsomes is stereoselective and highly variable. Since formoterol is a potent long-acting β2-adrenoceptor agonist, such variability could partially explain the differences in duration of effect observed clinically. Our study provides impetus for further studies on the metabolism of formoterol enantiomers by purified UDPGT isoforms or whole cell homogenates of recombinant cell lines expressing single UDPGTcDNA. In addition, formoterol may be a useful substrate for the assessment of glucuronidation polymorphism.
Acknowledgments
The authors wish to thank Mr Richard Stubbs, Consultant Surgeon, the Wakefield Clinic, Wellington for assistance in obtaining human liver tissue. Ms Zhang also thanks the University of Otago for a PhD Scholarship.
References
- 1.Morgan DJ. Clinical pharmacokinetics of β-agonists. Clin Pharmacokinet. 1990;18:270–294. doi: 10.2165/00003088-199018040-00002. [DOI] [PubMed] [Google Scholar]
- 2.Nyberg L. Pauwels R, O’Byrne PM. Lung Biology in Health and Disease. Vol. 106. New York: Marcel Dekker Inc; 1997. β2-agonists in asthma treatment; pp. 87–130. [Google Scholar]
- 3.Boulton DW, Fawcett JP. Enantioselective disposition of salbutamol in man following oral and intravenous administration. Br J Clin Pharmacol. 1996;41:35–40. doi: 10.1111/j.1365-2125.1996.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 4.Borgstrom L, Nyberg L, Jonsson S, Lindberg C, Paulson J. Pharmacokinetic evaluation in man of terbutaline given as separate enantiomers and as the racemate. Br J Clin Pharmacol. 1989;27:49–56. doi: 10.1111/j.1365-2125.1989.tb05334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walle T, Walle UK. Stereoselective sulphate conjugation of racemic terbutaline by human liver cytosol. Br J Clin Pharmacol. 1990;30:127–133. doi: 10.1111/j.1365-2125.1990.tb03752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walle UK, Pesola GR, Walle T. Stereoselective sulphate conjugation of salbutamol in humans: Comparison of hepatic, intestinal and platelet activity. Br J Clin Pharmacol. 1993;35:413–418. doi: 10.1111/j.1365-2125.1993.tb04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson AA, Wang J, Koch P, Walle T. Stereoselective sulphate conjugation of fenoterol by human phenolsulphotransferases. Xenobiotica. 1997;27:1147–1154. doi: 10.1080/004982597239903. [DOI] [PubMed] [Google Scholar]
- 8.Boulton DW, Fawcett JP. Pharmacokinetics and pharmacodynamics of single oral doses of albuterol and its enantiomers in humans. Clin Pharmacol Ther. 1997;62:138–144. doi: 10.1016/S0009-9236(97)90061-8. [DOI] [PubMed] [Google Scholar]
- 9.Newman SP, Woodman G, Clarke SW, Sackner MA. Enhanced drug delivery from metered dose inhalers with InspirEase. Am Rev Respir Dis. 1985;131:96. [Google Scholar]
- 10.Newman SP, Pavia D, Moren F, Sheahan NF, Clarke SW. Deposition of pressurised aerosols in the human respiratory tract. Thorax. 1981;36:52–55. doi: 10.1136/thx.36.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trofast J, Osterberg K, Kallstrom BL, Waldeck B. Steric aspects of agonism and antagonism at β-adrenoceptors: Synthesis of and pharmacological experiments with the enantiomers of formoterol and their diastereomers. Chirality. 1991;3:443–450. doi: 10.1002/chir.530030606. [DOI] [PubMed] [Google Scholar]
- 12.Kamimura H, Sasaki H, Higuchi S, Shiobara Y. Quantitative determination of the β-adrenoceptor stimulant formoterol in urine by gas chromatography mass spectrometry. J Chromatogr. 1982;229:337–345. doi: 10.1016/s0378-4347(00)84276-0. [DOI] [PubMed] [Google Scholar]
- 13.Butter JJ, Berg BTJ, Portire EJG, Kaiser G, Boxtel CJ. Determination by HPLC with electrochemical detection of formoterol enantiomers in urine of healthy human subjects after single dose racemate inhalations. Pharm World Sci. 1995;17(Suppl):D6. [Google Scholar]
- 14.Robson RA, Mathews AP, Miners JO, et al. Characterisation of theophylline metabolism in human liver microsomes. Br J Clin Pharmacol. 1987;24:293–300. doi: 10.1111/j.1365-2125.1987.tb03172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 16.Henderson PJF. Enzyme Assays. A Practical Approach. In: Eisenthal R, Danson MJ, editors. The Practical Approach Series. New York: Oxford University Press; 1992. pp. 277–313. [Google Scholar]
- 17.Arvidsson P, Larsson S, Lofdahl CG. Objective and subjective bronchodilation over 12 hours after inhaled formoterol: individual responses. J Asthma. 1993;30:459–465. doi: 10.3109/02770909309056755. [DOI] [PubMed] [Google Scholar]
- 18.Temellini A, Giuliani L, Pacifici GM. Interindividual variability in the glucuronidation and sulphation of ethinyloestradiol in human liver. Br J Clin Pharmacol. 1991;31:661–664. doi: 10.1111/j.1365-2125.1991.tb05589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lacarelle B, Rajaonarison JF, Catalin J, Durand A, Cano JP. UDP-glucuronosyltransferase activity toward digitoxigenin monodigitoxoside in human liver microsomes. Drug Metab Dispos. 1992;21:338–341. [PubMed] [Google Scholar]
- 20.Burchell B, Coughtrie MWH. UDP-Glucuronosyltransferases. In: Kalow W, editor. International Encyclopedia of Pharmacology and Therapeutics. New York: Pergamon Press; 1992. pp. 195–225. Section 137. [Google Scholar]
- 21.Kroemer HK, Klote U. Glucuronidation of drugs. Clin Pharmacokinet. 1992;23:292–310. doi: 10.2165/00003088-199223040-00005. [DOI] [PubMed] [Google Scholar]
- 22.Dahl-Puustinen ML, Dumont E, Bertilsson L. Glucuronidation of E-10-hydroxynortriptyline in human liver, kidney and intestine: Organ-specific differences in enantioselectivity. Drug Metab Dispos. 1989;17:433–436. [PubMed] [Google Scholar]
- 23.Dumont E, Bahr CV, Perry TJ, Bertilsson L. Glucuronidation of the enantiomers of E-10-hydroxynortriptyline in man and rat liver microsomes. Pharmacol Toxicol. 1987;61:335–341. doi: 10.1111/j.1600-0773.1987.tb01831.x. [DOI] [PubMed] [Google Scholar]
- 24.Batt AM, Magdalou J, Vincent–viry M, et al. Drug metabolizing enzymes related to laboratory medicine: cytochromes P–450 and UDP–glucuronosyltransferases. Clin Chim Acta. 1994;226:171–190. doi: 10.1016/0009-8981(94)90214-3. [DOI] [PubMed] [Google Scholar]


