Abstract
Aims
To evaluate the effectiveness of a health authority/pharmaceutical company collaborative intervention to influence the choice of proton pump inhibitors
Methods
Randomized controlled trial, with general practices forming the unit of allocation and analysis.
Results
Constructive working relationships were achieved with five of six pharmaceutical companies involved. One hundred and two out of 140 practitioners in intervention group practices received at least one visit from an industry representative. There were no reports of representatives operating outside their agreed remit. Prescribing in both the intervention and control group moved towards that recommended by the guidelines but there was no difference between the groups in either the proportion of prescriptions in line with the guidelines or the overall cost.
Conclusions
Health authorities can achieve professional working relationships with the pharmaceutical industry although no changes in practice attributable to the intervention are achieved. Further work is required to develop effective means to influence prescribing in line with independent guidelines especially in the context of the development of Primary Care Groups.
Keywords: outreach visits, pharmaceutical industry/health authority collaboration, prescribing practice, randomized evaluation
Introduction
There is widespread concern that the recommendations from evidence based guidelines do not translate automatically into changes in practice [1]. However, substantial changes in prescribing behaviour do occur, and these are often accompanied by considerable marketing activities from the pharmaceutical industry [2]. There are occasions when the goals of the NHS and the pharmaceutical industry are clearly in tandem, such as in attempting to increase the use of angiotensin converting enzyme inhibitors in patients with heart failure [3]. Successful collaboration may be beneficial to patients while meeting the needs of both the NHS and industry.
There is growing health policy interest in ‘managed care’ or ‘disease management’ in the UK [4]. In an Executive letter, the National Health Service Executive indicated to Health Authorities that there had been ‘a number of approaches to the NHS at Regional or local level by pharmaceutical companies offering disease management packages or similar agreements for the preferential purchase of drugs and other pharmaceutical products’ [5]. The Executive letter counselled caution in the development of such arrangements on the grounds that these may run counter to the aims of the NHS, and also may be in conflict with other specific health policies such as the Pharmaceutical Pricing Regulatory Scheme. Many health authorities have developed collaborative agreements on specific projects such as the sponsorship of guidelines development or support for clinical audit. At least three health authorities in the UK, Warwickshire HA, Calderdale and Kirklees HA and North-west Anglia HA have developed more extensive projects. Uniquely in Warwickshire these efforts have been subjected to rigorous evaluation that aimed to establish if the approach was worthy of development in the NHS. This paper describes the results of a randomized controlled trial evaluating the effectiveness of a collaborative approach between the pharmaceutical industry and a health authority in modifying prescribing practice in line with evidence based guidelines for prescribing proton pump inhibitors in primary care.
Methods
Intervention
The main aims of the intervention were
to test the feasibility of a Health Authority working in partnership with pharmaceutical companies to achieve agreed objectives
to establish whether the marketing approach for the delivery of Health Authority messages significantly influences prescribing decisions made by GPs;
to achieve a strategic shift in the allocation of prescribing resources from one therapeutic area to another.
The feasibility of the approach was investigated in all practices in Warwickshire between October 1997 and April 1998 for cardiovascular prescribing in collaboration with six companies who were invited by the health authority to take part. These were Bayer, Glaxo-Wellcome, Hoechst Marion Roussel, Merck Sharpe & Dohme, Rhône Poulenc Rorer, Wyeth. Merck Sharpe & Dohme participated only in the development stage of the project. Prescribing for the management of dyspepsia was also targeted during this period in the context of a randomized trial in which practices were randomized to the collaborative intervention or to control. The management of dyspepsia was selected since a major prescribing intervention was straight forward—aiming for substitution of one drug for an alternative deemed therapeutically equivalent but less costly.
The specific prescribing behaviour targeted was to promote a switch from prescribing omeprazole to lansoprazole, within the criteria for prescribing advised by the independently produced guidelines from the Warwickshire Multi-disciplinary Audit Advisory Group [6]. It was also in line with price information provided alongside these guidelines by the health authority. The rationale for the switch in prescribing behaviour was that savings could be achieved that could subsequently be invested in other clinical areas (notably in cardiovascular disease) without compromising the quality of care for patients appropriately receiving proton pump inhibitors.
The intervention involved attempting to facilitate visits by pharmaceutical company representatives to individual practices at which time messages derived from the MAAG guidelines would be detailed. Practices received a letter from the chief executive of the health authority endorsing the project, and postgraduate educational allowance accreditation was granted for the visits. A letter from the pharmaceutical advisor asking the practice to agree to see the representative preceded approaches by company representatives to specific practices. All practices received the MAAG guidelines, and routine health authority advice. Similarly, the pharmaceutical industry continued its routine marketing activity. Thus the evaluation estimated the additional benefits from a collaborative approach in which pharmaceutical company marketing activity was supported and encouraged actively by the health authority in intervention practices.
A steering group for the project included representatives of the health authority, each company concerned, the Local Medical Committee the Multidisciplinary Audit Advisory Group and representatives from General Practice. Health authority personnel conducted a series of presentations to local general practitioners describing the rationale and aims of the collaborative project. All visits were undertaken within the terms of the industry code of practice and in line with relevant legislation.
Qualitative assessment
The company representative concerned recorded visits undertaken under the aegis of the project. Where visits had taken place or where representatives had attempted but failed to gain access to general practitioners, a short standardized semistructured telephone interview lasting about 5 min was conducted to elicit the views of participating doctors where possible. Practices were only contacted once regardless of the number of attempts made by representatives to arrange visits, or the number of successful visits made.
Quantitative assessment
Practices formed the unit of allocation and analysis [7]. Practices were randomized to intervention or control using computer generated random numbers in a stratified scheme that took into account proximity to secondary care facilities and was concealed from those involved in the provision of care or with local knowledge. We collected practice specific prescribing reimbursement data from the Prescriptions Pricing Authority for the proton pump inhibitors. Our main analyses were based upon the proportion of items (prescriptions) reimbursed for each practice for lansoprazole against those for proton pump inhibitors as a whole. These data were analysed using a generalized linear model with a logit link and binomial error [8]. The logit of the proportion of lansoprazole items against total proton pump inhibitors reimbursed for the month prior to the beginning of the intervention, fundholding status and dispensing practice status were all identified as potentially important factors a priori that might predict variability in prescribing, and were considered in the model. The extra-binomial variance associated with differences between practices was accounted for by inflating the scale parameter (which equals 1 for the binomial distribution) by the mean residual deviance on the appropriate stratum, using the ideas of quasi-likelihood. The effect of randomization to the intervention group on the mean cost of prescribing proton pump inhibitors per practice was also examined. Since cost data collected in trials is often not normally distributed, we used the bootstrap percentile method to obtain a non parametric estimate of 95% confidence intervals for the difference in means [9]. Bootstrapping recognizes that the best source of an estimate for the confidence limits of a data set is based upon the data themselves, and uses computer intensive random sampling with replacement to build a distribution that best describes the probabilistic range of likely outcomes. We also used conventional parametric methods to check the results. Statistical analyses were conducted using SAS 6.12 (Cary, NC, USA: 1996).
Results
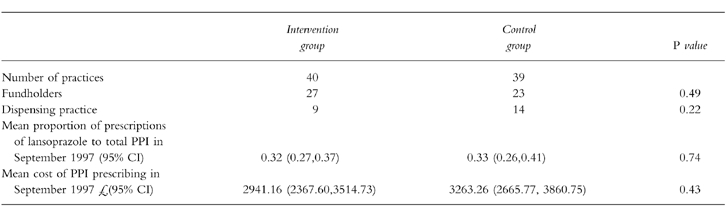
All 79 practices in Warwickshire participated in the trial. These were evenly balanced for the major characteristics identified as potential predictors of prescribing behaviour (seeTable 1).
Table 1.
Baseline characteristics of practices.
Delivery of the intervention
Altogether company representatives reported visiting 102 doctors in the intervention group on at least one occasion. No visits were achieved with the remaining 38 doctors in the intervention group. A second visit was achieved with 39 doctors, and a third or more with 9.
Qualitative assessment
The project received a mixed reaction from general practitioners. This ranged from comments that: visits were ‘a very positive development’; through ‘more positive than the usual type of visit with quite informative information—drug reps [usually] bring me out in spots’; to ‘any collaboration between drug companies and the health authority is coercion’. All of the practices that saw company representatives in the context of the study reported that they normally saw company representatives in the course of their work.
Quantitative evaluation
Effect upon choice of drug
Overall the intervention had no measurable effect upon the prescribing of proton pump inhibitors in the intervention practices. At the end of the intervention period, the mean proportion of prescriptions of lansoprazole against total proton pump inhibitor prescribing was 0.42 (95% CI 0.35,0.50) in the control group and 0.44 (95% CI 0.38,0.50) in the intervention group. Thus both groups demonstrated changes in prescribing practice in line with the guidelines recommendations.
There was substantial over-dispersion in the base logistic regression model with the ratio of deviance divided by the degrees of freedom equal to 5.39 (χ2 with 75 degrees of freedom; P < 0.0001). The variance estimates were therefore adjusted by this factor, leading to wider confidence intervals, to reflect the extra uncertainty introduced by this heterogeneity. By far the most important predictor in the model was prior prescribing which was highly statistically significant. The odds ratio for the factor in the model that described the predictive value of preintervention prescribing on the probability of prescribing lansoprazole in preference to omeprazole after the intervention was 1.86 (95% CI 1.65,2.09). Neither fundholding nor dispensing status contributed to the model individually and so they were not included, although this information may be encoded in the preintervention prescribing rate. The odds ratio describing the effect of the intervention was 1.04 (95% CI 0.83,1.31) (seeFigure 1). In other words, the intervention was associated with a 4% increase in the odds of prescribing lansoprazole which may simply be explained by chance (P = 0.73).
Figure 1.
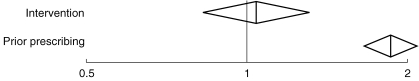
Odds ratio and 95% confidence intervals describing the predictive value of the effects of the intervention, and of prior prescribing.
Effect upon prescribing costs
The mean cost of prescribing proton pump inhibitors was £2580.36 (95% CI 2035.97,3124.76) in the control group, and £3024.78 (95% CI 2438.53,3611.03) in the intervention group in March 1998. Over the 6 month period of the study, the cost of prescribing proton pump inhibitors decreased by £122.32 more in the control group than in the intervention group (95% CI–94.91,342.91). Thus the intervention was not associated with less costly overall prescribing. Analysing these data using standard t-tests provided similar, although not identical, results (95% CI–104.24,348.88).
Discussion
We undertook a randomized trial to examine the potential of harnessing the marketing skills of the pharmaceutical industry to influence prescribing within the context of a substantial project addressing the feasibility of the approach. Major pharmaceutical companies collaborated actively in the intervention, helping to produce materials to aid the marketing of evidence based guidelines and allocated members of their sales force. In general, relationships with companies were constructive and developed over time. In the randomized controlled trial, 67.5% of practices in the intervention group received a visit from an industry representative during the course of the study. However, although we demonstrated that it was possible for a health authority to work in partnership with the pharmaceutical industry, in our primary analysis we observed no effect upon the proportion of targeted drugs prescribed attributable to the intervention. Similarly, there was no evidence of a saving in prescribing costs as a result of the intervention. The experimental design of the evaluation enabled the attribution of any effects observed to the intervention [10].
The confidence intervals for the estimate of effect include the possibility that results may range from a 17% reduction to a 31% increase in the odds of prescribing lansoprazole. However, the most likely estimate is a 4% increase. Similarly, the analysis of the costs of prescribing proton pump inhibitors indicates no saving in the overall cost of prescribing attributable to the intervention although the confidence intervals are wide. Warwickshire is an average sized health authority, and the potential for improving the efficiency of prescribing appears substantial and is considered a priority. A striking feature of the practices in Warwickshire was the level of heterogeneity between practices in the prescribing of proton pump inhibitors during this period.
We experienced none of the apparent ‘dangers’ associated with collaboration with the pharmaceutical industry identified by the NHS Executive [5]. Good working relationships were achieved with 5 companies. However, although company sales forces were a potentially important resource for the health authority, no additional benefits were achieved, above the changes in prescribing that were happening anyway, to support their deployment in a collaborative project.
The main limitation of our study is that it is not possible to determine whether some aspect of the message derived from the guideline prevented uptake, and that a different message (for example one that did not focus on cost reduction) may have been implemented more effectively. However, the complete absence of a benefit from the intervention suggests that it is unlikely that further work repeating the same intervention will prove fruitful.
There are a number of possible explanations for the lack of success of this method for influencing prescribing behaviour in Warwickshire. Perhaps the most persuasive of these concerns the access to practices achieved by the industry representatives. The encouragement of the health authority had at best limited success in achieving access to practices that did not usually see industry representatives, although all the companies collaborating in the project reported gaining access to practices that they did not usually visit. Thirty-eight intervention group doctors did not see the industry representatives (27%). If those doctors who did participate in visits were likely to see industry representatives, the collaborative intervention may have simply formalized a process that would have taken place in any case.
It is apparent that the aims of the pharmaceutical industry and those of the UK NHS or other health providers do not always sit comfortably together, and it is notable that about one quarter of doctors did not see an industry representative. However, this refusal of a minority of general practitioners to take part in visits does not explain the lack of effect. In a seminar in which the results of the project were discussed with local practitioners, one that had received visits commented
‘I usually do see reps because my brother is in sales, and he has a rotten time making appointments—I don’t think that the reps influence me but they must because otherwise they would not bother to see me … The collaborative visits were different … it was not like being marketed to at all. It was hard to see what was different from a standard visit, but [the rep] seemed factual and lacking self confidence’.
Thus an explanation for the lack of impact of the visits may simply be that industry representatives felt constrained and did not use their techniques and skills in a credible manner.
Avorn & Soumerai [11] have described the importance of credibility in the delivery of prescribing messages to general practice in the context of independent pharmacists undertaking outreach visits. It seems likely that neither the pharmaceutical company nor the health authority was perceived as credible (our feedback suggested both reservations on the part of responding prescribers). Indeed, the reader may note that we did not specify who the enemy is, in the title of this report. Avorn & Soumerai also identify the importance of investigating baseline knowledge and motivations for current prescribing patterns, highlighting and repeating essential messages and providing positive reinforcement of improved practices in follow up visits. Baseline knowledge and motivations for current prescribing were addressed through the explicit involvement of local general practitioners in the development of the MAAG guidelines, and the intervention. The pharmaceutical company representatives who attempted additional visits in all intervention group practices addressed repeating messages and reinforcing improved practices. Although Avorn & Soumerai [11] identify a positive effect from outreach visits by independent outreach workers, attempting to achieve similar benefits was unsuccessful in our trial of a collaborative health authority/pharmaceutical intervention. Indeed, there has to date been no completed large-scale evaluation of the effectiveness and cost effectiveness of educational outreach in primary care in the UK. Such a trial is currently underway [12].
There was some evidence of counter-detailing, in which companies not involved in the project who were in danger of losing market share as a result of the intervention increased their marketing activities. This may have cancelled out positive effects of the intervention.
It has been suggested that the effectiveness of educational visits may be dependent upon the affiliation of the outreach worker [13–15], and in particular that the effect of the intervention may be reduced if sponsored by the pharmaceutical industry [15]. While this may go some way towards explaining the absence of an effect in our quantitative evaluation, the qualitative evaluation does not support it. We received similar concerns expressed about the motivations of the health authority as the industry. Had a truly independent worker delivered the outreach visits our findings may have been different, and we are currently investigating this question in a large randomized trial of independent outreach visits [12].
The development of Primary Care Groups [16] may provide the opportunity for general practitioners to benefit from more constructive relationships with the pharmaceutical industry. However, these will only be achieved when the overall objectives in primary care are in line with those of specific companies given the differences in the objectives of a health care system and the market place.
Given the importance of achieving high quality prescribing, the quest for effective means to implement evidence based guidance for prescribing in primary care should continue. Collaborative working with the pharmaceutical industry appears feasible although our trial provides no support for further development of interventions similar to that used in our evaluation. Further evaluations that are currently underway in the NHS, including a large scale randomized trial of academic detailing [12] using the approach described by Avorn & Souermai [11], should identify the extent to which alternative intervention strategies may achieve worthwhile changes in prescribing practice.
Acknowledgments
Nick Freemantle was funded by Warwickshire Health Authority for his contribution to this project. We are most grateful to Anne Burton for her assistance with the qualitative interviews and preparation of the manuscript.
References
- 1.Effective Health Care. Implementing Clinical Practice Guidelines. Leeds: University of Leeds; 1994. Bulletin No 8. [Google Scholar]
- 2.Freemantle N, Mason JM, Watt I. Evidence into practice? Prescribing selective serotonin reuptake inhibitors. Int J Technol Assessment Health Care. 1998;14:387–391. doi: 10.1017/s0266462300012332. [DOI] [PubMed] [Google Scholar]
- 3.Eccles M, Freemantle N, Mason JM. for the North of England Evidence Based Guideline Development Project. Evidence based clinical practice guideline: angiotensin converting-inhibitors in the primary care management of adults with symptomatic heart failure. Br Med J. 1998;316:1369–1375. doi: 10.1136/bmj.316.7141.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maynard A, Bloor K. Managed Care: Panacea or palliation? London: Nuffield Trust; 1998. Nuffield Occasional Papers Health Economics Series. Paper No 8. [Google Scholar]
- 5.Liddell A. Commercial approaches to the NHS regarding disease management packages. Vol. 94. Leeds: NHS Executive; 1994. EL (94) [Google Scholar]
- 6.MAAG. 1998. Steps to Better Practice? Warwick: Multi-disciplinary Audit Advisory Group.
- 7.Wood J, Freemantle N. Choosing an appropriate unit of analysis in trials of interventions that attempt to influence practice. Journal of Health Services Research and Policy. 1999;4:44–88. doi: 10.1177/135581969900400111. [DOI] [PubMed] [Google Scholar]
- 8.Baker RJ, Nelder JA. The GLIM System Release 3 General Linear Interactive Modelling Manual. London: Royal Statistical Society; 1978. [Google Scholar]
- 9.Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Statist Sci. 1986;1:54–77. [Google Scholar]
- 10.Freemantle N, Wood J, Crawford F. Evidence into practice, experimentation and quasi experimentation: are the methods up to the task? J Epidemiol Community Health. 1998;52:75–81. doi: 10.1136/jech.52.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soumerai SB, Avorn J. Principles of educational outreach (‘academic detailing’) to improve clinical decision-making. JAMA. 1990;263:549–556. [PubMed] [Google Scholar]
- 12.Freemantle N, Eccles M, Wood J, et al. A randomised trial of Evidence Based OutReach (EBOR): rationale and design. Controlled Clinical Trial. 1999;20:479–492. doi: 10.1016/s0197-2456(99)00023-9. [DOI] [PubMed] [Google Scholar]
- 13.Schaffner W, Ray WA, Federspiel C, et al. Improving antibiotic prescribing in office practice: a controlled trial of three educational methods. JAMA. 1983;250:1728–1732. [PubMed] [Google Scholar]
- 14.Pippalla RS, Riley DA, Chin Burapa V. Influencing the prescribing behaviour of physicians: a meta evaluation. J Pharmac Ther. 1995;20:189–198. doi: 10.1111/j.1365-2710.1995.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 15.Friis H, Bro F, Mabeck CE, et al. Changes in prescription of antibiotics in general practice in relation to different strategies for drug information. Dan Med Bull. 1991;38:380–332. [PubMed] [Google Scholar]
- 16.Department of Health. 1997. The New NHS Modern—Dependable Leeds: Department of Health, Cmnd 3807.


