Abstract
IL-1β is an endogenous pyrogen that is induced during systemic lipopolysaccharide (LPS)- or IL-1-induced fever. We have examined the fever and cytokine responses following i.p. injection of IL-1 agonists, IL-1α and IL-1β, and compared these with response to LPS (i.p.) in wild-type and IL-1β-deficient mice. The IL-1β deficient mice appear to have elevated body temperature but exhibit a normal circadian temperature cycle. Exogenously injected IL-1β, IL-1α, or LPS induced hyperresponsive fevers in the IL-1β-deficient mice. We also observed phenotypic differences between wild-type and IL-1β-deficient mice in hypothalamic basal mRNA levels for IL-1α and IL-6, but not for IL-1β-converting enzyme or IL-1 receptor type I or type II. The IL-1α mRNA levels were down-regulated, whereas the IL-6 mRNA levels were up-regulated in the hypothalamus of IL-1β-deficient mice as compared with wild-type mice. The IL-1β-deficient mice also responded to LPS challenge with significantly higher serum corticosterone and with lower serum tumor necrosis factor type α levels than the wild-type mice. The data suggest that, in the redundant cascade of proinflammatory cytokines, IL-1β plays an important but not obligatory role in fever induction by LPS or IL-1α, as well as in the induction of serum tumor necrosis factor type α and corticosterone responses either by LPS or by IL-1α or IL-1β.
IL-1β is a proinflammatory cytokine with multiple cellular and systemic effects (see for review ref. 1). Systemic effects of IL-1β include fever, sickness syndrome, activation of the hypothalamic–pituitary–adrenal axis, and induction of cytokines mediating the acute phase response. The expression of IL-1β is highly inducible by bacterial and viral infections and by several inflammatory mediators. IL-1β is one of the three ligands to the two known IL-1 receptors: type I and type II IL-1 receptors (IL-1RI and IL-1RII, respectively). IL-1β and IL-1α are agonists, whereas the IL-1 receptor antagonist (IL-1ra) is a bona fide antagonist at these receptors (2). The induction of IL-1α, IL-1β, and two other early proinflammatory cytokines, tumor necrosis factor type α (TNFα) and IL-6, involves an ordered cascade of events. In this cascade, IL-1β and TNFα induce each other, and each of these cytokines can induce IL-6, which, in turn, can induce the acute phase response. IL-6 induced by IL-1 or TNFα in the central nervous system is also thought to mediate the febrile response (3, 4).
The exact role played by a single cytokine in this pattern of intersecting crossinductions is difficult to delineate, as it is very much dependent on the cell type studied and on the type of infectious or inflammatory stimulus used. IL-1β-deficient mice, generated by null mutation of the single IL-1β gene (5), provide an opportunity to assess the relative contribution of IL-1β expression to certain inflammatory and endocrine responses. It has been shown in recent experiments that IL-1β-deficient mice mount a reduced febrile response to lipopolysaccharides (LPSs) and an impaired defense response to influenza virus (6). The IL-1β-deficient mice were also resistant to fever induction and to anorexia by local turpentine injection and developed a reduced acute phase response to this stimulus (5). IL-1β-deficient mice also showed a reduced production of IL-1α when macrophages were in vitro-stimulated by LPS, whereas TNFα and IL-6 production by macrophages from the IL-1β-deficient mice were normal (7).
This paper addresses the following question: What happens to IL-1 receptor-mediated responses when one of the two IL-1 agonists (IL-1β) is not expressed, neither during development nor during immune challenge, due to a null mutation of the single IL-1β gene? We used IL-1α and IL-1β as stimuli to examine whether the absence of the endogenous agonist (IL-1β) modified the receptor expression, receptor coupling, and function, as assessed by the cytokine, corticosterone, and fever responses to exogenously administered agonists, IL-1α and IL-1β, and to LPS.
MATERIALS AND METHODS
Materials.
LPS was derived from Escherichia coli (055:B5, purified by phenol extraction and gel filtration chromatography; Sigma). Recombinant rat IL-1 (rrIL-1)α and rrIL-1β were from the CEC European Concerted Action “Cytokines in the Brain.” These rat cytokines were available in LPS-free high-purity quality, in sufficient quantities for the in vivo work. Rompun was from Bayer (Wuppertal, Germany), and Ketalar was from Parke-Davis. All substances were diluted in pyrogen-free saline solution the day of the experiment.
Animals.
IL-1β-deficient (−/−) male mice and wild-type (+/+) littermates (7–12 weeks old) were used. The generation of this strain of mice and its genetic background have been described by Zheng et al. (5). The mice were housed one per cage in a temperature-controlled room with food and water ad libitum, and they were kept under a 12 h light/12 h dark diurnal cycle (light phase began at 8:00 a.m.). The temperature in the room was maintained at 30 ± 1°C. The animals were acclimated for at least 1 week before experimental procedures were begun. The experiments were initiated when the mice reached a body weight of 30 ± 5 g. Differences in body weight were not observed between IL-1β-deficient and wild-type mice.
Measurement of Body Temperature.
The radiotransmitter was inserted in the mouse peritoneal cavity under anesthesia with ketamine (Ketalar, 50 mg/kg) and xylazine (Rompun, 10 mg/kg). Mice with inserted radiotransmitters were indistinguishable, in terms of body weight and activity, from normal mice 4 days after the surgery. After implantation, the animals were allowed to recover for at least 5 days before testing.
The core body temperatures of the animals were measured using battery-operated biotelemetry devices (model no. XM-FH, Mini-Mitter, Sunriver, OR) as described earlier (8). Body temperature values were recorded at 10-min intervals, beginning at least 24 h before the injection of LPS, rrIL-1α, or rrIL-1β and continued for at least 48 h after the injections. Injections began between 9:00 and 10:30 a.m., during the light period.
Poly(A)+ RNA Purification from the Mouse Hypothalamus and Reverse Transcriptase-PCR.
The mice were sacrificed by decapitation 105 min after the injection of LPS, rrIL-1α, rrIL-1β, or saline. Purification of total RNA and consecutive preparation of cDNA from the hypothalami of mice and RT-PCR for comparative analysis of IL-1α, IL-1β, IL-6, TNFα, β-actin, IL-1ra, IL-1RI, IL-1RII, and IL-1β-converting enzyme (ICE) mRNA levels were carried out as described earlier (9). A variation of the protocol was made to improve the possibility of evaluating the results: after the reverse transcriptase reaction, each cDNA was added to a mix with PCR buffers, salts, nucleotides, and Taq polymerase enzyme. This mix was then aliquoted to individual PCR tubes, where only the primers had to be added before starting the PCR cycling. This allows for the same amount of cDNA template for each of the PCR products of a given cDNA. Each PCR product could then be evaluated according to its ratio compared with β-actin. All the PCR primers used were run in individual tubes.
The sequences of the PCR primers used have previously been described (9–11), except for the following (the annealing temperature used is given in parentheses). IL-1α: forward PCR primer, 5′-GCCAGTTGAGTAGGATAAAGG-3′, and reverse PCR primer, 5′-CAGTCTGTCTCCTTCTTGAGG-3′ (53°C); IL-1β, forward PCR primer, 5′-CTCCATGAGCTTTGTACAAGG-3′, and reverse PCR primer, 5′-TGCTGATGTACCAGTTGGGG-3′ (53°C); and IL-1ra, forward PCR primer, 5′-GACCCTGCAAGATGCAAGCC-3′, and reverse PCR primer, 5′-GAGCGGATGAAGGTAAAGCG-3′ (52°C).
Since we wanted to compare the amount of original template, cycles of PCR were primarily within the exponential range, as not to reach a plateau, where comparisons between different treatments cannot be accurately determined.
Cytokine Serum Measurements.
Blood was collected by orbital bleeding during light ether anesthesia, and serum was prepared 105 min after the injection of LPS, rrIL-1α, rrIL-1β, or saline. IL-1α, IL-6, and TNFα levels were measured using ELISAs specific for murine cytokines, kindly provided by Endogen (Cambridge, MA), and carried out according to manufacturer’s instructions. For all three ELISAs, the intraassay variability was <10% and the interassay variability was also <10%. The sensitivity of the ELISAs were as follows: IL-1α, <6 pg/ml; IL-6, 15 pg/ml; and TNFα, <10 pg/ml.
Serum Corticosterone Measurements.
Serum corticosterone was measured by radioimmunoassay, using an antiserum (C-8784) for corticosterone purchased from Sigma, following the manufacturer’s instructions. [3H]Corticosterone was purchased from Amersham. The intraassay variability was <5% and interassay variability was <10%. Sensitivity was ≈50 pg/ml.
Serum Glucose Measurements.
Serum glucose was measured using a kit from Sigma Diagnostics carried out according to the manufacturer’s instructions.
Receptor Binding Experiments.
125I-labeled recombinant human interleukin-1β [125I-rhIL-1β; specific activity, 120 μCi/μg (1 Ci = 37 Gbq)] was purchased from DuPont/NEN. IL-1β-deficient mice and wild-type mice were sacrificed, and hippocampi were dissected and stored on dry ice. The tissues were placed in ice-cold buffer (5 mM Tris·HCl, pH 7.2/1 mM MgCl2/0.25 M sucrose) and disrupted using a Teflon-glass homogenizer. The homogenates were centrifuged at 500 × g for 3 min, and the supernatants were again centrifuged at 14,500 × g for 2 min. The membrane fractions from the high-speed centrifugation were resuspended in binding buffer [RPMI 1640 medium (GIBCO/BRL)/25 mM Hepes, pH 7.2/1% BSA/0.1% sodium azide). Saturation binding was performed in a final volume of 60 μl for 1 h, at room temperature, with or without 200 nM unlabeled rhIL-1β. Receptor-bound 125I-rhIL-1β was separated from free 125I-rhIL-1β by centrifugation at 14,500 × g for 1 min. The resulting pellets were washed twice with binding buffer, and the radioactivity of the pellets was measured in a γ counter. The protein concentrations of the incubated membranes were determined by Bradford protein assay (Bio-Rad), after the pellets were washed twice with 0.1 M NaCl.
Statistical Analysis.
All data were reported as means ± SEs. ANOVA test followed by Fisher’s protected least significant difference (PLSD) test was used to analyze statistical differences. In the temperature measurements, animals with consecutive missing temperature recordings, due to failure of the telemetry system, were excluded from the statistical analysis.
RESULTS
Fever Response in the IL-1β-Deficient and Wild-Type Mice to IL-1α, IL-1β, and LPS.
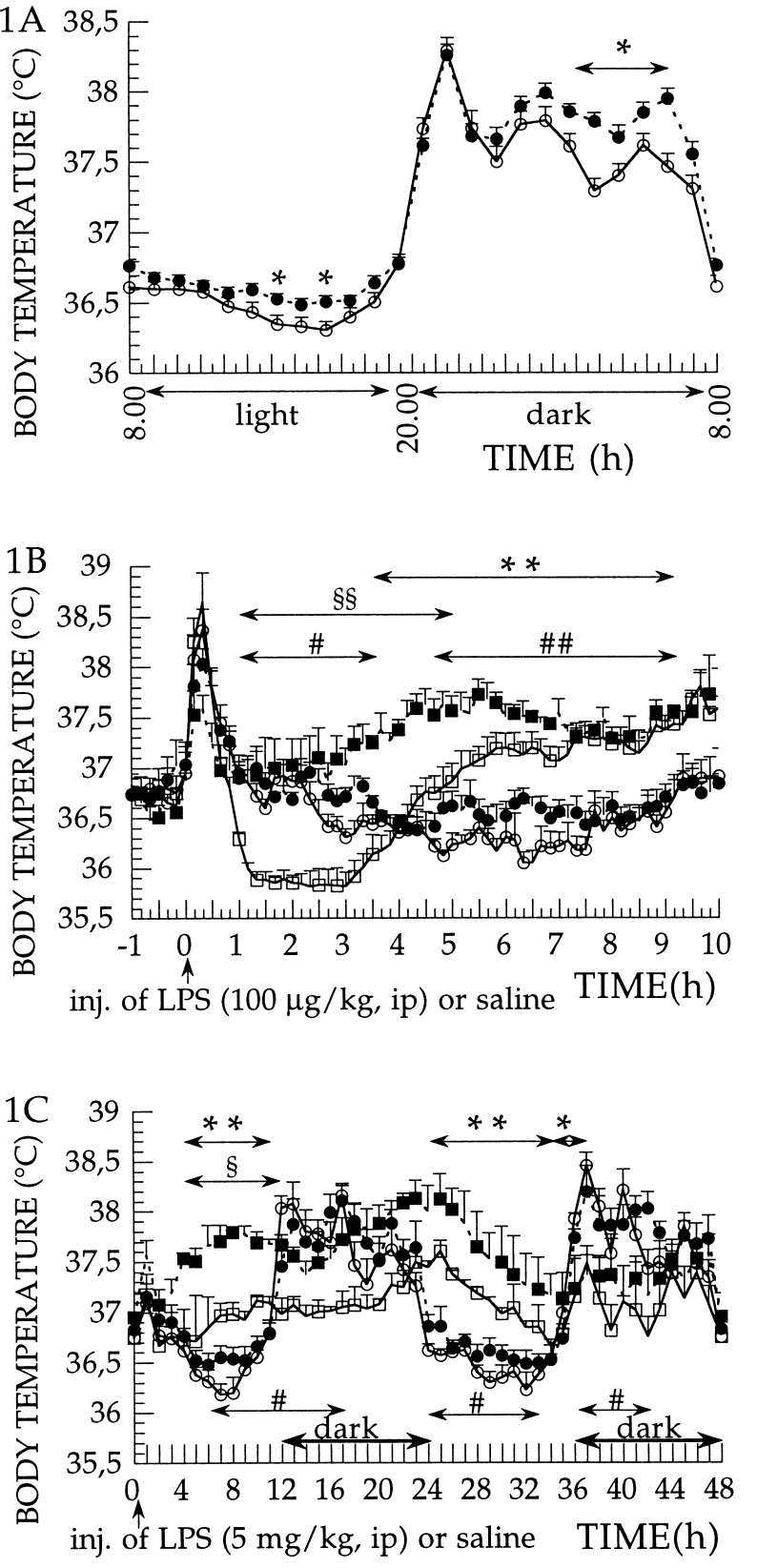
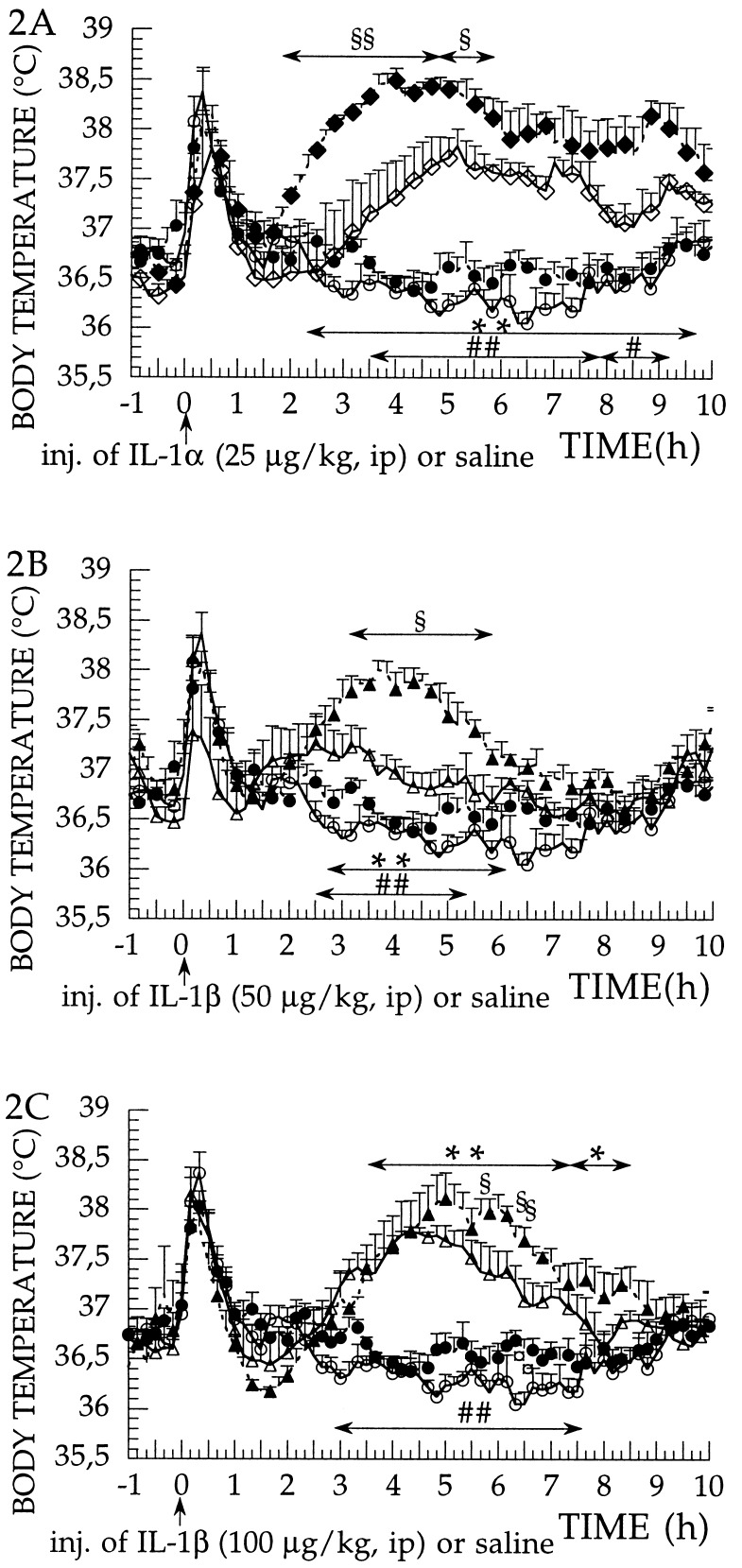
To examine whether the absence of IL-1β in the IL-1β-deficient mice resulted in supersensitivity of the IL-1 receptors to exogenous IL-1α and IL-1β, we injected rrIL-1α (25 μg per kg of body weight, i.p.) or rrIL-1β (50 and 100 μg per kg of body weight, i.p.) and examined the fever response in the IL-1β-deficient mice and in the wild-type mice. Comparison of the diurnal rhythm of temperature variation in the wild-type and in the IL-1β-deficient mice shows that the latter mice have a slightly elevated body temperature during 50% of the light and 50% of the dark period when the animals are kept at 30°C ambient temperature, which is thermoneutral temperature for these mice (Fig. 1A). When the febrile response of IL-1β-deficient and wild-type mice to exogenous pyrogen LPS (100 μg per kg of body weight, i.p.) was compared, the febrile responses were higher in IL-1β-deficient than in wild-type mice (Fig. 1B). Another way of describing the febrile reaction of IL-1β-deficient mice is to say that they lack the first hypothermic phase that develops in wild-type mice. When a much higher dose of LPS (5 mg per kg of body weight, i.p.) was used, the IL-1β-deficient mice showed a higher fever response, on both day 1 and day 2, than the wild-type mice (Fig. 1C). When IL-1α (25 μg per kg of body weight, i.p.) was administered as an exogenous pyrogen, the febrile reaction in the IL-1β-deficient mice was clearly hyperresponsive. The fever response of the IL-1β-deficient mice had a faster onset and a significantly higher amplitude, during a 4-h period following the injection of IL-1α (2–6 h after injection), than that observed in the wild-type mice (Fig. 2A). When IL-1β (50 μg per kg of body weight, i.p.) was administered as an exogenous pyrogen, the febrile reaction in the IL-1β-deficient mice was again hyperresponsive with a significantly higher amplitude of fever response, during a 3-h period following the injection, than that observed in the wild-type mice (Fig. 2B). When using 100 μg per kg of body weight of IL-1β, the amplitude of the fever response in both wild-type and in the IL-1β-deficient mice reached 1.8°C, which is the maximum fever we have observed at this ambient temperature in these mouse strains, using any pyrogen. Even at this maximum response, a larger, more prolonged fever response is evident in the IL-1β-deficient mice as compared with wild-type mice (Fig. 2C).
Figure 1.
(A–C) Diurnal temperature rhythm and LPS-induced fever in IL-1β-deficient and wild-type mice and measurement of the core body temperature of IL-1β-deficient (−/−) and wild-type (+/+) mice, measured at 30 ± 1°C of ambient temperature. (A) Recordings of basal temperatures of the animals for 24 h. •, IL-1β-deficient mice (n = 36); and ○, wild-type mice (n = 36). One-hour average (mean ± SE) of temperature recordings over 24 h are shown. ∗, P < 0.05, wild-type vs. IL-1β-deficient mice. (B) Injection of LPS (100 μg per kg of body weight, i.p.) or saline. Effects on body temperature in saline-injected IL-1β-deficient mice (n = 8; •), saline-injected wild-type mice (n = 9; ○), LPS-injected IL-1β-deficient mice (n = 4; ▪), and LPS-injected wild-type mice (n = 5; □). Injection at time 0. Data were recorded at 10-min intervals and are presented as mean ± SE. ∗∗, P < 0.01, LPS-injected IL-1β-deficient vs. saline-injected IL-1β-deficient mice; ##, P < 0.01, and #, P < 0.05, LPS-injected wild-type vs. saline-injected wild-type mice; and §§, P < 0.01, LPS-injected wild-type vs. LPS-injected IL-1β-deficient mice. (C) Injection of LPS (5 mg per kg of body weight, i.p.) or saline. Effects on body temperature in saline-injected IL-1β-deficient mice (n = 8; •), saline-injected wild-type mice (n = 9; ○), LPS-injected IL-1β-deficient mice (n = 3; ▪), and LPS-injected wild-type mice (n = 3; □). Injection at time 0. One-hour average (mean ± SE) of temperature recordings, over 48 h, are shown. ∗∗, P < 0.01, and ∗, P < 0.05, LPS-injected IL-1β-deficient vs. saline-injected IL-1β-deficient mice; #, P < 0.05, LPS-injected wild-type vs. saline-injected wild-type mice; and §, P < 0.05, LPS-injected wild-type vs. LPS-injected IL-1β-deficient mice.
Figure 2.
(A–C) IL-1α- and IL-1β-induced fever responses in IL-1β-deficient and wild-type mice. Effect of injections on the core body temperature of IL-1β-deficient (−/−) and wild-type (+/+) mice, measured at 30 ± 1°C of ambient temperature. (A) Injection of rrIL-1α (25 μg per kg of body weight, i.p.) or saline. Effects on body temperature in saline-injected IL-1β-deficient mice (n = 8; •), saline-injected wild-type mice (n = 9; ○), rrIL-1α-injected IL-1β-deficient mice (n = 4; ♦), and rrIL-1α-injected wild-type mice (n = 4; ⋄). Injection at time 0. Data were recorded at 10-min intervals and are presented as mean ± SE. ∗∗, P < 0.01, IL-1α-injected IL-1β-deficient vs. saline-injected IL-1β-deficient mice; ##, P < 0.01, and #, P < 0.05, IL-1α-injected wild-type vs. saline-injected wild-type mice; and §§, P < 0.01, and §, P < 0.05, IL-1α-injected wild-type vs. IL-1α-injected IL-1β-deficient mice. (B) Injection of rrIL-1β (50 μg per kg of body weight, i.p.) or saline. Effects on body temperature in saline-injected IL-1β-deficient mice (n = 8; •), saline-injected wild-type mice (n = 9; ○), rrIL-1β-injected IL-1β-deficient mice (n = 5; ▴), and rrIL-1β-injected wild-type mice (n = 5; ▵). Injection at time 0. Data were recorded at 10-min intervals and are presented as mean ± SE. ∗∗, P < 0.01, IL-1β-injected IL-1β-deficient vs. saline-injected IL-1β-deficient mice; ##, P < 0.01, IL-1β-injected wild-type vs. saline-injected wild-type mice; §, P < 0.05, IL-1β-injected wild-type vs. IL-1β-injected IL-1β-deficient mice. (C) Injection of rrIL-1β (100 μg per kg of body weight, i.p.) or saline. Effects on body temperature in saline-injected IL-1β-deficient mice (n = 8; •), saline-injected wild-type mice (n = 9; ○), rrIL-1β-injected IL-1β-deficient mice (n = 4; ▴), and rrIL-1β-injected wild-type mice (n = 4; ▵). Injection at time 0. Data were recorded at 10-min intervals and are presented as mean ± SE. ∗∗, P < 0.01, and ∗, P < 0.05, IL-1β-injected IL-1β-deficient vs. saline-injected IL-1β-deficient mice; ##, P < 0.01, IL-1β-injected wild-type vs. saline-injected wild-type mice; and §, P < 0.05, IL-1β-injected wild-type vs. IL-1β-injected IL-1β-deficient mice; and ¤, P < 0.05, saline-injected wild-type vs. saline-injected IL-1β-deficient mice.
Receptor Binding Study.
We have examined the number of IL-1 receptors in the central nervous system of wild-type and IL-1β-deficient mice by determining the saturation binding curve for 125I-rhIL-1β in hippocampal membranes. The hippocampus contained a sufficient amount of tissue with high IL-1 receptor density to carry out a binding study. A full saturation binding curve using hypothalamic membranes was not possible, due to scarcity of tissue. To determine whether the lack of the agonist IL-1β induced an overexpression of the IL-1 receptors, binding of 125I-rhIL-1β was studied. However, we did not observe a significant difference in the IL-1β-deficient mice as compared with wild-type mice, neither in terms of Bmax (11 ± 3 and 9 ± 6 fmol of bound 125I-rhIL-1β per mg of protein for wild-type and IL-1β-deficient mice, respectively) nor in terms of Kd (2.2 ± 0.8 nM for wild-type and 1.7 ± 2.0 nM for IL-1β-deficient mice).
Serum Levels of Corticosterone and Cytokines.
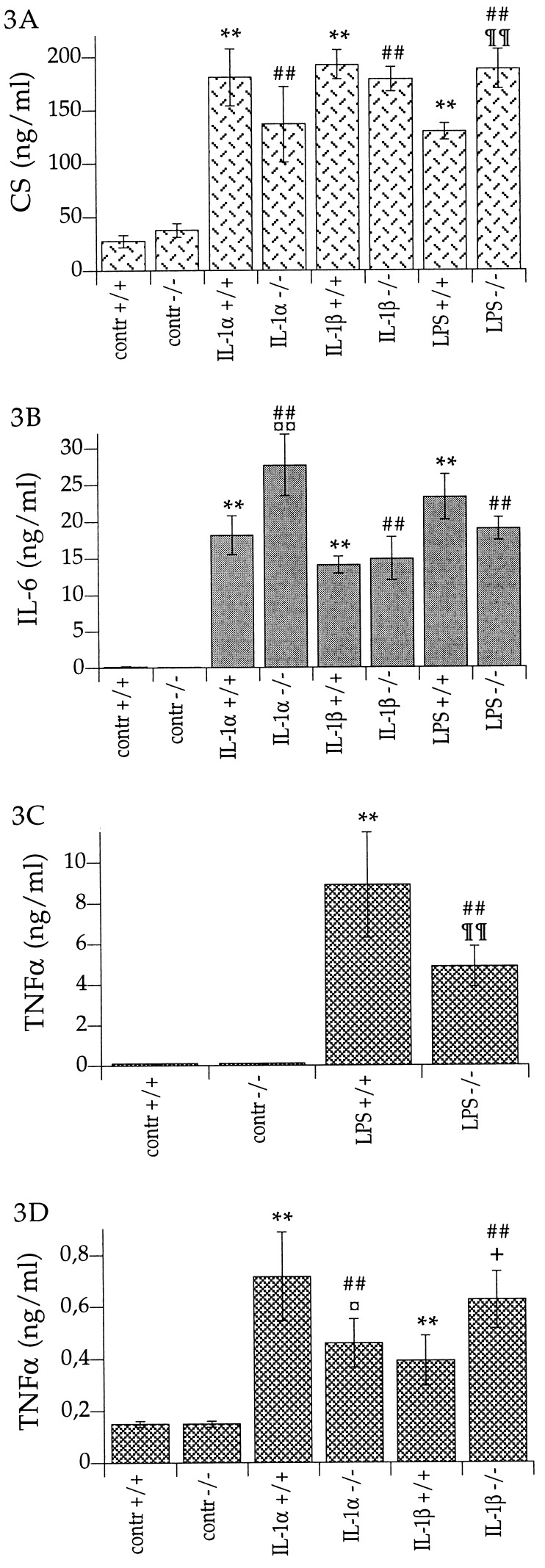
Serum corticosterone responses to LPS were statistically different in the IL-1β-deficient mice compared with the wild-type mice. LPS resulted in significantly higher (45%) levels of serum corticosterone in the IL-1β-deficient mice than in the wild-type mice (Fig. 3A). IL-1α-induced serum IL-6 levels were 53% higher in the IL-1β-deficient mice than in the wild-type mice (Fig. 3B); IL-1β and LPS were equally potent in elevating serum IL-6 levels in the IL-1β-deficient mice as in the wild-type mice (Fig. 3B). Serum TNFα levels were significantly less elevated in response to IL-1α and also to LPS in the IL-1β-deficient mice than in the wild-type mice (36% lower for IL-1α and 45% lower for LPS; Fig. 3 C and D), whereas IL-1β was more potent in elevating serum TNFα levels in the IL-1β-deficient mice than in the wild-type mice (60% higher; Fig. 3D). No statistically significant differences in serum IL-1α levels were observed after LPS stimulation (data not shown). IL-1α (i.p.) and IL-1β (i.p.) caused the same degree of hypoglycemia in the IL-1β-deficient mice as in the wild-type mice (data not shown).
Figure 3.
(A) Serum corticosterone levels in IL-1β-deficient mice (−/−) and wild-type mice (+/+) after injection of rrIL-1α (10 μg per kg of body weight, i.p.) [IL-1α]; rrIL-1β (10 μg per kg of body weight, i.p.) [IL-1β]; LPS (5 mg per kg of body weight, i.p.) [LPS]; or saline [contr]. ∗∗, P < 0.01 vs. control +/+; ##, P < 0.01 vs. control −/−; and ¶¶, P < 0.01 vs. LPS +/+. Control +/+, n = 12; control −/−, n = 12; IL-1α +/+, n = 4; IL-1α −/−, n = 3; IL-1β +/+, n = 5; IL-1β −/−, n = 5; LPS +/+, n = 7; and LPS −/−, n = 7. (B) Serum IL-6 levels in IL-1β-deficient mice (−/−) and wild-type mice (+/+), after injection of rrIL-1α (10 μg per kg of body weight, i.p.) [IL-1α]; rrIL-1β (10 μg per kg of body weight, i.p.) [IL-1β]; LPS (5 mg per kg of body weight, i.p.) [LPS]; or saline [contr]. ∗∗, P < 0.01 vs. control +/+; ##, P < 0.01 vs. control −/−; ¤¤, P < 0.01, vs. IL-1α +/+. Control +/+, n = 12; control −/−, n = 12; IL-1α +/+, n = 4; IL-1α −/−, n = 3; IL-1β +/+, n = 5; IL-1β −/−, n = 5; LPS +/+, n = 7; and LPS −/−, n = 7. (C and D) Serum TNFα levels in IL-1β-deficient mice (−/−) and wild-type mice (+/+). (C) TNFα levels in serum after injection of LPS (5 mg per kg of body weight, i.p.) [LPS] or saline [contr]. ∗∗, P < 0.01, vs. control +/+; ##, P < 0.01, vs. control −/−; and ¶¶, P < 0.01, vs. LPS +/+. Control +/+, n = 12; control −/−, n = 12; LPS +/+, n = 7; and LPS −/−, n = 7. Note that the scale on the ordinate is 10-fold higher than in D. (D) TNFα levels in serum after injection of rrIL-1α (10 μg per kg of body weight, i.p.) [IL-1α]; rrIL-1β (10 μg per kg of body weight, i.p.) [IL-1β]; or saline [contr]. ∗∗, P < 0.01, vs. contr +/+; ##, P < 0.01, vs. control −/−; ¤, P < 0.05, vs. IL-1α +/+; and +, P < 0.05, vs. IL-1β +/+. Control +/+, n = 12; control −/−, n = 12; IL-1α +/+, n = 5; IL-1α −/−, n = 5; IL-1β +/+, n = 5; and IL-1β −/−, n = 5.
Cytokine mRNA Levels in the Hypothalamus.
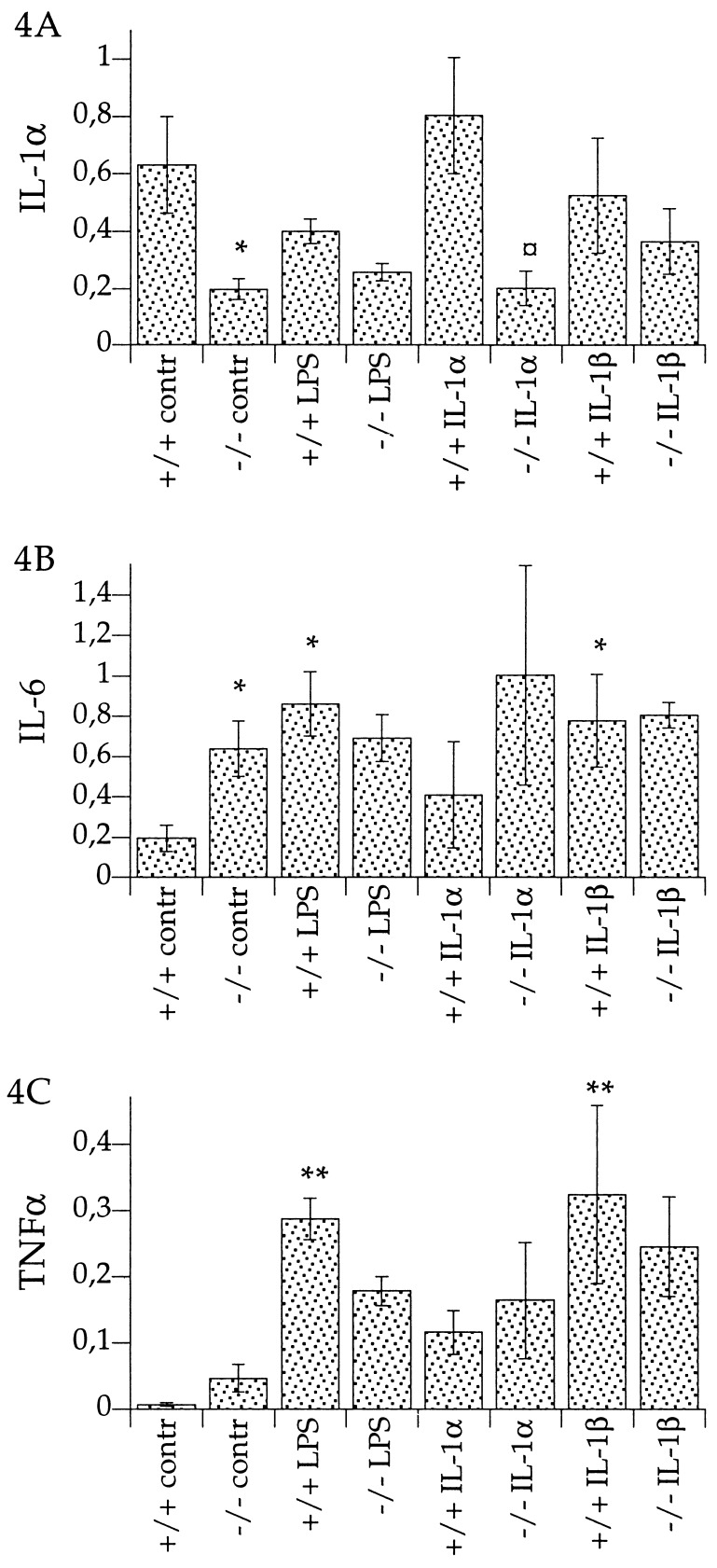
In the absence of any immune challenge—i.e., when injected with saline—mRNA levels for IL-1α were significantly lower (69% lower than wild-type), but mRNA levels for IL-6 were significantly higher (227% higher) in the hypothalamus of IL-1β-deficient mice than in the wild-type mice (Fig. 4). Steady-state mRNA levels for other members of the IL-1 family, such as ICE, IL-1RI, IL-1RII, and IL-1ra, were not significantly different, under these conditions, in IL-1β-deficient and wild-type mice (data not shown).
Figure 4.
(A–C) Relative mRNA levels in hypothalamus of IL-1β-deficient mice (−/−) and wild-type mice (+/+), after injection of LPS (5 mg per kg of body weight, i.p.) [LPS]; rrIL-1α (10 μg per kg of body weight, i.p.) [IL-1α]; rrIL-1β (10 μg per kg of body weight, i.p.) [IL-1β]; or saline [contr]. Ratios of respective mRNA and β-actin mRNA are presented. ∗∗, P < 0.01, and ∗, P < 0.05, vs. control +/+; and ¤, P < 0.05, vs. IL-1α +/+. (A) IL-1α mRNA levels. Control +/+, n = 7; control −/−, n = 7; LPS +/+, n = 3; LPS −/−, n = 3; IL-1α +/+, n = 3; IL-1α −/−, n = 3; IL-1β +/+, n = 5; IL-1β −/−, n = 5. (B) IL-6 mRNA levels. Control +/+, (n = 7); control −/−, (n = 7); LPS +/+, n = 3; LPS −/−, n = 3; IL-1α +/+, n = 3; IL-1α −/−, n = 3; IL-1β +/+, n = 5; and IL-1β −/−, n = 5. (C) TNFα mRNA levels. Control +/+, n = 8; control −/−, n = 7; LPS +/+, n = 3; LPS −/−, n = 3; IL-1α +/+, n = 3; IL-1α −/−, n = 3; IL-1β +/+, n = 5; and IL-1β −/−, n = 5.
The IL-1β-deficient mice showed a significantly lower response to IL-1α challenge in terms of the induction of IL-1α mRNA in the hypothalamus (75% lower than in wild-type; Fig. 4A). When IL-1β or LPS was used to stimulate cytokine mRNA levels in the hypothalami of IL-1β-deficient and wild-type mice, we observed no significant difference in the magnitude of the IL-1α, IL-6, or TNFα mRNA levels in the two mouse genotypes.
DISCUSSION
The febrile response to the exogenous pyrogen LPS involves the activity of endogenous pyrogens IL-1α and/or IL-1β, as demonstrated by the ability of IL-1ra (12) or antibodies to IL-1β (13, 14) to attenuate or block LPS-induced fever. Furthermore, in IL-6-deficient mice, there is no fever following LPS or IL-1β i.p. injection. This demonstrates that central expression of IL-6 is obligatory for both pyrogens (3).
The major finding of the present study is a phenotypical difference in febrile response between wild-type and IL-1β-deficient mice when challenged by LPS (Fig. 1 B and C), IL-1α (Fig. 2A), or IL-1β (Fig. 2 B and C). An increased febrile response may reflect altered IL-1 receptor number, due to lack of one of the agonists, IL-1β, in the IL-1β-deficient mice; or it may reflect enhanced coupling of IL-1 receptors in the IL-1β-deficient mice. The hyperresponsive febrile reaction may also be a consequence of altered metabolic response, at the level of heat generation, in the IL-1β-deficient mice. We have examined these possibilities and were unable to detect a difference, neither in the total number, nor in the affinity of IL-1 receptors, as measured by 125I-rhIL-1β equilibrium binding. The coupling efficacy of IL-1 receptors in the central nervous system was examined by measuring IL-1-mediated changes in IL-1α, TNFα, and IL-6 mRNA levels in the hypothalamus, and it was found (Fig. 4) that there is no supersensitive response to IL-1α or IL-1β in the IL-1β-deficient mice. At the level of the periphery, exogenous IL-1α induces higher IL-6 serum levels and exogenous IL-1β induces higher TNFα serum levels in IL-1β-deficient mice (Figs. 3 B and D, respectively). These observations, together with an elevated body temperature (Fig. 1A), may suggest that IL-1β is required for feedback regulation of IL-6 and TNFα levels, as well as for normal thermoregulation. When challenged with the exogenous pyrogen LPS or with IL-1α or IL-1β, the impaired thermoregulation is manifested by a hyperresponsive fever in the IL-1β-deficient mice. It is noteworthy that the IL-1β-deficient mice show hyperresponsive fever to systemic challenge, such as intraperitoneal injection of LPS, IL-1α, or IL-1β, while to local challenge, such as turpentine injection, these mice respond with reduced amplitude of acute phase response (5).
In contrast to our results, the IL-1β-deficient mice were observed by Kozak et al. (6) to respond to LPS (2.5 mg per kg of body weight, i.p.) by a fever of lower magnitude than in the wild-type mice (6). The difference in those and the present results is unclear, other than by noting that the LPS used in the different laboratories were from different strains of E. coli. Furthermore, we observed a hyperresponse to exogenous IL-1β and IL-1α (cf. below). Thus, the observation of higher LPS induced fever in the IL-1β-deficient mice than in wild-type mice is part of a series of congruent observations in our hands, each suggesting some degree of enhanced, supersensitive responsiveness to pyrogens.
The differential response in corticosterone levels between IL-1β-deficient and wild-type mice after LPS stimulation (Fig. 3A) was not found in earlier reports, although basal unstimulated CS levels were similar in both reports (7).
It is noteworthy that IL-1α levels are not only not elevated in the absence of IL-1β expression to compensate for the lack of one of the other IL-1 receptor agonists, but they tend to be lower in the IL-1β-deficient mice. This finding is consistent with those made in the ICE-deficient mice, which because of the lack of this key pro-IL-1β-processing enzyme, do not produce mature IL-1β. These ICE-deficient mice, from the point of view of the IL-1β occupancy of their IL-1 receptors, are similar to IL-1β-deficient mice (15). Data from both the IL-1β-deficient mice (cf. this paper and ref. 7) and ICE-deficient mice suggest that the full expression (and release) of IL-1α requires the presence of 17-kDa IL-1β gene product.
Induction of serum TNFα by both IL-1α and by LPS is significantly lower in the IL-1β-deficient mice than in the wild-type mice, strongly suggesting the need for IL-1β expression for full TNFα systemic response. It is only with IL-1β as stimulant that higher serum TNFα levels can be reached in the IL-1β-deficient mice than in the wild-type controls. Serum IL-6 levels seem to be more responsive to IL-1α, but not to IL-1β or LPS stimulation, in the IL-1β-deficient mice.
In the brain, the pro-IL-1β processing enzyme ICE mRNA levels were similar in the IL-1β-deficient mice and in the wild-type mice, under these experimental conditions (cf. ref. 11). mRNA levels for IL-1ra and for the two IL-1 receptor subtypes were similar in the brains of IL-1β-deficient and wild-type mice. Although this was only determined under basal or LPS stimulated conditions, it is an interesting finding, as in several systems it has been shown that IL-1β can induce its own receptors (10, 16), and thus the absence of IL-1β could have been reflected by lower mRNA levels for the type I and type II IL-1 receptors.
In summary, the febrile response in IL-1β-deficient mice to systemic LPS or to IL-1α or IL-1β is altered, and there are numerous differences in the cytokine responses at both mRNA and gene product level between the IL-1β-deficient and wild-type mice. These findings suggest that there must be several conditions under which these animals will not be able to muster a proper immune response. Turpentine injections appear to be an example of such a local immune challenge (5), while the present LPS, IL-1α, and IL-1β injections are examples of systemic stimulation; these reveal phenotypic differences as a result of lack of the IL-1β gene product during development and immune response. The hyperresponsive end points to systemic stimulation, such as higher fever, greater induction of serum corticosterone by LPS, and greater induction of serum TNFα by IL-1β are giving a consistent picture of hyperresponsivity to certain stimuli in the IL-1β-deficient mice, which is in apparent contradiction to the blunted LPS response and turpentine response in these mice found by Kozak and coworkers (5, 6). These differences may stem from different strain and batch of E. coli LPS, animal handling, and/or ambient temperature at fever measurements.
Acknowledgments
The IL-1β-deficient mice were kindly provided by Merck Research Laboratories. This study was supported by Riksbankens Jubileumsfond, Swedish Medical Research Council, and National Institutes of Health Grant AI-15614. Z.C. is a teacher at Peking University, Beijing, China.
ABBREVIATIONS
- IL
interleukin
- IL-1RI and IL-1RII
IL-1 receptor type I and type II, respectively
- IL-1ra
IL-1 receptor antagonist
- TNFα
tumor necrosis factor type α
- LPS
lipopolysaccharide
- rrIL-1
recombinant rat IL-1
- ICE
IL-1β-converting enzyme
- 125I-rhIL-1β
125I-labeled recombinant human interleukin-1β
References
- 1.Dinarello C A. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 2.Eisenberg S P, Evans R J, Arend W P, Verderber E, Brewer M T, Hannum C H, Thompson R C. Nature (London) 1990;343:341–346. doi: 10.1038/343341a0. [DOI] [PubMed] [Google Scholar]
- 3.Chai Z, Gatti S, Toniatti C, Poli V, Bartfai T. J Exp Med. 1996;183:311–316. doi: 10.1084/jem.183.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kluger M J. Physiol Rev. 1991;71:93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng H, Fletcher D, Kozak W, Jiang M, Hofmann K J, Conn C A, Soszynski D, Grabiec C, Trumbauer M E, Shaw A, Kostura M J, Stevens K, Rosen H, North R J, Chen H Y, Tocci M J, Kluger M J, Van der Ploeg L H T. Immunity. 1995;3:9–19. doi: 10.1016/1074-7613(95)90154-x. [DOI] [PubMed] [Google Scholar]
- 6.Kozak W, Zheng H, Conn C A, Soszynski D, Van der Ploeg L H T, Kluger M J. Am J Physiol. 1995;269:R969–R977. doi: 10.1152/ajpregu.1995.269.5.R969. [DOI] [PubMed] [Google Scholar]
- 7.Fantuzzi G, Zheng H, Faggioni R, Benigni F, Ghezzi P, Sipe J D, Shaw A R, Dinarello C A. J Immunol. 1996;157:291–296. [PubMed] [Google Scholar]
- 8.Kozak W, Conn C A, Kluger M J. Am J Physiol. 1994;266:R125–R135. doi: 10.1152/ajpregu.1994.266.1.R125. [DOI] [PubMed] [Google Scholar]
- 9.Chai Z, Alheim K, Lundkvist J, Gatti S, Bartfai T. Cytokine. 1996;8:227–237. doi: 10.1006/cyto.1996.0032. [DOI] [PubMed] [Google Scholar]
- 10.Bristulf J, Gatti S, Malinowsky D, Björk L, Sundgren A K, Bartfai T. Eur Cytokine Network. 1994;5:319–330. [PubMed] [Google Scholar]
- 11.Tingsborg S, Zetterström M, Alheim K, Hasanvan H, Schultzberg M, Bartfai T. Brain Res. 1996;712:153–158. doi: 10.1016/0006-8993(95)01525-6. [DOI] [PubMed] [Google Scholar]
- 12.Smith B K, Kluger M J. Am J Physiol. 1992;263:R653–R655. doi: 10.1152/ajpregu.1992.263.3.R653. [DOI] [PubMed] [Google Scholar]
- 13.Long N C, Otterness I, Kunkel S L, Vander A J, Kluger M J. Am J Physiol. 1990;259:R724–R728. doi: 10.1152/ajpregu.1990.259.4.R724. [DOI] [PubMed] [Google Scholar]
- 14.Klir J J, McClellan J L, Kluger M J. Am J Physiol. 1994;266:R1845–R1848. doi: 10.1152/ajpregu.1994.266.6.R1845. [DOI] [PubMed] [Google Scholar]
- 15.Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J, Towne E, Tracey D, Wardwell S, Wei F Y, Wong W, Kamen R, Seshadri T. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 16.Bristulf J, Bartfai T. Neurosci Lett. 1995;187:53–56. doi: 10.1016/0304-3940(95)11336-u. [DOI] [PubMed] [Google Scholar]