Abstract
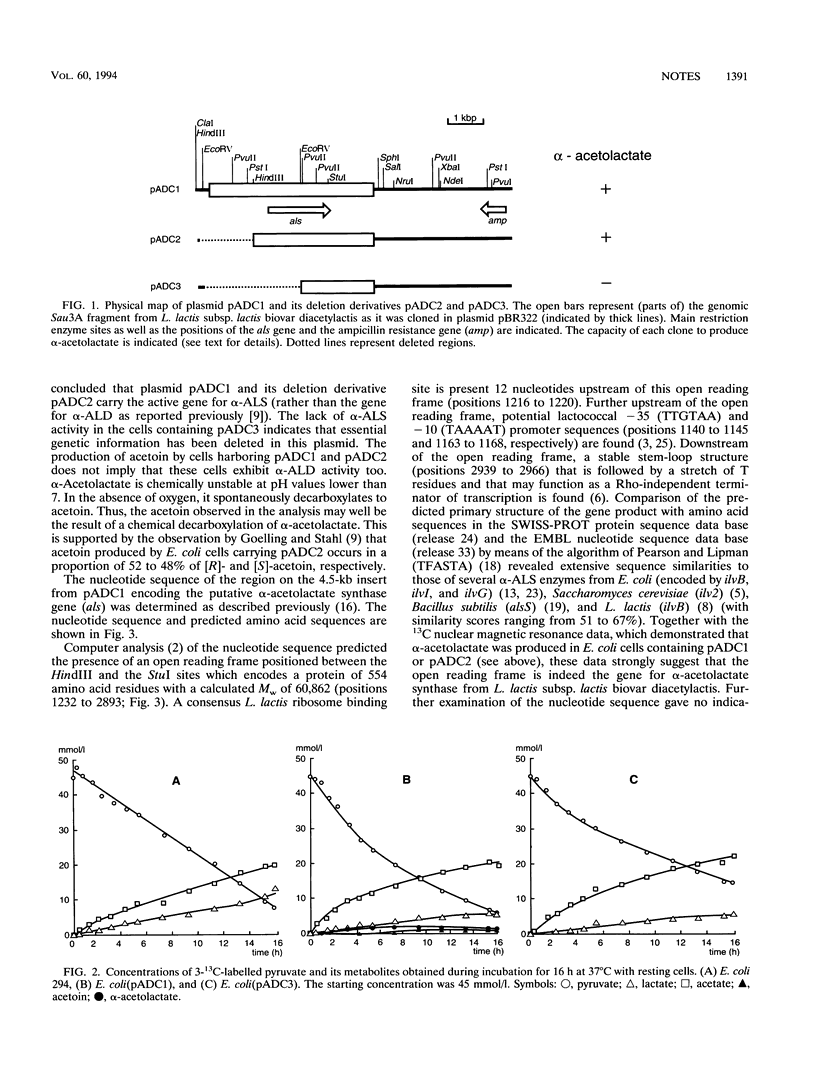
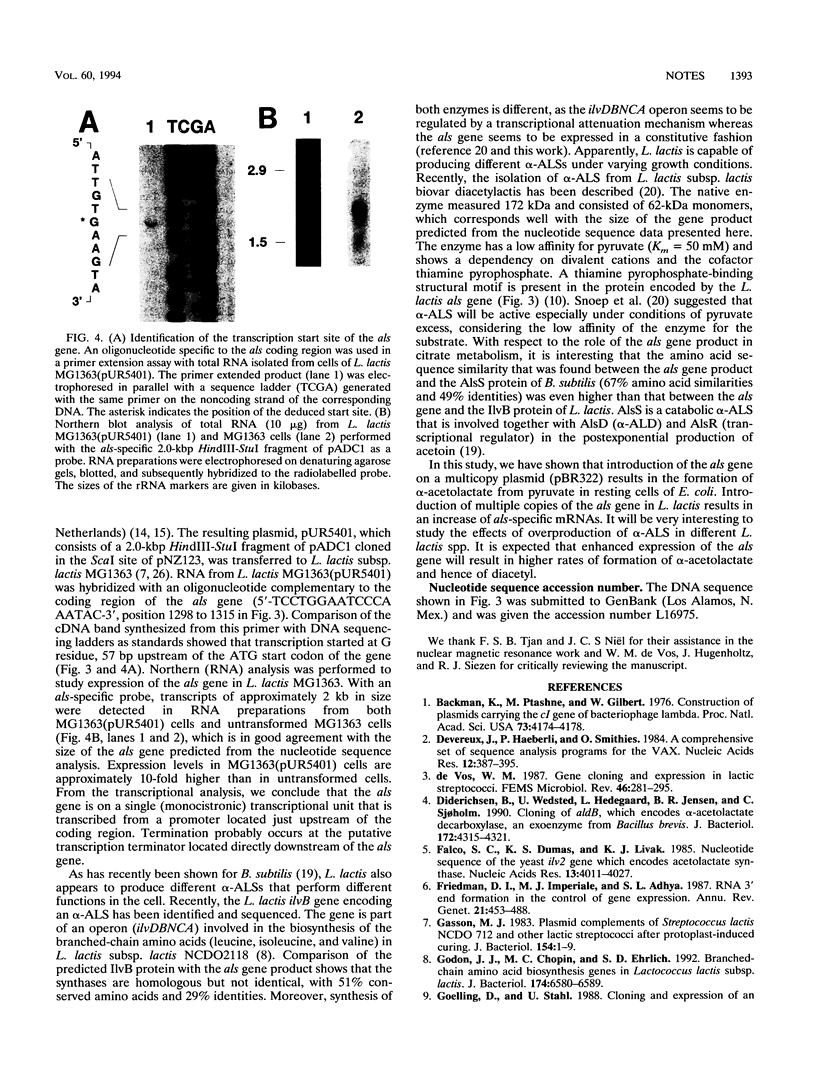
The conversion of 3-13C-labelled pyruvate in an acetoin-producing clone from a Lactococcus lactis subsp. lactis biovar diacetylactis strain DSM 20384 plasmid bank in Escherichia coli was studied by 13C nuclear magnetic resonance analysis. The results showed that alpha-acetolactate was the first metabolic product formed from pyruvate, whereas acetoin appeared at a much slower rate and reached only low concentrations. This alpha-acetolactate production shows that the cells express the gene for alpha-acetolactate synthase (als). Nucleotide sequence analysis identified an open reading frame encoding a protein of 554 amino acids. The deduced amino acid sequence exhibits extensive similarities to those of known alpha-acetolactate synthases from both prokaryotes and eukaryotes. The als gene is expressed on a monocistronic transcriptional unit, which is transcribed from a promoter located just upstream of the coding region.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backman K., Ptashne M., Gilbert W. Construction of plasmids carrying the cI gene of bacteriophage lambda. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4174–4178. doi: 10.1073/pnas.73.11.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diderichsen B., Wedsted U., Hedegaard L., Jensen B. R., Sjøholm C. Cloning of aldB, which encodes alpha-acetolactate decarboxylase, an exoenzyme from Bacillus brevis. J Bacteriol. 1990 Aug;172(8):4315–4321. doi: 10.1128/jb.172.8.4315-4321.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco S. C., Dumas K. S., Livak K. J. Nucleotide sequence of the yeast ILV2 gene which encodes acetolactate synthase. Nucleic Acids Res. 1985 Jun 11;13(11):4011–4027. doi: 10.1093/nar/13.11.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. I., Imperiale M. J., Adhya S. L. RNA 3' end formation in the control of gene expression. Annu Rev Genet. 1987;21:453–488. doi: 10.1146/annurev.ge.21.120187.002321. [DOI] [PubMed] [Google Scholar]
- Gasson M. J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983 Apr;154(1):1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godon J. J., Chopin M. C., Ehrlich S. D. Branched-chain amino acid biosynthesis genes in Lactococcus lactis subsp. lactis. J Bacteriol. 1992 Oct;174(20):6580–6589. doi: 10.1128/jb.174.20.6580-6589.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins C. F., Borges A., Perham R. N. A common structural motif in thiamin pyrophosphate-binding enzymes. FEBS Lett. 1989 Sep 11;255(1):77–82. doi: 10.1016/0014-5793(89)81064-6. [DOI] [PubMed] [Google Scholar]
- Lawther R. P., Wek R. C., Lopes J. M., Pereira R., Taillon B. E., Hatfield G. W. The complete nucleotide sequence of the ilvGMEDA operon of Escherichia coli K-12. Nucleic Acids Res. 1987 Mar 11;15(5):2137–2155. doi: 10.1093/nar/15.5.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenhouts K. J., Kok J., Venema G. Campbell-like integration of heterologous plasmid DNA into the chromosome of Lactococcus lactis subsp. lactis. Appl Environ Microbiol. 1989 Feb;55(2):394–400. doi: 10.1128/aem.55.2.394-400.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marugg J. D., Gonzalez C. F., Kunka B. S., Ledeboer A. M., Pucci M. J., Toonen M. Y., Walker S. A., Zoetmulder L. C., Vandenbergh P. A. Cloning, expression, and nucleotide sequence of genes involved in production of pediocin PA-1, and bacteriocin from Pediococcus acidilactici PAC1.0. Appl Environ Microbiol. 1992 Aug;58(8):2360–2367. doi: 10.1128/aem.58.8.2360-2367.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renna M. C., Najimudin N., Winik L. R., Zahler S. A. Regulation of the Bacillus subtilis alsS, alsD, and alsR genes involved in post-exponential-phase production of acetoin. J Bacteriol. 1993 Jun;175(12):3863–3875. doi: 10.1128/jb.175.12.3863-3875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoep J. L., Teixeira de Mattos M. J., Starrenburg M. J., Hugenholtz J. Isolation, characterization, and physiological role of the pyruvate dehydrogenase complex and alpha-acetolactate synthase of Lactococcus lactis subsp. lactis bv. diacetylactis. J Bacteriol. 1992 Jul;174(14):4838–4841. doi: 10.1128/jb.174.14.4838-4841.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone H., Fujii T., Kondo K., Shimizu F., Tanaka J., Inoue T. Nucleotide sequence and expression of the Enterobacter aerogenes alpha-acetolactate decarboxylase gene in brewer's yeast. Appl Environ Microbiol. 1988 Jan;54(1):38–42. doi: 10.1128/aem.54.1.38-42.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speckman R. A., Collins E. B. Diacetyl biosynthesis in Streptococcus diacetilactis and Leuconostoc citrovorum. J Bacteriol. 1968 Jan;95(1):174–180. doi: 10.1128/jb.95.1.174-180.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C. H., De Felice M., Devereux J., Calvo J. M. Molecular structure of ilvIH and its evolutionary relationship to ilvG in Escherichia coli K12. Nucleic Acids Res. 1983 Aug 11;11(15):5299–5313. doi: 10.1093/nar/11.15.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starrenburg M. J., Hugenholtz J. Citrate Fermentation by Lactococcus and Leuconostoc spp. Appl Environ Microbiol. 1991 Dec;57(12):3535–3540. doi: 10.1128/aem.57.12.3535-3540.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhue W. M., Tjan F. S. Study of the Citrate Metabolism of Lactococcus lactis subsp. lactis Biovar Diacetylactis by Means of C Nuclear Magnetic Resonance. Appl Environ Microbiol. 1991 Nov;57(11):3371–3377. doi: 10.1128/aem.57.11.3371-3377.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Guchte M., Kok J., Venema G. Gene expression in Lactococcus lactis. FEMS Microbiol Rev. 1992 Feb;8(2):73–92. doi: 10.1111/j.1574-6968.1992.tb04958.x. [DOI] [PubMed] [Google Scholar]
- van der Lelie D., van der Vossen J. M., Venema G. Effect of Plasmid Incompatibility on DNA Transfer to Streptococcus cremoris. Appl Environ Microbiol. 1988 Apr;54(4):865–871. doi: 10.1128/aem.54.4.865-871.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vossen J. M., Kodde J., Haandrikman A. J., Venema G., Kok J. Characterization of transcription initiation and termination signals of the proteinase genes of Lactococcus lactis Wg2 and enhancement of proteolysis in L. lactis. Appl Environ Microbiol. 1992 Sep;58(9):3142–3149. doi: 10.1128/aem.58.9.3142-3149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]




