Abstract
Aims
To investigate the pharmacodynamics of milnacipran in healthy young and elderly volunteers.
Methods
Randomized double-blind crossover designs were employed and a standardized psychometric battery was administered pre and post dose for both studies. In the first study 10 healthy young volunteers received milnacipran 12.5 mg, 25 mg, 50 mg, 100 mg as a single dose or matched placebo. The test battery was administered at baseline and at 1, 2, 4 and 6 h post dose. The second study compared the effects of milnacipran 75 mg (50 mg+25 mg) per day, amitriptyline 50 mg (25 mg+25 mg) per day and placebo for 3 days’ dosing in healthy volunteers aged over 65 years. The test battery was administered at baseline and at 2, 10 and 24 h post dose. The psychometric battery included critical flicker fusion (CFF), choice reaction time (CRT), compensatory tracking (CTT) and tests of short-term memory (STM), subjective sedation (LARS) and subjective sleep (LSEQ).
Results
Milnacipran produced no significant dose related effects in the young volunteers. For the elderly, milnacipran significantly (P < 0.05) raised CFF scores compared with placebo but had no significant effects on any of the other measures used. Amitriptyline, in contrast, significantly (P < 0.05) lowered CFF threshold, lengthened CRT and increased error on the CTT. On the subjective variables, LARS and LSEQ, amitriptyline increased ratings both of sedation and of difficulty in waking from sleep.
Conclusions
The results showed that milnacipran at single doses of up to 100 mg in healthy young volunteers is free from disruptive effects on cognitive function and psychomotor performance. In addition, milnacipran 75 mg (50+25 mg) appears to be free of negative effects on cognitive function in elderly volunteers, where it seemingly improves performance on CFF. In contrast, the tricyclic antidepressant amitriptyline, used here as a positive internal control, significantly impaired performance in the elderly on the majority of psychometric measures used in this study. This finding not only validated the sensitivity of this current test battery but also indicates the potential behavioural toxicity of amitriptyline in clinical use in the elderly.
Keywords: amitriptyline, antidepressant, cognitive function, elderly volunteers, milnacipran, psychomotor performance
Introduction
Milnacipran (1-phenyl-1-diethyl aminocarbonyl-2-aminomethyl cyclopropane-2-hydorochloride) exerts its antidepressant activity by inhibiting the uptake of serotonin (5-HT) and noradrenaline (NA) at presynaptic sites and may therefore be regarded as one of the new class of serotonin and noradrenaline reuptake inhibitors (SNRIs). Milnacipran has been shown not to affect postsynaptic cholinergic, adrenergic, histaminergic H1, dopaminergic D2 or serotonergic receptor sites [1–4]. The biochemical profile of milnacipran and its lack of interaction with other neurotransmitters indicates that the drug may be maximally effective in the treatment of depression, while being free of the side-effects associated with other antidepressant drugs.
In clinical trials with depressed patients, milnacipran (50 mg twice daily) has been shown to have significant antidepressant activity compared with placebo [5, 6]. It has also been shown to be at least as efficacious as typical tricyclic antidepressants (TCAs) [7, 8] and the selective serotonin reuptake inhibitors (SSRIs), fluvoxamine and fluoxetine [9, 10].
Apart from clinical efficacy the decision to prescribe a particular antidepressant must also be based on its side-effect profile and although studies indicate that the TCAs are effective in the treatment of severe depression [11, 12] the use of the SSRIs are more generally favoured because of their superior tolerability profile [13, 14]. The nonspecific pharmacological action of TCAs has been found to result in adverse behavioural and cognitive effects [15, 16] and in a variety of somatic adverse events including orthostatic hypotension, cardiac dysrhythmias and tachycardia, convulsions, dry mouth, tremor and blurred vision [17, 18]. When compared with the SSRIs, milnacipran was associated with a lower incidence of gastrointestinal disturbances and anxiety but with a higher incidence of dysuria [19, 20]. Although the SSRIs as a group are relatively free of adverse behavioural effects [13, 15, 16] the profile of milnacipran is unknown.
Behavioural toxicity is an aspect of tolerability which is often overlooked. It can be defined as the extent to which a drug disrupts those abilities necessary for the safe performance of the cognitive and psychomotor tasks of everyday life. A significant level of behavioural toxicity can also be countertherapeutic. It may exacerbate the cognitive dysfunction associated with depression, so hindering improvement of some of the psychological manifestations of the illness [13, 21] and, in general terms, reduce compliance. The issue of behavioural toxicity is especially important in the elderly. Pharmacotherapy for this age group presents a particular set of difficulties. Pharmacokinetic profiles vary with age, and accumulation of drug and active metabolites is frequently a feature of use of a particular drug in the elderly [22]. The less efficient metabolism and elimination in the elderly means that the drug and/or its metabolites may persist in the body for longer. This may well increase the duration of clinical activity, and/or result in a greater number or increased severity of undesirable side-effects which will in turn lower tolerability. Furthermore it has been suggested that there is an increasing sensitivity of the brain, to psychoactive drugs with advancing age [23] resulting in an augmented response for a given tissue concentration. Certainly, geriatric patients are often very sensitive to drugs, and to their side-effects. The combination of these factors may result in a significant level of behavioural toxicity—not a trivial matter in the elderly in whom simple accidents such as falls can have very serious consequences [24].
Two experiments were conducted. The first experiment was designed to assess the dose-response effects of a single dose of milnacipran (dose range was 12.5 mg–100 mg) on cognitive and psychomotor function in healthy volunteers, compared with placebo. The second experiment in healthy elderly volunteers was designed to assess the effects on cognitive and psychomotor function of a repeated dose of milnacipran (50 mg+25 mg ‘boost’) compared with placebo and a low dose of amitriptyline, a positive internal control, because of its known adverse effects on cognitive and psychomotor performance in both patients [15] and volunteers [16].
Methods
Subjects
Ten healthy male volunteers with a mean age of 26.4 years (range 19–36) were recruited to the first study. Twelve healthy elderly volunteers (7 males, 5 females) were recruited to the second study. Their mean age was 71.6 years (range 66–80).
All subjects were in good physical and mental health and free from concomitant medication. No volunteers were dependent on social substances and all refrained from alcohol, caffeine and nicotine for a 24 h period prior to the study day and during the study day. All subjects gave their written informed consent. Ethical approval was obtained from the Ethics Committee of the Isle of Wight Area Health Authority and the studies were carried out at the Royal Isle of Wight County Hospital.
Design and procedure
In the first study volunteers received milnacipran 12.5 mg, 25 mg, 50 mg, 100 mg or matched placebo in a randomized double-blind 5-way crossover design in which each subject acted as their own control. The order of treatment was randomized. Each medication was administered at 09.00 h, following baseline measures on the test battery. Assessments were repeated at 2, 4 and 6 h post dose. Additionally, critical flicker fusion was administered at 1 h post dose.
Volunteers in the second study received milnacipran 75 mg day−1 (50 mg at 09.00 h and 25 mg at 13.00 h); amitriptyline 50 mg day−1 (25 mg administered at 09.00 and 13.00 h); and matched placebo. A randomized double-blind three-way crossover design was employed, the use of latin squares ensuring balanced groups. The dose regimen was chosen to reflect the clinical situation where 50 mg milnacipran would be given as the unit dose with an ‘extra’ 25 mg ‘boost’ to ensure that adequate blood levels would be maintained during the investigational period. Amitriptyline 50 mg day−1 is not a clinically recommended dose for depression but was used primarily as a positive internal control to impair performance on the psychometric tests and so establish the validity of the test battery. Medication was given on each test day at 09.00 h and 13.00 h. The test battery was administered at predrug baseline and at 2, 10, 24, 48, 50, 58 and 72 h. All medications and placebo were packaged in identical capsules and at least a 7 day washout period was allowed between each treatment period. Before participation in both studies subjects were trained to a performance plateau on the psychometric tests to avoid any learning effects.
Psychometric test battery
The test battery comprised previously validated tests shown to be reliable measures of cognitive and psychomotor function in pharmacodynamic assessments of psychoactive drugs. It consisted of:
Critical flicker fusion (CFF )
The CFF task assesses the integrative capacity of the central nervous system (CNS), and more specifically, the ability to discriminate discrete ‘bits’ of sensory information [25]. Subjects were required to discriminate flicker from fusion, and vice versa, in a set of four light emitting diodes arranged in a 1 cm square. The diodes were held in foveal fixation at a distance of 1 m. Individual thresholds were determined by the psychophysical method of limits on three ascending (flicker to fusion) and three descending (fusion to flicker) scales [26]. CFF has been shown to be sensitive to psychoactive compounds [27] and to the effects of ageing [28]. A decrease in the CFF threshold is indicative of a reduction in the overall integrative activity of the CNS [29].
Short-term memory (STM)
High speed scanning and retrieval from short-term memory were assessed using a technique based on a reaction time method [30]. Subjects memorized a random series of four digits (the stimulus set) which were presented sequentially at a rate of 1.2 s per digit. One second after the final digit of the stimulus set was presented an auditory warning signal sounded. This was followed by a series of 12 single probe digits. Subjects indicated whether each probe digit was contained within the original stimulus set or not, by pressing one of two mouse buttons as quickly and as accurately as possible. The rate of presentation of the probe digits was determined by the subject’s rate of response. Twenty trials were carried out. Response time and accuracy were recorded. Performance on the STM is sensitive to psychoactive compounds [31] and to the effects of ageing [32].
Choice reaction time (CRT)
The CRT task [25, 29] is used as an indicator of sensorimotor performance, assessing the ability to attend and respond to a critical stimulus. Subjects placed the index finger of their preferred hand on a central starting button, and were instructed to extinguish one of six equidistant red lights, illuminated at random, by pressing the response button immediately in front of the light as quickly as possible. The mean of 20 consecutive presentations was recorded as a response measure of three components of reaction time: recognition, motor and total reaction time. Recognition reaction time (RRT) is the time it takes for the subject to notice the light, being the time between stimulus onset and the subject lifting their finger from the start button. Motor reaction time (MRT) indexes the movement component of this task, and is the time between the subject lifting their finger from the start button and touching the response button. The total reaction time (TRT) is the sum of RRT and MRT. CRT is sensitive to a variety of psychoactive agents [27, 29] and to the effects of ageing [33].
Compensatory Tracking Test (CTT)
This interactive task of psychomotor function entails tracking a moving arrow on a VDU screen using a joystick. The response measure (RMS) was the mean deviation from the track program in pixels over a one minute trial period, with lower scores indicative of more accurate tracking. A peripheral awareness task (PRT) was included in which the subject responded to a stimulus presented in the periphery of vision, while simultaneously attending to the tracking test. The mean reaction time in milliseconds to these stimuli over the trial period was taken as the response measure for this component of the divided attention task [34].
Line analogue rating scale for sedation (LARS)
The LARS is employed as a measure of subjective effects of psychoactive drugs. Subjects marked a series of 10 cm line analogue scales, indicating their present feeling with regards to a mid-point, which represents their normal state of mind before treatment began. The mean scores of ratings of ‘tiredness’, ‘drowsiness’, and ‘alertness’, presented among several distracter scales, are taken as a measure of perceived sedation [35]. The higher the score (in mm), the less alert and more tired and drowsy the subject feels.
Leeds sleep evaluation questionnaire
The LSEQ assesses the effects of psychoactive compounds on sleep and early morning behaviour [25]. The subjects marked a series of 10 cm line analogue scales, indicating the direction and magnitude of any changes in behavioural state they experience following the administration of a drug. More specifically, the LSEQ considers the perceived ease of getting to sleep, the quality of sleep, and any hangover effect the following morning.
The LSEQ was used in experiment 2 only.
Adverse events
Adverse events were elicited by an open question, ‘Have you noticed anything different?’ Details were recorded on the appropriate case record form.
Statistical analysis
Both experiments were analysed separately. Data were entered as difference-from-baseline scores. This is an alternative to analysis of covariance using baseline scores as the covariate [36]. Data on all the psychometric tests were analysed using repeated measures analyses of variance with the factors treatment and time. Post hoc comparisons between means were performed using two-tailed 95% confidence intervals. Means representing the levels of each factor were compared using Tukey’s LSD tests of least significant difference.
Results
Experiment 1
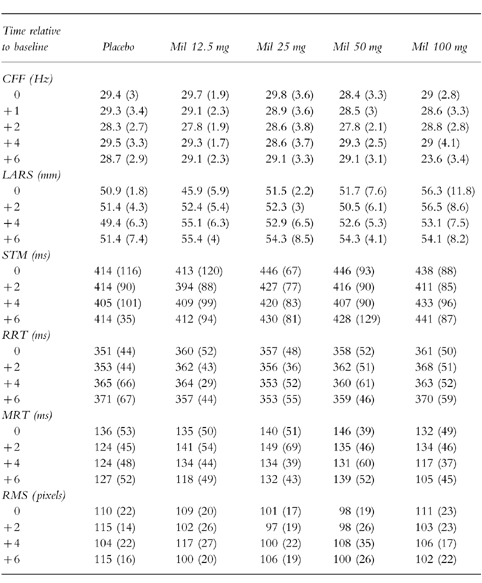
Table 1 shows the mean results of milnacipran 12.5 mg, 25 mg, 50 mg, 100 mg and placebo for CFF, LARS, STM, RRT, MRT and RMS. There were no significant main effects of treatment or time and no significant interactions between these factors on any of the psychometric measures (P > 0.05). Milnacipran was not significantly different to placebo and displayed no dose response effects.
Table 1.
Means, with standard deviations in parentheses, for cognitive and psychomotor tests in experiment 1.
The main adverse events reported for milnacipran were nausea and vomiting, with four volunteers experiencing nausea and three volunteers vomiting at the highest dose (100 mg). Vomiting occurred only at the highest dose and nausea was less frequently reported for the lower doses. There were two reports of nausea for milnacipran 50 mg, one report of nausea at 25 mg and none at 12.5 mg. Four volunteers experienced a feeling of ‘wellbeing’ at lower doses of milnacipran. There was one report of drowsiness for milnacipran which was present at the highest dose. Headache [1] and nausea [1] were reported for placebo.
Experiment 2
The results for the CFF task showed a significant main effect of treatment (F [2, 220]=22.19, P < 0.05). When post hoc comparisons between treatment means were performed, there were significant differences between amitriptyline and placebo (CI 95%=−1.03, −0.25) and between milnacipran and placebo (CI 95%=0.28, 1.07). Compared with placebo, amitriptyline caused a significant overall decline in CFF threshold while milnacipran significantly increased CFF threshold (Figure 1).
Figure 1.
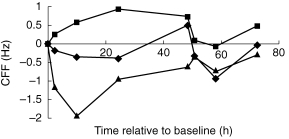
The effects of milnacipran (▪), amitriptyline (▴) and placebo (♦) cognitive function as measured by critical flicker fusion (CFF). Higher scores represent better cognitive function. Values are plotted as differences from baseline over time from the first dose on day 1. Milnacipran was significantly better than placebo overall; amitriptyline was significantly worse.
The RRT component of the choice reaction time task was also significantly affected by treatment (F [2, 215]=8.85, P < 0.05). Comparisons between treatment means showed that amitriptyline caused a significant slowing of reaction time compared with placebo (CI 95%=21.43, 69.59) whereas milnacipran was not significantly different to placebo (CI 95%=−21.89, 26.27) (Figure 2).
Figure 2.
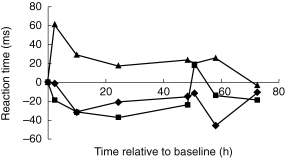
The effects of milnacipran (▪), amitriptyline (▴) and placebo (♦) on psychomotor performance as measured by recognition reaction time (RRT). Lower scores indicate faster performance. Values are plotted as differences from baseline over time from the first dose on day 1. Amitriptyline produced significantly slower RT than placebo overall. Milnacipran was not significantly different from placebo at any time.
Similarly, there were significant treatment effects for tracking accuracy (RMS) (F [2, 220]=15.27, P < 0.05), subjective ratings of sedation (LARS) (F [2, 15]=11.31, P < 0.05) and the AFS component of the LSEQ (F [2, 55]=9.90, P < 0.05). Post hoc comparisons of each treatment with placebo revealed that amitriptyline significantly worsened RMS in the tracking test (CI 95%=0.99, 2.30), significantly increased subjective ratings of sedation (LARS) (CI 95%=2.71, 6.63) and produced a significant increase in the difficulty of Awakening From Sleep (CI 95%=0.68, 12.08) (Figure 3). Milnacipran was not significantly different from placebo for tracking accuracy (CI 95%=0.5, 0.8), sedation (CI 95%=−0.22, 3.70) or Awakening From Sleep (CI 95%=−12.26,0.86). Finally, there were no significant main effects of time and no significant interactive effects between treatment and time on any of the psychometric measures.
Figure 3.
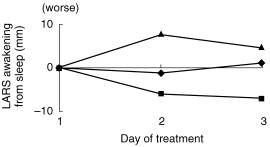
The effects of milnacipran (▪), amitriptyline (▴) and placebo (♦) on subjective ease of awakening from sleep, as measured by the AFS component of the LSEQ. A higher score represents more subjectively perceived difficulty in waking up. Values are plotted as differences from baseline, over the three dose days. There were no significant differences between milnacipran and placebo at any time point. Amitriptyline, however, produced greater subjective difficulty in waking compared to placebo.
The main adverse events reported by volunteers with amitriptyline were drowsiness (11) and dry mouth (3); with milnacipran, headache (9) and nausea (7); and with placebo, drowsiness (6) and headache (6). Contrary to the first study which had no reports of headache following milnacipran, headache was the most frequently reported adverse effect of milnacipran in the elderly. The number of reports of drowsiness was comparable for milnacipran and placebo, while amitriptyline was associated with a significantly higher number of drowsiness reports.
Discussion
The data presented here demonstrate that milnacipran at doses of up to 100 mg daily in young volunteers and repeated doses of 75 mg (50 mg and 25 mg) daily in elderly volunteers is free from disruptive effects on salient aspects of cognitive function and psychomotor performance. For the young volunteers no dose-response effects were observed with the drug, and there were no significant differences between milnacipran and placebo, indicating that the molecule was intrinsically free from detrimental effects and that increasing the dose (within the range studied) would be unlikely to produce any significant change to psychomotor performance. In the elderly group, milnacipran also showed no significant impairing effects on any of the psychometric measures used. Milnacipran significantly raised CFF threshold, a measure of central arousal. In contrast, a subclinical dose of amitriptyline significantly lowered CFF threshold, increased recognition reaction time, worsened RMS in the tracking test, and increased subjective ratings of sedation and difficulty of Awakening From Sleep.
The difference in CFF threshold between milnacipran and placebo was maintained for the duration of the study. Since a low CFF is a characteristic of depressive illness [37], the ability of milnacipran to raise CFF is an encouraging feature for the clinical use of this drug. The significant increase in CFF thresholds in the elderly, which was not present in the young, is possibly associated with the fact that healthy young volunteers are performing optimally to begin with while performance in the elderly may be in decline. Hindmarch [28] has shown that CFF thresholds decrease with age and suggests that this decrease is a reflection of age related changes in information processing capacity.
The impairment seen with amitriptyline in this study is a well established and necessary sequelae to the use of TCAs. The impairment of cognitive and psychomotor functioning, and memory and high sedation [15, 16] is a direct result of their pharmacological action, i.e. anticholinergic, antihistaminic activity and α1-receptor antagonism. This pharmacological profile accounts for the clinical findings of increased drowsiness, impaired memory and cognitive and psychomotor functioning. The consequences of these effects are an increase in accidents, particularly in the elderly [38]. Ray et al. [38] showed that elderly patients taking amitriptyline 125 mg day−1 (or TCA equivalent) are six times more likely to be involved in a road traffic accident than patients taking other drugs. Currie et al. [39] analysed blood samples from patients presenting at casualty centres with accidental injuries. Independent assessors divided the patients into those who were deemed responsible for the accident (e.g. solo car driver) and those who were not (e.g. hit by falling masonry) and found that there was a significantly greater representation of TCAs in the bloods from those who were responsible for the accidents than those who were not. Similar results were found for alcohol and benzodiazepines. Despite a very poor tolerability profile and a raised incidence of accidents, TCAs are widely prescribed, even though the dose used is subclinical, for the treatment of depression in the elderly [40, 41].
There is growing evidence that dual action antidepressants, such as milnacipran and venlafaxine, which act to increase both noradrenergic and serotonergic activity may combine the efficacy of TCAs with the benign side-effect profile of SSRIs [42, 43]. The results of the present studies show that milnacipran is without any notable effects on a battery of psychometric tests designed to detect impairment in performance and countertherapeutic activity. The lack of adverse psychomotor effects of milnacipran is a product of its pharmacological profile, just as the behavioural toxicity of TCAs can be explained via their intrinsic pharmacological action.
Given that that most antidepressants currently available are clinically efficacious and that the British Association for Psychopharmacology guidelines [44] recommend that prescription choice for antidepressants should be based on side-effect profiles as well as clinical efficacy, then milnacipran’s lack of countertherapeutic side-effects represents a considerable advantage in an antidepressant, since impairment of mental function is not only a component of depressive illness but also a cause of reduced quality of life, poor compliance and increased accident risk.
Acknowledgments
This study was funded by Institut de Recherche Pierre Fabre, 81106 Castres, France.
References
- 1.Kitamura Y, Nagatani T, Takao K. Pharmacological study of milnacipran, a novel antidepressant. Jpn J Neuropsychopharmacol. 1995;17:25–34. [Google Scholar]
- 2.Matsubara R, Matsubara S, Koyama T. Effect of chronic treatment with milnacipran, a novel antidepressant, on the β-adrenergic receptor-adenylate cyclase system and serotonin2 receptor in the rat cerebral cortex. Jpn J Neuropsychopharmacol. 1993;15:119–126. [Google Scholar]
- 3.Moret C, Briley M. Effect of antidepressant drugs on monoamine synthesis in brain in vivo. Neuropharmacol. 1992;31:679–684. doi: 10.1016/0028-3908(92)90146-g. [DOI] [PubMed] [Google Scholar]
- 4.Moret C, Charveron M, Finberg JP, Couzinier JP, Briley M. Biochemical profile of midalcipran (2207), 1–phenyl–1–diethyl aminocarbonyl–2–aminomethyl cyclopropane–2–hydorochloride, a potential fourth generation antidepressant drug. Neuropharmacol. 1985;24:1211–1219. doi: 10.1016/0028-3908(85)90157-1. [DOI] [PubMed] [Google Scholar]
- 5.Lecrubier Y, Pletan Y, Solles A, Tournoux A, Magne V. Clinical efficacy of milnacipran: Placebo controlled trials. Int Clin Psychopharmacol. 1996;11:29–33. doi: 10.1097/00004850-199609004-00004. [DOI] [PubMed] [Google Scholar]
- 6.Macher J-P, Sichel J-P, Serre C, von Frenckell R, Huck JC, Demarez JP. Double blind placebo-controlled study of milnacipran in hospitalized patients with major depressive disorders. Neuropsychobiol. 1989;22:77–82. doi: 10.1159/000118596. [DOI] [PubMed] [Google Scholar]
- 7.Clerc GE, Pagot R, Bouchard JM, et al. Therapeutic contribution of milnacipran and clomipramine during a 3-month treatment: Results of a comparative study. Psychiatr Psychobiol. 1990;5:137–143. [Google Scholar]
- 8.Kaspar S, Pletan Y, Solles A, Tournoux A. Comparative studies with milnacipran and tricyclic antidepressants in the treatment of patients with major depression: a summary of clinical trial results. Int Clin Psychopharmacol. 1996;11:35–39. doi: 10.1097/00004850-199609004-00005. [DOI] [PubMed] [Google Scholar]
- 9.Ansseau M, von Frenckell R, Gérard MA, et al. Interest of a loading dose of milnacipran in endogenous depressive inpatients: Comparison with the standard regimen and with fluvoxamine. Eur Neuropsychopharmacol. 1991;1:113–121. doi: 10.1016/0924-977x(91)90712-4. [DOI] [PubMed] [Google Scholar]
- 10.Ansseau M, Papart P, et al. Controlled comparison of milnacipran and fluoxetine in major depression. Psychopharmacol. 1994;114:131–137. doi: 10.1007/BF02245454. [DOI] [PubMed] [Google Scholar]
- 11.Danish University Antidepressant Group. Paroxetine, a selective serotonin reuptake inhibitor showing better tolerance, but weaker antidepressant effect than clomipramine in a controlled multicentre study. J Affect Dis. 1990;18:289–299. doi: 10.1016/0165-0327(90)90081-i. [DOI] [PubMed] [Google Scholar]
- 12.Anderson IM, Tomenson BM. The efficacy of selective serotonin re-uptake inhibitors in depression: a meta-analysis of studies against tricyclic antidepressants. J Psychopharmacol. 1994;8:239–249. doi: 10.1177/026988119400800407. [DOI] [PubMed] [Google Scholar]
- 13.Kerr JS, Sherwood N, Hindmarch I. The comparative psychopharmacology of 5- HT re-uptake inhibitors. Hum Psychopharmacol. 1991;6:313–317. [Google Scholar]
- 14.De Vane CL. Pharmacokinetics of the newer antidepressants: Clinical relevance. Am J Med. 1997;6A:13S–23S. doi: 10.1016/0002-9343(94)90359-x. [DOI] [PubMed] [Google Scholar]
- 15.Fairweather DB, Kerr JS, Harrison DA, Moon CA, Hindmarch I. A double-blind comparison of the effects of fluoxetine and amitriptyline on cognitive function in elderly depressed patients. Hum Psychopharmacol. 1993;8:41–47. [Google Scholar]
- 16.Sherwood N, Hindmarch I. A comparison of five commonly prescribed antidepressants with particular reference to their behavioural toxicity. Hum Psychopharmacol. 1993;8:417–422. [Google Scholar]
- 17.Steffens DC, Krishman KR, Helms MJ. Are SSRIs better than TCAs? Comparison of SSRIs and TCAs: a meta-analysis. Depress Anxiety. 1997;6:10–18. doi: 10.1002/(sici)1520-6394(1997)6:1<10::aid-da2>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 18.Rudorfer MV, Potter WZ. Antidepressants: a comparative review of the clinical pharmacology and therapeutic use of the ‘newer’ versus the ‘older’ drugs. Drugs. 1989;3:713–738. doi: 10.2165/00003495-198937050-00006. [DOI] [PubMed] [Google Scholar]
- 19.Puech A, Montgomery SA, Prost JF, Solles A, Briley M. Milnacipran, a new serotonin and noradrenaline reuptake inhibitor: an overview of its antidepressant activity and clinical tolerability. Int Clin Psychopharmacol. 1997;12:99–108. doi: 10.1097/00004850-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Montgomery SA, Prost JF, Solles A, Briley M. Efficacy and tolerability of milnacipran: and overview. Int Clin Psychopharmacol. 1996;11:47–51. doi: 10.1097/00004850-199609004-00007. [DOI] [PubMed] [Google Scholar]
- 21.Cohen RM, Weingartner H, Smallberg SA, Pickar D, Murphy DL. Effort and cognition in depression. Arch Gen Psychiat. 1982;39:593–597. doi: 10.1001/archpsyc.1982.04290050061012. [DOI] [PubMed] [Google Scholar]
- 22.Greenblatt DJ, Sellers EM, Shader RI. Drug disposition in old age. N Engl J Med. 1982;306:1081–1087. doi: 10.1056/NEJM198205063061804. [DOI] [PubMed] [Google Scholar]
- 23.Castleden CM, George CF, Hallett C, Marcer D. Increased sensitivity to nitrazepam in old age. Br Med J. 1977;1:10–12. doi: 10.1136/bmj.1.6052.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lord SR, Anstey KJ, Williams P, Ward JA. Psychoactive medication use, sensorimotor function and falls in older women. Br J Clin Pharmacol. 1995;39:227–234. doi: 10.1111/j.1365-2125.1995.tb04441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hindmarch IA. 1, 4–benzodiazepine temazepam (K3917) its effects on some psychological parameters of sleep and behaviour. Arzneimittel–Forsch (Drug Res) 1975;11:1836–1839. [PubMed] [Google Scholar]
- 26.Woodworth RS, Schlosberg H. Experimental Psychology. New York: Holt, Rinehart & Winston; 1954. pp. 192–233. [Google Scholar]
- 27.Hindmarch I, Haller J, Sherwood N, Kerr JS. Comparison of five anxiolytic benzodiazepines on measures of ‘qlpsychomotor performance and sleep. Neuropsychobiol. 1991;24:84–89. doi: 10.1159/000119466. [DOI] [PubMed] [Google Scholar]
- 28.Hindmarch I. Critical Flicker Fusion Thresholds as a useful measure of circadian rhythmicity. In: Marks V, Parker SW, editors. The Biological Clock- Current Approaches. Duphar: Southampton; 1988. pp. 57–61. [Google Scholar]
- 29.Hindmarch I. Psychomotor function and psychoactive drugs. Br J Clin Pharmacol. 1980;10:189–209. doi: 10.1111/j.1365-2125.1980.tb01745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sternberg S. High speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- 31.Subhan Z, Hindmarch I. Effects of zopiclone and benzodiazepine hypnotics on search in short-term memory. Neuropsychobiol. 1984;12:244–248. doi: 10.1159/000118146. [DOI] [PubMed] [Google Scholar]
- 32.Anders TR, Fozard JL, Lillyquist TD. Effects of age upon retrieval from short-term memory. Dev Psychol. 1972;6:214–217. [Google Scholar]
- 33.Frewer LJ, Hindmarch I. The effects of time of day, age, and anxiety on a choice reaction time task. In: Hindmarch I, Aufdembrinke B, Ott H, editors. Psychopharmacology and Reaction Time. Chichester: John Wiley and Sons; 1988. pp. 103–143. [Google Scholar]
- 34.Parkin C, Fairweather DB, Shamsi Z, Stanley N, Hindmarch I. The effects of cigarette smoking on overnight performance. Psychopharmacol. 1998;136:172–178. doi: 10.1007/s002130050553. [DOI] [PubMed] [Google Scholar]
- 35.Hindmarch I, Gudgeon AC. The effects of clobazam and lorazepam on aspects of psychomotor performance and car handling ability. Br J Clin Pharmacol. 1980;10:145. doi: 10.1111/j.1365-2125.1980.tb01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gnanadesikan R. Methods for Statistical Data Analysis of Multivariate Observations. New York: John Wiley & Sons; 1977. [Google Scholar]
- 37.Hindmarch I. Antidepressant drugs and cognitive function. In: Biziere K, Garattini S, Simon P, editors. Quo Vadis- Diagnosis and Treatment of Depression. Paris: McGraw-Hill; pp. 356–365. [Google Scholar]
- 38.Ray WA, Fought RL, Decker MD. Psychoactive drugs and the risk of injurious motor vehicle crashes in elderly drivers. Am J Epidemiol. 1992;136:873–883. doi: 10.1093/aje/136.7.873. [DOI] [PubMed] [Google Scholar]
- 39.Currie D, Hashemi K, Fothergill J, Findlay A, Harris A, Hindmarch I. The use of benzodiazepines in the ‘qlperpetrators and victims of accidents. Occup Med. 1995;45:323–325. doi: 10.1093/occmed/45.6.323. [DOI] [PubMed] [Google Scholar]
- 40.Koenig HG, George LK, Meador KG. Use of antidepressants by nonpsychiatrists in the treatment of medically ill hospitalized depressed elderly patients. Am J Psychiatry. 1997;154:1369–1375. doi: 10.1176/ajp.154.10.1369. [DOI] [PubMed] [Google Scholar]
- 41.Barbone F, McMahon AD, Davey PG, et al. Association of road-traffic accidents with benzodiazepine use. Lancet. 1998;352:1331–1336. doi: 10.1016/s0140-6736(98)04087-2. [DOI] [PubMed] [Google Scholar]
- 42.Puech A, Montgomery SA, Prost JF. Milnacipran, a new serotonin and noradrenaline reuptake inhibitor: an overview of its antidepressant activity and clinical tolerability. Int Clin Psychopharmacol. 1997;12:99–108. doi: 10.1097/00004850-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Clerc GE, Ruimy P, Verdeau-Pailles J. A double blind comparison of venlafaxine and fluoxetine in patients hospitalised for major depression and melancholia. Int Clin Psychopharmacol. 1994;9:139–143. doi: 10.1097/00004850-199409000-00001. [DOI] [PubMed] [Google Scholar]
- 44.British Association for Psychopharmacology. Guidelines for treating illness with antidepressants. J Psychopharmacol. 1993;7:19–23. doi: 10.1177/0269881193007001041. [DOI] [PubMed] [Google Scholar]


