Abstract
Aims
To explore drug exposure, frequency of adverse drug reactions (ADRs), types of ADRs, predisposing risk factors and ADR-related excess hospital stay in medical inpatients.
Methods
Structured data regarding patient characteristics, ‘events’ (symptoms, laboratory results), diagnoses (ICD10) and drug therapy were collected using a computer-supported data entry system and an interface for data retrieval from electronic patient records. ADR data were collected by ‘event monitoring’ to minimize possible bias by the drug monitor. The causality of each event was assessed in relation to disease(s) and drug therapy.
Results
The analysis included 4331 (100%) hospitalizations. The median observation period was 8 days. The median number of different drugs administered per patient and day was 6 and varied between 4 (Q1) and 9 (Q3) different drugs in 50% of all hospital days. In 41% of all hospitalizations at least one disease-unrelated event could be possibly attributed to drug therapy. Clinically relevant ADRs occurred in 11% of all hospitalizations. In 3.3% of all hospitalizations ADRs were the cause of hospital admission. The incidence of possibly ADR-related deaths was 1.4. Factors predisposing for clinically relevant ADRs were female gender and polypharmacy. ADR-related excess hospital stay accounted for 8.6% of hospital days.
Conclusions
These data demonstrate the feasibility of the developed ‘event monitoring’ system for quantitative analysis of ADRs in medical inpatients. With increasing numbers of recorded patients the pharmacoepidemiological database provides a valuable tool to study specific questions regarding drug efficacy and safety in hospitalized patients.
Keywords: adverse drug reactions, event monitoring, hospital pharmacoepidemiology, inpatients, medication usage
Introduction
Adverse drug reactions (ADRs) are a major burden on health care. It has been estimated that approximately 3–5% of all hospital admissions are related to ADRs [1, 2]. Additionally, 5–8% of all hospitalized patients experience serious ADRs and 5–10% of inhospital costs are related to ADRs [1–3]. These data were obtained in different settings, in the USA or in Europe, and may not reflect current medical practice in Switzerland.
The epidemiology of adverse drug reactions in inpatients was a major research focus in the late sixties and seventies in several countries [4, 5]. Later, most researchers shifted their focus to large data bases on outpatients, which generally lack detailed information about inpatient care such as for example inhospital drug therapy [6] and laboratory results. Inpatients differ considerably from outpatients, since they suffer generally from more acute disease and might be treated with more concomitant drugs than outpatients. Also, some drugs are preferentially or even exclusively used in hospitals, and the data collected in the seventies do not adequately reflect current medical practice. Therefore, we developed a pharmacoepidemiological database for inpatients in two Swiss hospitals. This database provides a basis for the ongoing assessment of adverse drug reactions during hospital stay and as a cause of hospital admission in Switzerland.
In this study, the developed database was used (1) to study drug exposure in medical inpatients, (2) to determine the frequency of medication-related events, (3) to estimate the contribution of ADRs to hospital admissions, (4) to characterize types of ADRs observed, (5) to determine predisposing risk factors and (6) to estimate the costs of ADRs in terms of ADR-related excess hospital stay.
Methods
Monitored cohort
This study includes data recorded in a defined cohort of medical inpatients between January 1996 and December 1998. The cohort includes all patients admitted to defined units within the Departments of Internal Medicine of the University Hospital Zurich and the Kantonsspital (State Hospital) St Gallen, both of which are teaching hospitals. The University Hospital Zurich serves as a tertiary referral centre and also in part as a primary city hospital. The Kantonsspital St Gallen is a primary city hospital as well as a secondary referral centre for the north-eastern region of Switzerland. In the University Hospital Zurich admission to specific units is independent of a subspecialty, whereas in St Gallen the monitored units belong to one of three divisions of the Department of Internal Medicine with a focus on infectious, endocrine and pulmonary diseases.
Data recording
All data are entered into a relational database built in MSAccess under WindowsNT. This database has a front end for manual data entry based on structured lists and an electronic interface to the computerized patient records for direct data retrieval.
Drug data are recorded by selection from an electronically available list which includes all drugs registered in Switzerland as well as any unregistered drugs provided by the hospital pharmacies. Additional lists relate drug identification numbers to chemical names and to the ATC-code. Drug brand names, duration of therapy and route of administration are recorded for all medications given within one week prior to entry and/or during cohort stay. Medication data during cohort stay also include dosages, dates and times of drug administration.
Clinical event data are recorded by selection from an electronically available structured list of symptoms pertaining to all organ systems (e.g. skin: pruritus, pupura, …, nervous system: headache, confusion, tremor, …, liver: jaundice, …). Each record includes separate structured data items for the time of occurrence, the time of cessation and the event’s association with the patient’s disease(s) and drug therapy. Additional information such as for example a more detailed description of the event and its temporal development can be added as ‘free text’.
Figure 1 summarizes the data recording procedure. At entry into the monitored units, patient characteristics (i.e. personal data, age, gender, body weight, height, etc.), drug therapy prior to admission and medical history are recorded. During the entire stay in the monitored units, all medications administered and all clinical events are recorded daily and entered into the database by the drug monitor. Data relating to blood pressure (BP), heart rate (HR) and temperature (T) are also recorded. To minimize any possible bias by the monitoring physician defined events are collected independent of their association with diseases and/or drug therapy. The patients' laboratory data are retrieved electronically from the laboratory computer.
Figure 1.

Event monitoring of ADRs in medical inpatients
After hospital discharge, the patients' ICD10 diagnoses are recorded as additional structured data items. The monitoring physician then evaluates the cause(s) for hospital admission and its(their) relationship(s) to drug therapy prior to hospitalization. Subsequently, he assesses the relationship of each clinical event and pathological laboratory result to the patient’s disease(s) and drug therapy and adds the corresponding ICD10 diagnosis codes and drug names in the appropriate data entry area. An event is considered as possibly drug-related, if a temporal relationship to drug therapy exists, and if similar events have previously been reported for the same drug in the standard literature of adverse drug reactions [7] or in the Swiss Drug Compendium [8]. An event is considered as disease-related, if it has previously been reported to be associated with one of the patient’s diagnoses according to standard reference textbooks [9, 10]. Thus, possibly drug-related events include all possible, probable or definite ADRs according to the Karch and Lasagna criteria and possibly drug-related, disease-unrelated events include only probable and definite ADRs [11]. Yet unknown ADRs (conditional ADR according to the Karch/Lasagna criteria) are categorized as possibly drug- and disease-unrelated events. This category contains potentially novel ADRs and therefore represents an important target for future research. The present analysis focuses on the general epidemiology of known ADRs and thus includes only possibly drug-related events. The latter were further evaluated with respect to their clinical significance and characterized as clinically relevant ADRs, if they resulted in considerable discomfort, drug withdrawal or dose reduction and/or initiation of therapeutic measures. While intentional drug overdoses were not regarded as ADRs, involuntary overdosing was included, such as for example inadequate dose adjustments to impaired renal function. All cases with drug-related admissions, suspected clinically relevant ADRs and especially difficult cases were routinely re-evaluated by a senior physician specialized in clinical pharmacology. Finally, data are anonymised by extracting all personal data and then stored in a separate archive database. Only the anonymised database is accessible for data analysis.
Data analysis
The present analysis included all patients monitored until the end of 1998. The program SAS (Release 6.11) under Windows NT was used for data analysis accessing the anonymised archive database by the open database connectivity (ODBC) interface. The relevant data were extracted, tabulated or plotted and the appropriate statistical tests were performed. Variables with skewed distributions were log-transformed before their inclusion into regression models and/or analysis of covariance.
For descriptive analyses of hospitalization duration, discharge diagnosis, frequency of ADRs and estimation of total ADR-related hospital stay, repeated hospitalizations of the same patients were counted individually. In contrast, only one (randomly selected) hospitalization per patient was included in all analyses where different patient groups were compared in order to avoid interdependent data bias. Data are generally described as median and (1st=Q1, 3rd=Q3) quartile.
The present analysis on polypharmacy included only drugs with a clear chemical identity. Although, data on herbal medicines, vitamins, minerals and electrolytes were recorded and are available in the database, they were excluded from this analysis to avoid inappropriate inflation of the estimates of polypharmacy, for example, by single ingredients of multivitamin supplements. Moreover, different salts of the same drug were counted as one drug only. If a preparation contained two or more active substances (for example a tablet containing two different diuretics), each active component was counted. The concurrent use of different drugs was evaluated on a per day and not on a per hospitalization basis in order to control for different hospitalization durations. Because medication recorded on admission and discharge days might be incomplete, the first and the last day of the cohort stay were excluded. For analysis of risk factors and/or the effects of polypharmacy on ADRs, the patient’s median number of drugs per day was used as an individual quantitative estimate of polypharmacy. Exposure to specific medications was evaluated by estimating the number of hospitalizations during which at least one dose of the corresponding drugs was administered during the cohort stay.
If at least one clinical event or pathological laboratory result was considered to be possibly drug-related, the corresponding hospitalization was defined as with possibly drug-related event(s). The laboratory results evaluated for that purpose included serum sodium, potassium, chloride, calcium, creatinine, uric acid, albumin, total proteins, cholesterol, triglycerides, glucose, bilirubin, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, γ-glutamyltranspeptidase, lactate dehydrogenase, creatine kinase, amylase, lipase, C-reactive protein, thyroid stimulating hormone, haemoglobin, leucocytes, eosinophils and platelets. BP, HR or T values outside the normal range were evaluated only if two or more consecutive recordings were abnormal. If at least one of the possibly drug-related events could not be attributed additionally to a diagnosed disease, the corresponding hospitalization was classified as with possibly drug-related, disease-unrelated event(s). If one of the possibly drug-related events was considered to be clinically relevant (see above), the corresponding hospitalization was recorded as with clinically relevant ADRs. For determination of incidence rates of clinically relevant ADRs, only ADRs arizing during the cohort stay were included.
Possible risk factors predisposing to clinically relevant ADRs were identified by comparing (1) patients without ADRs (2) patients admitted for ADRs and (3) patients with clinically relevant ADRs during cohort stay. The latter group included only patients admitted for ADR-unrelated causes. For patients admitted more than once, the included hospitalization was selected with a randomization procedure favouring hospitalizations with a clinically relevant ADR. The different groups were compared using Chi-square for bivariate variables and Kruskal–Wallis for continuous variables with P values adjusted according to Bonferoni. Chi-square or Mann–Whitney-U-tests were performed for posthoccomparisons. Thereafter, the data on hospital stay and on the patients' median number of drugs per day were log-transformed and any direct influence of identified ADR-related risk factors on hospital stay was estimated from the data of patients without clinically relevant ADRs by analysis of covariance. The obtained model was used to predict expected hospital stay based on patient characteristics and drug therapy for patients with clinically relevant ADRs but without ADR-related admission. The total ADR-related excess hospital stay was then estimated as the sum of the differences between observed and expected hospital stay and the sum of the durations of all hospitalizations with ADR-related admissions.
Results
Cohort stay and patient characteristics
Among the total of 4331 hospitalizations, 2763 (64%) hospitalizations were recorded in Zurich and 1568 (36%) in St Gallen. The median (Q1, Q3) cohort stay was 8 (4, 15) days with the corresponding distribution given in Figure 2. The cohort stay was 7 (4, 12) days in Zurich and 11 (6, 18) days in St Gallen. The overall duration of hospitalization in internal medicine was 10 (5, 18) days with the exact values amounting to 9 (5, 16) days for Zurich and 13 (8, 21) days for St Gallen. The difference between cohort stay and duration of total hospitalization arose because some patients were transferred to the cohort from other units of internal medicine such as for example from the intensive care units (479 (11%) hospitalizations).
Figure 2.

Distribution of duration of cohort stay in days for all 4331 hospitalizations recorded. ▄ male,  female.
female.
The 4331 hospitalizations included 3624 individual patients. 3144 patients were hospitalized once in the cohort, 343 patients twice, 91 three times and 46 four times or more. 194 (5%) patients died during hospital stay.
The patient's median age was comparable for both hospitals, but the age distribution was more widespread in St Gallen than in Zurich (St Gallen: 62 (44, 96), Zurich 60 (47, 72)). In 65% of all hospitalizations (St Gallen: 58%, Zurich: 69%) at least one discharge diagnosis involved the cardiovascular system (ICD10, I00-I99). In 42% (St Gallen: 41%, Zurich: 43%) at least one diagnosis involved the endocrine system or nutrition (ICD10, E00-E90). In 30% (St Gallen; 47%, Zurich: 21%) at least one diagnosis was related to the respiratory system (ICD10, J00-J99). In 21% (St Gallen: 24%, Zurich: 20%) and 21% (St Gallen: 24%, Zurich: 19%) of hospitalizations at least one diagnosis was related to neoplasias (ICD10, D50-D89) or to infectious diseases (ICD10, A00-B99), respectively. Thus, besides a predominance of respiratory diseases in St Gallen and cardiovascular diseases in Zurich, the disease distribution was quite comparable in both hospitals.
The 3624 patients included 2154 men and 1470 women. The overrepresentation of male patients originated mostly from Zurich, whereas there was only a minor difference between the number of female (640) and male (714) patients in St Gallen. The duration of cohort stay was 9 (5, 17) days for women and 8 (4, 14) days for men (Figure 2). The median age was 59 (46, 71) years for male and 64 (46, 77) for female patients. The corresponding numbers for weight were 74 (65, 83) kg and 62 (53, 72) kg, respectively. Information about smoking and alcohol consumption was available for 3299 (2047 male, 1298 female) and 3103 (1873 male, 1230 female) patients, respectively. Among the male patients, there were 800 (39% of available data) smokers, 619 (30%) former smokers and 387 (21%) patients with alcohol abuse. The corresponding numbers for female patients were 328 (25%) smokers, 185 (14%) former smokers and 83 (7%) patients with alcohol abuse. The frequency of most discharge diagnoses were comparable for male and female patients except that 26% of all female patients had at least one discharge diagnosis concerning the musculoskeletal system and the connective tissue (M00-M99) while a similar diagnosis was attributed to only 16% of male patients.
Drug exposure
Figure 3 illustrates the distribution of the number of different drugs administered per patient and day. The median number of drugs administered per day was 6 and varied between 4 (Q1) and 9 (Q3) for 50% of the monitored days. No difference in the numbers of drugs administered was present between the two hospitals (St Gallen: 6 (4, 9), Zurich: 6 (4, 9)). Also the frequency distribution of the patients' median number of drugs per day was similar (5 (3, 8)).
Figure 3.

Drug use in medical inpatients: Number of different drugs per patient and day in 4331 hospitalizations in Internal Medicine.
The following statistically significant, but rather small influence of duration of hospital stay (DH) and age on the median number of drugs (ND) per patient and day could be estimated by regression analysis:
These numbers imply that median number of drugs per patient and day for patients at the 1st (46 years) and 3rd (74 years) age quartile in case of median cohort stay increased from 5 to 6.4. The corresponding numbers for hospital stay (Q1=5 days, Q3=18 days) in case of median age were 5 and 6.3 drugs per patients and day.
Table 1 shows that drugs for symptomatic therapy such as paracetamol, oxazepam, tramadol and metoclopramide were among the most frequently administered compounds. Lactulose was used frequently in Zurich, whereas another similar laxative, lactitol, was used instead in St Gallen (346 (22%) hospitalizations). These symptomatic drugs were started during cohort stay in more than 80% of the exposed patients. In contrast, cardiovascular drugs such as acetylsalicylic acid, frusemide, enalapril and digoxin were started in more than 50% of exposed patients before cohort entry. Corresponding to the rather high prevalence of respiratory diagnoses, the antiasthmatics ipratropium and salbutamol were used more frequently in St Gallen than in Zurich. Also systemic antibacterials as a group (ATC, J01) were more frequently given in St Gallen (43%) as compared with Zurich (34%). In St Gallen amoxicillin/clavulanic acid was among the 10 most frequently used drugs, whereas cotrimoxazole (8%), ciprofloxacin (8%) and amoxicillin/clavulanic acid (7%) were used with similar frequencies in Zurich. Most cardiovascular drugs were less frequently used in St Gallen as compared with Zurich. The difference was most predominant for β-adrenoceptor blocking agents (ATC, C07). This drug class was used in St Gallen only during 9% of all hospitalizations, whereas the corresponding number for Zurich was 28%. No relevant difference was present for the utilization of calcium channel blockers (ATC, C08) between both hospitals (St Gallen 14%, Zurich: 16%). In Zurich, in 9.5% of all hospitalizations antiemetics (ATC, A04) were used, whereas these drugs were rarely administered in St Gallen (1%). Anti-inflammatory and antirheumatic products (ATC, M01) were used in 11% and in 15% of all hospitalizations in Zurich and St Gallen, respectively.
Table 1.
The 20 drugs most frequently used

Frequency of medication-related events, clinically relevant ADRs and ADR-related admissions
At least one possibly drug-related event was recorded in 48% of the hospitalizations (Table 2). If all events attributable to the patient's disease(s) were excluded, i.e. if only possibly drug-related, disease-unrelated events were counted, the corresponding number was reduced to 41%. These hospitalizations included mainly abnormal laboratory values (31%), whereas clinical events were present in 16% and abnormal BP, HR and/or T in 6% of hospitalizations. At least one clinically relevant ADR was recorded in 11% of hospitalizations (Table 2). The majority of these ADRs presented with clinical symptoms (8%), while only a minority exhibited clinically relevant abnormal laboratory results (4%). These findings were similar for both hospitals monitored. If cancer-chemotherapy-induced nausea/vomiting and leukopenia, common adverse reactions generally considered as acceptable in relation to the severity of the disease, were excluded, the total number of hospitalizations with clinically relevant ADRs decreased to 8%.
Table 2.
Frequency of possibly drug-related events and clinically relevant ADRs.

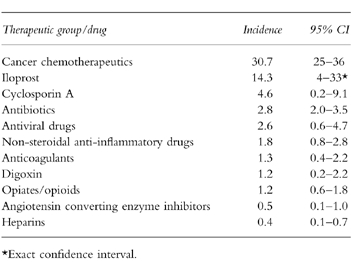
The incidence rates of clinically relevant ADRs for some therapeutic drug classes and individual drugs are given in Table 3. These numbers show that cancer chemotherapy was most frequently associated with adverse drug reactions. During one third of all hospitalizations with cancer chemotherapy at least one clinically relevant ADR was reported. Also a high incidence rate was obtained for iloprost. However, since iloprost was only given during a few hospitalizations, the corresponding 95% confidence interval is quite large. For cyclosporin A, antibiotics, antivirals and nonsteroidal anti-inflammatory drugs, clinically relevant ADR(s) occurred at frequencies between 4.6 and 1.8%. For the other drugs and drug classes studied incidence rates of approximately 1% or less were obtained.
Table 3.
Incidence rates of clinically relevant ADRs for some drug classes and individual drugs.
In 144 (3.3%) of the 4331 hospitalizations, ADR(s) were the cause of hospital admission. If cancer-chemotherapy-induced leukopenia is excluded, the number of ADR-related hospitalizations is reduced to 117 (2.7%).
Types of clinically relevant ADRs
Figure 4 illustrates that clinically relevant ADRs and ADR-related admissions concerned most frequently the gastrointestinal tract and the haematological systems. While cardiovascular ADRs were also frequent causes of hospital admission, clinically relevant ADRs involving the skin and the nervous system occurred predominantly during cohort stay.
Figure 4.

Systems and organs involved in clinically relevant ADRs present during cohort stay ( ) and involved in ADR-related hospital admissions (
) and involved in ADR-related hospital admissions ( ) ADRs during cohort stay: Number of hospitalizations with at least one clinically relevant ADR involving the corresponding organ or system. Note that during some hospitalizations more than one clinically relevant ADR occurred and that some ADRs involved several organs or systems. ADR-related hospital admissions: Number of hospitalizations caused by an ADR involving mainly the corresponding organ or systems. Note that some ADRs involved several organs or systems.
) ADRs during cohort stay: Number of hospitalizations with at least one clinically relevant ADR involving the corresponding organ or system. Note that during some hospitalizations more than one clinically relevant ADR occurred and that some ADRs involved several organs or systems. ADR-related hospital admissions: Number of hospitalizations caused by an ADR involving mainly the corresponding organ or systems. Note that some ADRs involved several organs or systems.
The 131 hospitalizations with gastrointestinal clinically relevant ADRs included 89 cases with nausea and/or vomiting caused by cancer chemotherapy (73), morphine (8), digoxin (3), nonsteroidal anti-inflammatory drugs (3), antiretroviral drugs (1) and cyclosporin A (1). Six patients complained of diarrhoea. Twenty-one of the 30 patients admitted for gastrointestinal ADRs had gastrointestinal bleeding and/or ulcers associated with nonsteroidal anti-inflammatory drugs (16), anticoagulants (7) and/or methotrexate (1). Possibly drug-induced gastrointestinal bleeding during cohort stay occurred in only seven cases.
Among the 112 hospitalizations with clinically relevant haematological ADRs 97 presented with leukopaenia/agranulocytosis, eight with anaemia, four with pancytopaenia, six with thrombocytopaenia and one with eosinophilia. The causing agents included anticancer drugs (84), cotrimoxazol (7), other antibiotics (6) and antiviral drugs (6). Leukopaenia/agranulocytosis was diagnosed in 31 of the 40 hospitalizations with admission for haematological ADRs.
Clinically relevant ADRs involving the skin occurred in 93 hospitalizations. They included 79 cases with rash and/or pruritus caused by various drugs including antibiotics (47), nonsteroidal anti-inflammatory drugs (6), diuretics (4) and antiviral drugs (2). Skin ADRs were the cause of hospitalization in 11 patients (maculopapular rash (3), hypersensitivity reactions (4), thrombocytopaenic purpura (3) and multiple large haematomas (1)).
Among the 61 hospitalizations with clinically relevant ADRs involving the nervous system, 14 had dyskinesias and/or tremor caused mainly by antipsychotic drugs (7) and metoclopramide (2). Six other patients suffered from confusion caused by antipsychotics (2), opioids (2) and cyclosporin (2). Cases of neurological ADRs causing hospital admission (15) mainly included somnolence, dyskinesia and peripheral neuropathy.
Clinically relevant cardiovascular ADRs were observed in 27 hospitalizations and were found to be the cause of 17 hospital admissions. They comprised mainly bradycardia, atrial fibrillation and other dysrhythmias (12), hypotension and syncopes (11). The responsible drugs were β-adrenoceptor blocking agents (5), antiarrhythmic drugs (2), antipsychotics (2), antidepressants (1), diuretics (3) and angiotensin converting enzyme inhibitors (1).
The 25 hospitalizations with clinically relevant musculoskeletal ADRs included seven with muscular haematomas/haemarthros caused by anticoagulants or heparin, 11 with fractures due to steroid-induced osteoporosis and two with diuretic-induced gout. Fractures due to steroid-induced osteoporosis was also the reason for admission in seven of the 10 cases admitted for musculoskeletal ADRs. Among the 24 cases with clinically relevant ADRs involving the kidneys and the urinary system, 16 had drug-induced worsening of renal function mainly due to nonsteroidal anti-inflammatory drugs (7) or amphotericin B (2). Five of the seven cases admitted for renal ADRs had acute renal failure caused by nonsteroidal anti-inflammatory drugs (4) or contrast agents (1). The 14 hospitalizations with clinically relevant respiratory ADRs included five with angiotensin converting enzyme inhibitor-induced cough/angiooedema and three with drug-induced pulmonary oedema. The 14 clinically relevant ADRs involving the mineral and fluid balance included severe changes in serum potassium and/or sodium concentrations caused by diuretics (7) or drug-induced syndromes of inappropriate ADH secretion (3). Ten cases suffered from clinically relevant ADRs involving the liver. The nine cases with clinically relevant ADRs involving special senses had quinine- or clarithromycin-induced reversible hearing loss (3), retinal bleeding due to drug-induced thrombocytopaenia (3), angiotensin converting enzyme inhibitor induced smell disturbances (1), ticlopidine-induced taste alterations (1) and digoxin-induced alterations of colour vision (1). Of the nine admissions caused by endocrine ADRs, four were due to hypoglycaemia caused by insulin (2) or glibenclamide (2). Among the five patients with miscellaneous clinically relevant ADRs were four cases of drug fever caused by piperacillin (2), azathioprine (1) and pyrazinamide (1).
ADR-related deaths
So far, we have observed eight possibly ADR-related deaths. In three cases, the ADR was the cause of hospital admission. Two of these patients were admitted for cancer-chemotherapy-induced agranulocytosis and died because of untreatable infection. A third patient was admitted for ACE-inhibitor induced angiooedema and died from the sequelae of hypoxic brain damage.
In the remaining five patients, the ADR occurred during hospital stay. Four of these five patients developed cancer-chemotherapy induced agranulocytosis and died because of untreatable infection. A fifth patient had lung cancer, was treated with several medications including fluconazole, mianserin and mefenaminic acid, developed agranulocytosis and died from pneumonia. If one relates these five fatal cohort cases to the total number of patients included in the cohort, the incidence of possibly ADR-related deaths is estimated at 1.4% (95% confidence interval: 0.2–2.6%).
Factors predisposing for clinically relevant ADRs
In Table 4, gender, age and polypharmacy were compared between (1) patients without clinically relevant ADRs (2) patients with ADR-related admissions and (3) patients with clinically relevant ADRs during cohort stay. Women were over-represented among patients with ADR-related admissions and among those with clinically relevant ADRs. No statistically significant age difference was observed between the various patient categories. The median number of drugs per day was significantly higher for patients experiencing a clinically relevant ADR during cohort stay as compared with patients without clinically relevant ADRs. Thus female gender and polypharmacy were identified as predisposing to clinically relevant ADRs, whereas no independent effect for age could be detected.
Table 4. Gender and polypharmacy as predisposing risk factors for clinically relevant ADRs.

Cancer chemotherapy is associated with a high incidence of clinically relevant ADRs and therefore patients with cancer chemotherapy were over-represented among the categories with ADR-related admissions (25 patients, 19%) and with clinically relevant ADRs during cohort stay (84 patients, 28%) as compared with those without clinically relevant ADRs (130 patients, 4%). The exclusion of patients with cancer-chemotherapy from the analysis did not change the results of the risk factor analysis in Table 4 (data not shown).
ADR-related excess hospital stay
The median (Q1, Q3) duration of hospitalizations without clinically relevant ADRs was 10 (5, 17) days. The corresponding numbers for hospitalizations with ADR-related admissions and with clinically relevant ADRs during cohort stay were 11 (6.75, 18) and 19 (9, 30), respectively. ADR-related admission had no effect on the duration of hospitalization, whereas clinically relevant ADRs during cohort stay prolonged hospital stay in the median by 9 days. After correction for the predisposing risk factors female gender and polypharmacy (Table 4) and for the overrepresentation of cancer chemotherapy in patients with clinically relevant ADRs (see above), the median excess hospital stay was reduced to 8.2 days. Overall, the adjusted estimate for ADR-related prolongation of hospital stay was 3′309 days. This represents 5.3% of the total of 62′389 hospital days recorded. ADR-related hospital admissions additionally contributed 2′101 (3.3%) hospital days. Thus, 5410 (8.6%) out of 62′389 hospital days were caused by ADRs or, expressed in other words, ADRs on average prolonged each hospitalization by 1.2 days.
Discussion
This study explores drug exposure and adverse drug reactions in hospital inpatients and demonstrates that it is feasible to collect ADR data by event monitoring. Thus, we successfully built a pharmacoepidemiologic database which allows the answering of general as well as specific questions relating to ADRs in hospital inpatients.
Surprisingly, there was a considerable predominance of male patients in Zurich. This predominance occurred, because male patients on average stayed in the hospital for a shorter period (Figure 2). Hence, more male patients were monitored despite the fact that a comparable number of beds was available for men and women in the monitored units. With regard to the duration of hospitalizations, a mean value of 11.3 (range 1–50) days was reported for Le Havre (France) in 1997 [1]. The mean value for Zurich was 13.1 (median 9, Q1=5, Q3=16) days and thus comparable to the value reported for Le Havre. Mean hospital stay in St Gallen amounted to 16.7 days which was caused by a few patients staying for very long periods since the median (13 days) and the quartiles (Q1=8 days, Q3=21 days) were close to the values mentioned above.
The number of different drugs administered per patient and day was remarkably large. In more than half of all patient days at least six or more different drugs were administered. The maximal number of different drugs given on one single day was 23. Since the effect of hospital stay on the number of drugs per day was rather small (i.e. only 1.3 additional drugs for an increase of hospital stay from 5 (Q1) to 18 (Q3) days), these numbers were representative for our inpatient cohort and were not dominated by only a few patients taking a large number of different drugs and staying in hospital for a long time. This predominance of polypharmacy clearly demonstrates that hospital inpatients differ considerably from outpatients. Despite the fact that in Zurich the proportion of patients referred for secondary or tertiary care was probably higher as compared with St Gallen, no differences were found in the numbers of drugs administered per patient and day between the two hospitals. Moore et al. reported that the mean number of drug classes administered per patient was 2.9 [1] during the entire hospitalization. Hence, our data indicate that in Zurich and St Gallen probably more drugs were administered simultaneously than in Le Havre. Since the simultaneous coadministration of different drugs increases the risk of ADRs exponentially [12], ADRs must be considered as a possible cause of any new events in inpatients, and their investigation in hospitalized patients should be included as a special focus in drug safety assessments.
It was of no surprise that paracetamol is the most commonly used drug, since it is generally recommended as first line treatment for pain relief. In contrast, the finding that tramadol was used in 17% (St Gallen: 19%, Zurich: 16%) of hospitalizations was unexpected. Tramadol appears to be commonly used as a second line therapy for pain relief in the monitored hospitals. Benzodiazepines were also frequently given to inpatients (27% of the patients received oxazepam, 9% lorazepam and 8% diazepam). This reflects the common clinical observation that sleep disturbances are frequent in hospitals. The high prevalence of drugs used to treat cardiovascular and pulmonary diseases can be explained by the high prevalence of cardiovascular and respiratory diseases in our cohort. The results also clearly show that nowadays low molecular weight heparin (e.g. dalteparin) is more commonly used than standard heparin. It is surprising that omeprazole was used in 16% of hospitalizations and one wonders if its use was appropriate in each case. This latter question can now be more specifically studied by a detailed analysis of the ICD10 diagnoses in the monitored patients.
The estimate of a 11% frequency of at least one clinically relevant ADR is comparable to the values reported in the literature. A recent review of Pirmohamed et al. reported ADR frequencies between 10 and 20% in inpatients [13]. Moore et al. reported that in 6.6% of all hospitalizations significant ADRs occurred during hospital stay [1]. Smith et al. reported a value of 7% suspected ADRs, with one third being present at admission and two thirds occurring during hospital stay [14]. A considerably higher number (67%) was reported for nursing home residents although little information concerning the severity of the reported ADRs was given [15]. Hence the ADR frequency estimates for inpatients significantly depends on the definitions of ADRs and varied in our analysis between 11% for clinically relevant ADRs, 41% for possibly drug related, disease unrelated events and 48% for possibly drug-related events (Table 2). These estimates only include already known ADRs. Future studies are required to detect potentially new ADRs by analysis of the events characterized as possibly drug-and disease-unrelated (see Patients and Methods).
The estimate of 3.3% for ADR induced hospital admissions is comparable with the values reported in the literature. The review of Pirmohamed et al. reported values of 2–6% [13]. An overview by Hallas summarized the studies reported in the literature and found values between 1.7 and 17% [16]. A meta-analysis of studies performed in the U.S. by Lazarou et al. reported an estimate of 4.7% (95% C. I. 3.1–6.2%) [3]. Smith et al. reported that 10% of all emergency room patients had drug-related problems but that none of them was admitted to the hospital [17]. The fact that outpatient emergency room patients were not included in our cohort provides an explanation why we obtained a relatively low number of ADR induced hospital admissions.
Among the 3624 patients 194 deaths were recorded. Only five deaths were related to ADRs occurring during hospital stay. Thus, the incidence of possibly ADR-related deaths is estimated at 1.4 (2.6 for the upper limit of the 95% confidence interval). This incidence estimate is somewhat lower than the value of 3.5 (95% C. I. 2.3–4.1) reported in a meta-analysis of U.S. studies [3].
Similarly to the study by Moore et al. [1] female gender and polypharmacy were associated with an increased risk for clinically relevant ADRs. In contrast, in the present study age could not be identified as an additional risk factor. Most importantly, however, and even after correction for predisposing risk factors, clinically relevant ADRs were responsible for an excess hospital stay of 8.6% of all hospital days. This estimate is close to the estimate of 7.6% reported in a previous study [1] and confirms the notion that ADRs represent an economically important problem in inpatients.
In conclusion, this paper presents first results from a novel system for adverse drug reaction recording in hospitalized patients. The recorded medication data allows drug utilization studies. Recording of structured data on clinical events and laboratory results by ‘event monitoring’ provides a valuable tool to study types, frequency, predisposing risk factors and costs of ADRs in inpatients. Thus, the developed database provides a valuable tool for answering various questions regarding drug safety and efficacy in inpatients.
Acknowledgments
We thank Mrs Ilse Schalch for help with data entry and all participating residents for their co-operation. The project is supported by a grant from the foundation ‘Stiftung für Arzneimittelsicherheit/Comprehensive Hospital Drug Monitoring’. The foundation is supported by Bayer AG, Glaxo-Wellcome, Hoechst Marion Roussel, Roche, Novartis Pharmaceuticals, the Federation of Swiss Physicians (FMH) and the Association ‘H+The Hospitals of Switzerland’.
This paper is dedicated to Professor Rolf Hoigné on the occasion of his 75th birthday.
References
- 1.Moore N, Lecointre D, Noblet C, Mabille M. Frequency and cost of serious adverse drug reactions in a department of general medicine. Br J Clin Pharmacol. 1998;45:301–308. doi: 10.1046/j.1365-2125.1998.00667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Einarson TR. Drug-related hospital admissions. Ann Pharmacother. 1993;27:832–840. doi: 10.1177/106002809302700702. [DOI] [PubMed] [Google Scholar]
- 3.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–1205. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 4.Jones JK, Kurata JH, Miwa L. Inpatient Databases. In: Strom BL, editor. Pharmacoepdiemiology. 2. Chichester: John Wiley & Sons; 1994. pp. 257–276. [Google Scholar]
- 5.Lawson DH, Beard K. Intensive Hospital-Besed Cohort Studies. In: Strom BL, editor. Pharmacoepdiemiology. 2. Chichester: John Wiley & Sons; 1994. pp. 157–170. [Google Scholar]
- 6.Garcia Rodriguez LA, Perez Gutthann S. Use of the UK General Practice Research Database for pharmacoepidemiology. Br J Clin Pharmacol. 1998;45:419–425. doi: 10.1046/j.1365-2125.1998.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dukes MNG, editor. Side effects of drugs. 13. Amsterdam: Elsevier; 1996. Meyler's. [Google Scholar]
- 8.Morant J, Ruppaner H, editors. 19. Basel: Documed; 1998. Arzneimittelkompendium der Schweiz. [Google Scholar]
- 9.Isselbacher KJ, Braunwald E, Wilson JD, Martin JB, Fauci AS, Kasper DL, editors. Principles of Internal Medicine. 13. New York: McGraw-Hill Inc; 1994. Harrison’s. [Google Scholar]
- 10.Thomas L, editor. Labor und Diagnose. 14. Marburg: Die Medizinische-Verlagsgesellschaft; 1992. [Google Scholar]
- 11.Karch FE, Lasagna L. Toward the operational identification of adverse drug reactions. Clin Pharmacol Ther. 1977;21:247–254. doi: 10.1002/cpt1977213247. [DOI] [PubMed] [Google Scholar]
- 12.Atkin PA, Shenfield GM. Medication-related adverse reactions and the elderly: a literature review. Adverse Drug React Toxicol Rev. 1995;14:175–191. [PubMed] [Google Scholar]
- 13.Pirmohamed M, Breckenridge AM, Kitteringham NR, Park BK. Fortnightly review: adverse drug reactions. Br Med J. 1998;316:1295–1298. doi: 10.1136/bmj.316.7140.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith CC, Bennett PM, Pearce HM, et al. Adverse drug reactions in a hospital general medical unit meriting notification to the Committee on Safety of Medicines. Br J Clin Pharmacol. 1996;42:423–429. doi: 10.1046/j.1365-2125.1996.04376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper JW. Probable adverse drug reactions in a rural geriatric nursing home population: a four-year study. J Am Geriatr Soc. 1996;44:194–197. doi: 10.1111/j.1532-5415.1996.tb02439.x. [DOI] [PubMed] [Google Scholar]
- 16.Hallas J. Drug related hospital admissions in subspecialities of internal medicine. Dan Med Bull. 1996;43:141–155. [PubMed] [Google Scholar]
- 17.Smith KM, McAdams JW, Frenia ML, Todd MW. Drug-related problems in emergency department patients. Am J Health Syst Pharm. 1997;54:295–298. doi: 10.1093/ajhp/54.3.295. [DOI] [PubMed] [Google Scholar]


