Abstract
Aims
To investigate the effects of sibutramine in combination with alcohol in a double-blind, randomised, placebo-controlled, four-way crossover study in 20 healthy volunteers.
Methods
On each study day each volunteer received either: sibutramine 20 mg+0.5 g kg−1 alcohol; sibutramine 20 mg+placebo alcohol; placebo capsules+0.5 g kg−1 alcohol; or placebo capsules+placebo alcohol. Alcohol was administered 2 h following ingestion of the study capsules. During each study day, assessments of cognitive performance were made prior to dosing, and at 3, 4.5, 6 and 10 h post dosing. Blood alcohol concentration was estimated using a breath alcometer immediately prior to each cognitive performance test session. Each study day was followed by a minimum 7 day washout period.
Results
Alcohol was found to produce statistically significant impairments in tests of attention (maximum impairment to speed of digit vigilance=49 ms) and episodic memory (maximum impairment to speed of word recognition=74 ms). Alcohol also increased body sway (maximum increase 17.4 units) and lowered self rated alertness (maximum decrease 13.6 mm). These effects were produced by an inferred blood alcohol level of 53.2 mg dl−1.Sibutramine was not found to potentiate any of the effects of alcohol. There was a small, yet statistically significant, interaction effect observed on the sensitivity index of the picture recognition task. In this test, the combined effects of sibutramine and alcohol were smaller than the impairments produced by alcohol alone. Sibutramine, when dosed alone, was associated with improved performance on several tasks. Sibutramine improved attention (mean speed of digit vigilance improved by 21 ms), picture recognition speed (improvement at 3=81) and motor control (tracking error at 3 h reduced by 1.58 mm). Also sibutramine improved postural stability (reducing body sway at 3 h by 14.2 units). Adverse events reported were unremarkable and consistent with the known pharmacology of sibutramine and alcohol.
Conclusions
There was little evidence of a clinically relevant interaction of sibutramine with the impairment of cognitive function produced by alcohol in healthy volunteers. The single statistically significant interaction indicated a reduction, rather than a worsening, of alcohol-induced impairment when sibutramine is taken concomitantly. Sibutramine when administered alone is associated with improved performance on several tasks.
Keywords: alcohol, attention, cognitive function, drug interaction, secondary memory, sibutramine, working memory
Introduction
It is now well established that the monoamine neurotransmitters in the central nervous system (noradrenaline, dopamine and serotonin) are all involved in the regulation of food intake by animals and man [1, 2]. Sibutramine hydrochloride monohydrate (BTS 54 524) is a novel monoamine-reuptake inhibitor [3] which exerts its actions in vivo predominantly via its secondary and primary amine metabolites, viz. BTS 54 354 and BTS 54 505, respectively [4]. In both animals and man, sibutramine potently inhibits the reuptake of noradrenaline and serotonin [3, 5], but not dopamine [5, 6]. This drug has previously been shown to produce a dose-related improvement in the weight reduction of patients on a restricted calorie diet [7], and animal studies have indicated that sibutramine produces this effect by a dual action. Firstly, sibutramine reduces food intake by enhancing satiety [8], and secondly it increases energy expenditure by enhancing metabolic rate [9]. As such, sibutramine represents a new class of agent for the treatment of obesity that differs from drugs such as fenfluramine, (+)-fenfluramine and fluoxetine, which exclusively activate serotonin systems, and from drugs such as (+)-amphetamine, mazindol and the β3-adrenoceptor agonists which exclusively activate catecholamine systems [2, 10–12].
In a clinical context sibutramine is likely to be taken regularly for extended periods, and it is therefore likely that alcohol will be taken concomitantly. It was considered to be of relevant practical importance to consider the pharmacodynamic effects of sibutramine when administered in combination with alcohol.
The cognitive effects of alcohol are to impair sustained attention, disrupt the ability to learn new information, to impair coordination, increase bodysway and to lower self-rated alertness [13–17]. These effects occur within 1 h of administration, usually pass by 4–6 h after dosing, and can be seen with doses as low as 0.35 g kg−1. To study fully the potential of a drug to interact with alcohol, a number of possibilities have to be investigated. The first is that the peak effects of alcohol are increased. This means that testing should be conducted at the time of the peak effects of alcohol. The second is that the effects of alcohol could be prolonged. This means that testing should be conducted repeatedly over the study day. The third is that the range of functions affected by alcohol could be increased. This requires the use of a range of tests that also cover aspects of function such as working memory which are not usually affected by alcohol. Of course all of the above requirements apply equally well to the compound being studied, and thus it is essential to have a condition in which the study drug is administered alone. Further, where possible dosing of the study compound and alcohol should be arranged such that the time of peak absorption of both drugs coincides. Finally it is necessary to select a test system which has multiple parallel forms, which covers the major aspects of cognitive function which are important for the conduct of the activities of daily living, and also which has proven sensitivity to a variety of compounds, including alcohol. The system selected for this study was the Cognitive Drug Research computerized test system which is sensitive to a wide variety of compounds [18–20], including alcohol [13–15, 17, 21] and which also has proven sensitivity for detecting interactions between compounds [16].
The primary objective of this study was therefore to determine the pharmacodynamic effects of sibutramine 20 mg both alone and also when taken with a moderate dose of alcohol (0.5 g kg−1) using tests of cognitive function in healthy volunteers.
Methods
Study population
Twenty (10 male, 10 female) healthy volunteers, aged between 18 and 40 years of age (mean, 27.6 years; standard deviation (s.d.), 5.0 years), and weighing within 15% of their ideal weight (mean, 67.7 kg; s.d., 8.8 kg) were included in the study. To exclude volunteers with any evidence of clinically relevant diseases or who were pregnant, each volunteer was screened before drug administration. Screening included a medical history, physical examination, 12-lead ECG, routine laboratory tests (haematology, blood chemistry, urinalysis and drugs of abuse), and, where appropriate, a pregnancy test. Females were excluded from participation if pregnant or not taking contraceptive precautions. Volunteers were asked not to take pharmaceutical preparations 14 days prior to, and during, the study period and alcohol was prohibited for 48 h both prior to, and after each study day. Smoking and consumption of caffeine were not permitted during the study days. The study was conducted according to the principles of the ‘Declaration of Helsinki’ (as amended in Tokyo, Venice and Hong Kong), the protocol approved by the Besselaar Clinical Research Unit Institutional Review Board, and signed, written, informed consent was obtained from all volunteers before their participation in the study.
Study design and study medication
In this randomised, double-blind, placebo-controlled, four-way crossover study, all 20 volunteers completed four treatment periods of 1 day separated by a minimum 7 day wash-out period. The volunteers were assigned randomly to receive oral doses of each of the four treatment combinations on a single occasion. In the morning of the four study days, volunteers received either two sibutramine 10 mg capsules+0.5 g kg−1 alcohol, two sibutramine 10 mg capsules+placebo alcohol, two placebo capsules+0.5 g kg−1 alcohol, or two placebo capsules+placebo alcohol. The alcohol was diluted in ginger ale, the glass was covered with cling-film, and the mixture (400 ml) was administered through a straw 2 h after ingestion of the study capsules, so that peak alcohol concentration would coincide with peak sibutramine and sibutramine metabolite concentrations at 3 h (Boots Pharmaceuticals 1994: Research Report No. DT94032). The subjects received a standardized breakfast 30 min after taking their sibutramine capsules. Lunch was provided following the 4.5 h cognitive test session.
Assessments
Subjects underwent four training sessions on the cognitive performance tests prior to the first study day. Assessments were made before, and 3, 4.5, 6 and 10 h post sibutramine dosing. The assessments were performed in the following sequence: blood alcohol concentration, cognitive performance tests, and adverse event recordings.
Blood alcohol concentration Blood alcohol concentration was estimated using a breath alcometer immediately prior to each cognitive performance test session. To maintain the double-blind nature of the study the output from the alcometer was shielded from the investigator and the volunteers.
Cognitive performance tests A selection of tests from the Cognitive Drug Research Computerized Assessment System [13–20] was used, parallel forms being presented via a high-resolution VGA colour monitor at each 20 min testing session. Responses to the tests were made using one of two response buttons, ‘YES’ or ‘NO’, on a single response module. The following tasks were administered.
Immediate word recall—the volunteer had to remember a list of 15 words presented on the monitor at the rate of 1 word every 2 s. Immediately following the presentation the volunteer was given 1 min to write down as many of the 15 words as they could remember. The major outcome measure is the percentage of words recalled correctly.
Picture presentation—the volunteer had to remember a series of 20 pictures which were presented on the monitor at the rate of one picture every 3 s. Recognition of these was tested later.
Simple reaction time—the word ‘YES’ was presented on the monitor at random intervals (1–3.5 s). The volunteer was instructed to press the ‘YES’ response button on the response module as quickly as possible following recognition of the stimulus. There were 50 trials. The outcome measure was speed of response.
Digit vigilance task—a computer-generated random digit was constantly displayed on the right-hand side of the monitor. A series of digits was displayed in the centre of the monitor at the rate of 150 per minute, and the volunteer was required to press the ‘YES’ response button when the two digits displayed matched. There were 45 targets in this test. The outcome measures were accuracy (percentage of detections), the average speed of detections, and number of false alarms.
Choice reaction time—‘YES’ or ‘NO’ were presented randomly on the monitor and the corresponding response button had to be pressed as quickly as possible. There were 50 trials. The outcome measures were speed and accuracy of response.
Visual tracking—the volunteer had 1 min to track a randomly moving target presented on the monitor using a joystick. The outcome measure was the average distance off-target per second.
Spatial working memory task—volunteers were instructed to remember which windows were lit in a house presented on the monitor. Following this initial presentation the house was re-presented, each time with one window lit. The volunteer had to press the ‘YES’ button if the window had originally been lit, and ‘NO’ if it had not. The outcome measures on this task were spatial sensitivity and speed of response.
Numeric working memory task—a series of five digits were presented on the monitor for the volunteer to hold in memory. This was followed by a series of 30 probe digits. The volunteer had to decide if the probe digit was in the original series and press the ‘YES’ or ‘NO’ response button as appropriate. Three trials were presented. The outcome measures were scanning speed and sensitivity [22].
Delayed word recall—the volunteer was given one minute to write down as many words as possible from the list originally presented at the start of the session. The outcome measures were accuracy and number of errors and intrusions.
Delayed word recognition—the words presented at the start of the session (target words) were randomly re-presented one at a time, together with 15 distractor words. The volunteer had to respond to target and distractor words by pressing the ‘YES’ or ‘NO’ response button to indicate if the word had appeared in the original series. The outcome measures were speed and recognition sensitivity [22].
Delayed picture recognition—the original pictures presented in the picture presentation task (target pictures) were randomly re-presented one at a time, together with 20 distractor pictures. The volunteer was to respond to target and distractor pictures by pressing the ‘YES’ or ‘NO’ response button to indicate if the picture had appeared in the original series. The outcome measures were speed and recognition sensitivity [22].
Body sway—a cord was attached to the volunteer who was then required to stand as still as possible with their feet apart and eyes closed for 1 min. Body sway was measured in arbitrary units [23].
Bond-Lader visual analogue scales [24]—a questionnaire of 16 visual analogue scales from which three factors are derived was administered to assess change in subjective alertness, calmness and contentment.
Tolerability Reports and observations of adverse events were recorded.
Statistics
It was estimated that with 20 evaluable volunteers receiving all four treatment combinations, differences of 26 ms and 8.3% between active and placebo treatments could be detected for the average response time of the digit vigilance task and the percentage of correct responses of the immediate word recall task, respectively. These data calculations are based on data from a previous drug/alcohol interaction study and assume variabilities of 652 ms and 62.9%, respectively, a significance level of 5%, power of 90% and a two-tailed, one-sample t-test.
Analyses of the cognitive performance tests were carried out using SAS®software (SAS Institute Inc., Cary, NC, USA). At the outset of the study four separate analyses were planned, three based on derived scores using data from the 3, 4.5, 6 and 10 h time points, and one based on looking at treatment effects at the 3 h time point only. The three derived scores were minimum impairment (maximum improvement), maximum impairment, and ‘average’ performance. The linear model included terms for: treatment, volunteer (random effects) and day. Baseline data were used as a covariate. A baseline–treatment interaction term was included in the model to test for homogeneity of regression slopes. Where this was not statistically significant (P < 0.05) the term was dropped from the ancova and the analyses repeated. (The assumption of homogeneity of regression slopes was satisfactory for all the statistically significant treatment effects reported in this paper.) Linear contrasts were constructed for sibutramine (active vs placebo), alcohol (active vs placebo), and the sibutramine–alcohol interaction. Post-hoc t-tests (two-tailed) were performed to clarify any statistically significant interaction effects. Confidence intervals reported are based on adjusted least squares means generated from the linear model.
Due to a bereavement one volunteer did not complete the fourth study day. His data were omitted from the statistical analyses of the cognitive performance tests. Adverse events and side-effects were listed in full. No further statistical analyses of these data were undertaken.
Results
There were no statistically significant treatment effects for immediate and delayed word recall, simple and choice reaction time, spatial working memory, numeric working memory, and subjective ratings of contentment and calmness.
Minimum impairment (Table 1)
Table 1.
Statistically significant treatment effects: minimum impairment.
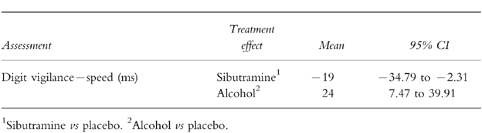
There were clear treatment effects for sibutramine and alcohol on speed of detections in the digit vigilance task (Figure 1). Sibutramine improved speed by 19 ms in contrast to alcohol which impaired speed by 24 ms.
Figure 1.
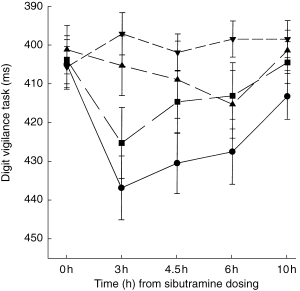
The speed of detections in the digit vigilance task (ms): illustration of the observed treatment effects (means±s.e. mean) over the time. ▴ placebo, ▾ sibutramine alone, • alcohol alone, ▪ alcohol+sibutramine.
Maximum impairment (Table 2)
Table 2.
Statistically significant treatment efects: maximum impairment.
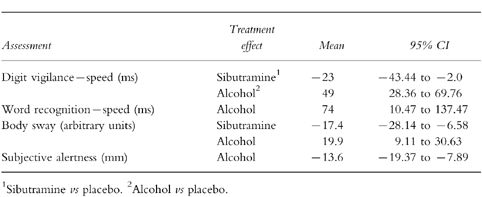
Again, there were clear treatment effects for sibutramine and alcohol on the digit vigilance task. Sibutramine significantly improved the speed of detections by 23 ms, in contrast to alcohol which produced a slowing of 49 ms.
Sibutramine reduced body sway (Figure 2) by 17.4 units, in contrast to alcohol which increased body sway by 19.9 units. Word recognition speed was impaired by alcohol. Volunteers felt less alert following alcohol.
Figure 2.
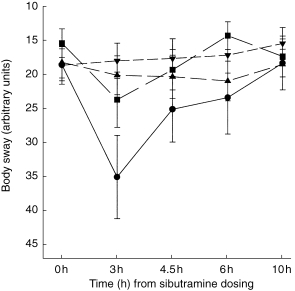
Body sway (arbitrary units): illustration of the observed treatment effects (mean±s.e. mean) over the time course of the study days. ▴ placebo, ▾ sibutramine alone, • alcohol alone, ▪ alcohol+sibutramine.
3 h performance (Table 3)
Table 3.
Statistically significant treatment efects: 3 h.
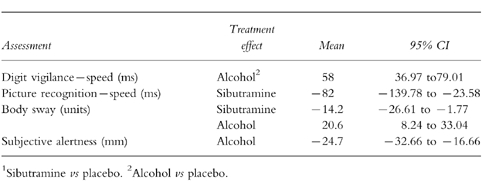
Again, there was a clear treatment effects for alcohol on the digit vigilance task, speed being slowed by 58 ms.
Sibutramine improved speed in the picture recognition task, and reduced body sway. In contrast alcohol increased body sway. Subjective alertness was again lowered following alcohol.
Mean performance (Table 4)
Table 4.
Statistically significant treatment efects: mean performance.
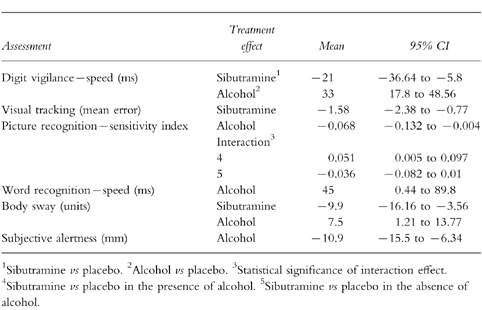
Once again, there were clear treatment effects for sibutramine and alcohol on digit vigilance. Sibutramine improved the speed of detections, in contrast to alcohol which impaired speed. Sibutramine also reduced the error on the visual tracking task, and reduced body sway.
Alcohol impaired the picture recognition sensitivity index, the speed of word recognition, and increased body sway. Subjective alertness was also lowered following alcohol.
There was a statistically significant interaction effect shown by the picture recognition sensitivity index. Performance was less impaired when alcohol was coadministered with sibutramine.
Blood alcohol
As expected, the average breath alcohol concentration was greatest at the 3 h assessment. The mean alcohol level following alcohol alone at this time was 53.2 mg dl−1 (s.d., 19.8 mg dl−1; range, 4–88 mg dl−1 ). This declined steadily, at 4.5 h it was 36.5 mg dl−1 (range 12–63), at 6 h it was 8.1 mg dl−1 (range 0–32) and was not detectable by 10.
Adverse events
The number of adverse events reported was highest in the treatment groups in which alcohol was taken. In the alcohol plus placebo treatment group, 16 (80%) volunteers reported 25 events, and in the sibutramine plus alcohol treatment groups 12 (60%) volunteers reported 18 events. Of these reports the highest number were in the category ‘Nervous’ including asthenia, dizziness and stupor.
Following sibutramine and placebo, five volunteers (25%) reported eight events, and in the placebo group five volunteers (26%) reported five events.
Only two events were moderate in severity, all other adverse events were mild in severity. None of the events was recorded as definitely related to the study treatments and no volunteer required withdrawal from the study because of adverse events.
Discussion
Alcohol was found to disrupt attention (speed of digit vigilance), to disrupt verbal (word recognition) and nonverbal (picture recognition) aspects of secondary memory, to increase body sway and to lower subjective alertness. This was achieved at a 0.5 g kg−1 dose of alcohol which produced an inferred blood alcohol level of 53.2 mg dl−1. These effects are entirely consistent with previous work [13–15, 17, 21]. For example in one previous study [13] a dose of alcohol of 40 g produced an inferred blood alcohol level of 64 mg dl−1, and both vigilance speed and verbal secondary memory were impaired. McClelland [25] has reviewed the literature on alcohol and body sway and has concluded that 0.54 g kg−1 is the threshold below which alcohol effects on sway will not be detected. This study indicates that this threshold can be lowered to 0.5 g kg−1. The present study has therefore extended previous work, illustrating that objective impairments to attention and secondary memory, disrupted ability to maintain postural stability and a lowered level of subjective alertness can be found following a dose of 0.5 g kg−1 alcohol.
Of all the variables examined in this study, only the picture recognition sensitivity index yielded a statistically significant interaction between sibutramine and alcohol. Co-administration of alcohol with sibutramine reduced the impairment observed when alcohol was administered alone. There were no interactions demonstrating worsening cognitive impairment when sibutramine and alcohol were taken together. Since the only observed effect was small, for the primary objective of this trial, sibutramine was not found to produce any clinically significant interactions with alcohol at the doses used in this study.
Sibutramine improved performance on several tasks. The drug significantly improved attention (digit vigilance speed). Tracking performance was also improved, as was speed of performance on the picture recognition task (nonverbal secondary memory) and postural stability as assessed with the body sway test. Cognition enhancing properties have previously been found with the monoamine oxidase inhibitor, moclobemide, these effects being seen in young volunteers given scopolamine [20, 26], elderly volunteers with or without concomitant alcohol administration [14, 27], and elderly depressed patients [27]. The observed effects of sibutramine and moclobemide might be the result of the actions of the two drugs on noradrenaline and serotonin; certainly the two compounds affected performance on the same tasks. Recent work with other compounds which inhibit the re-uptake of monoamines have also been associated with improvement on these and other tasks from the Cognitive Drug Research computerized test system [28, 29], although the relative contribution of the various monoamine neurotransmitters to these effects remains to be elucidated. A previous study, in which higher doses of sibutramine were studied (30, 45 and 60 mg), detected no effects of the drug on a variety of tasks from an automated battery [30]. However, only six volunteers were tested, which gave that study much less power than the present. In terms of the magnitude of the effects, the improvements with sibutramine were generally comparable with the impairments produced by alcohol, with the exception of the peak effects on the speed of detections in the vigilance task. The impairments produced by alcohol occurred with a dose which would exceed the limit for driving in most European countries. This magnitude of effect would be described as ‘relevant for everyday behaviour’, and thus the improvements with sibutramine should also be considered of clinical relevance.
Although the use of response feature analysis [31] using derived scores for minimum and maximum impairment, ‘average’ performance and the 3 h time point (this selection based on the known pharmacology of the study drugs) for the statistical analyses is not commonly used to assess cognitive function data, it did serve to identify the major effects seen in the present study. Whether or not this analysis has anything to offer over and above traditional repeated measures anova techniques is unclear.
In summary, there were no clinically significant interactions between alcohol and sibutramine on cognitive function, although a modest reduction in the alcohol impairment reached statistical significance in the picture recognition task sensitivity index. Sibutramine when administered alone is associated with improved performance on several tasks, whilst alcohol produced characteristic impairments. It should be emphasized that these findings are related to a laboratory investigation in normal volunteers, and extrapolation to the clinical situation is limited, so care should still be taken in coadministration of sibutramine and alcohol. The use of sibutramine to reduce impairments produced by alcohol is not justified by these data.
Acknowledgments
The conduct of this trial was funded by Knoll Pharmaceuticals.
References
- 1.Surge MF. Neuropharmacology of drugs affecting food intake. Pharmacol Ther. 1987;32:145–182. doi: 10.1016/0163-7258(87)90057-x. [DOI] [PubMed] [Google Scholar]
- 2.Silverstone T. Appetite suppressants: a review. Drugs. 1992;43:820–836. doi: 10.2165/00003495-199243060-00003. [DOI] [PubMed] [Google Scholar]
- 3.Bucket WR, Thomas PC, Luscombe GP. The pharmacology of sibutramine hydrochloride (BTS 54, 524), a new antidepressant which induces rapid noradrenergic down–regulation. Progr Neuropsychopharmacol Biol Psychiat. 1988;12:575–584. doi: 10.1016/0278-5846(88)90003-6. [DOI] [PubMed] [Google Scholar]
- 4.Luscombe GP, Hopcroft RH, Thomas PC, Buckett WR. The contribution of metabolites to the rapid and potent down-regulation of rat cortical β-adrenoceptors by the putative antidepressant sibutramine hydrochloride. Neuropharmacology. 1989;28:129–134. doi: 10.1016/0028-3908(89)90048-8. [DOI] [PubMed] [Google Scholar]
- 5.Luscombe GP, Slater NA, Lyons MB, Wynne RD, Scheinbaum ML, Buckett WR. Effect on radiolabelled-monoamine uptake in vitro of plasma taken from healthy volunteers administered the antidepressant sibutramine HCL. Psychopharmacology. 1990;100:345–349. doi: 10.1007/BF02244604. [DOI] [PubMed] [Google Scholar]
- 6.Heal DJ, Frankland ATJ, Gosden J. A comparison of the effects of sibutramine hydrochloride, bupropion and methamphetamine on dopaminergic function: evidence that dopamine is not a pharmacological target for sibutramine. Psychopharmacology. 1992;107:303–309. doi: 10.1007/BF02245152. et al. [DOI] [PubMed] [Google Scholar]
- 7.Weintraub M, Rubio A, Golik A, Byrne L, Scheinbaum ML. Sibutramine in weight control: a dose-ranging, efficacy study. Clin Pharmacol Ther. 1991;50:330–337. doi: 10.1038/clpt.1991.144. [DOI] [PubMed] [Google Scholar]
- 8.Halford JCG, Heal DJ, Blundell JE. 1994. Sibutramine suppresses food intake and preserves the behavioural sequence associated with satiety. Proceedings of Pharmacologic Treatment of Obesity, Satellite Symposium of the 7th International Congress on Obesity, Sainte-Adele.: 45.
- 9.Connoley IP, Heal DJ, Stock MJ. 1994. The thermogenic effects of sibutramine. Proceedings of Pharmacologic Treatment of Obesity, Satellite Symposium of the 7th International Congress on Obesity, Sainte-Adele.: 52.
- 10.Garattini S, Samanin R. Anorectic drugs and brain neurotransmitters. In: Silverstone T, editor. Appetite and Food Intake. Berlin: Dahlem Konferenzen; 1976. pp. 83–108. [Google Scholar]
- 11.Wilson CA, Stock MJ. Drugs for the treatment of obesity. Report on the Society for Drug Research Symposium held at the School of Pharmacy, University London, 6 July 1989. Pharmaceut Med. 1990;4:249–259. [Google Scholar]
- 12.Arch JRS, Kaumann AJ. β3 and atypical β-adrenoceptors. Med Res Rev. 1993;13:663–729. doi: 10.1002/med.2610130604. [DOI] [PubMed] [Google Scholar]
- 13.van Harten J, Stevens L, Raghoebar M, Holland R, Wesnes K, Cournot A. Fluvoxamine does not interact with alcohol or potentiate alcohol-related impairment of cognitive function. Clin Pharmacol Ther. 1992;52:427–435. doi: 10.1038/clpt.1992.166. [DOI] [PubMed] [Google Scholar]
- 14.Wesnes K, Simpson P, Christmas L, Anand R, McClelland GR. The cognitive effects of moclobemide and trazodone alone and in combination with ethanol in healthy elderly volunteers. Br J Clin Pharmacol. 1989;27:647P–648P. [Google Scholar]
- 15.Wesnes K, McEwen J, Pritchard G. The dose and time dependent profile of cognitive impairments of alcohol in young volunteers (Abstract) J Clin Pharmacol. 1994;34:1021. [Google Scholar]
- 16.Wesnes K, Simpson PM, Jansson B, Grahnén A, Weimann H-J, Küppers H. Moxonidine and cognitive function: Interactions with moclobemide and lorazepam. Eur J Clin Pharmacol. 1997;52:351–358. doi: 10.1007/s002280050300. [DOI] [PubMed] [Google Scholar]
- 17.Wilcox C, Wesnes K, Heiser J, Simpson P, Katz B, Happy J. A double blind, eight way crossover comparison of Abercarnil, lorazepam and placebo alone and after a single dose of alcohol, on psychometric performance in healthy volunteers. Psychopharmacol Bull. 1996;32:536. [Google Scholar]
- 18.Hanks GW, O’Neill WM, Simpson P, Wesnes K. The cognitive and psychomotor effects of opioid analgesics II. A randomised controlled trial of single doses of morphine, lorazepam and placebo in healthy subjects. Eur J Clin Pharmacol. 1995;48:455–460. doi: 10.1007/BF00194334. [DOI] [PubMed] [Google Scholar]
- 19.O’Neill WM, Hanks GW, White L, Simpson P, Wesnes K. The cognitive and psychomotor effects of opioid analgesics I. A randomised controlled trial of single doses of dextropropoxyphrene, lorazepam and placebo in healthy subjects. Eur J Clin Pharmacol. 1995;48:447–453. doi: 10.1007/BF00194333. [DOI] [PubMed] [Google Scholar]
- 20.Anand R, Wesnes KA. Cognition-enhancing effects of moclobemide, a reversible MAO inhibitor, in humans. In: Wurtman RJ, editor. Alzheimer’s Disease. New York: Raven Press; 1990. pp. 261–268. [PubMed] [Google Scholar]
- 21.Wesnes K, Lockton A, Rolan P, Stephenson N, Pincock C. Volunteer study of the potential interaction between remacemide 300 mg and alcohol (0.7 g/kg) J Psychopharmacol. 1997;11(Suppl):A59. [Google Scholar]
- 22.Frey PW, Colliver JA. Sensitivity and response measures for discrimination learning. Learning Motivation. 1973;4:327–342. [Google Scholar]
- 23.Wright BM. A simple mechanical ataxiameter. J Physiol. 1971;281:27P–28P. [PubMed] [Google Scholar]
- 24.Bond A, Lader M. The use of analogue scales in rating subjective feelings. Br J Med Psychol. 1974;80:1–46. [Google Scholar]
- 25.McClelland GR. Body sway and the effects of psychoactive drugs—a review. Human Psychopharmacol. 1989;4:3–14. [Google Scholar]
- 26.Wesnes K, Anand R, Lorscheid T. Potential of moclobemide to improve cerebral insufficiency identified using a scopolamine model of ageing and dementia. Acta Psychiat Scand. 1990;82:71–72. doi: 10.1111/j.1600-0447.1990.tb05338.x. [DOI] [PubMed] [Google Scholar]
- 27.Wesnes K, Simpson P, Christmas L, Anand R, McClelland GR. The effects of moclobemide on cognition. J Neural Transmission. 1989;29:91–102. [PubMed] [Google Scholar]
- 28.Jones S, Jensen NO, Oliver S, Wesnes KA. First in man cognitive effects of NS2330, a novel monoamine reuptake inhibitor, in volunteers. J Psychopharmacol. 1999;13(Suppl A):A26. [Google Scholar]
- 29.Bosworth J, Jensen NO, Oliver S, Wesnes KA. First cognitive effects of NS2359, a noradrenaline, dopamine & serotonin reuptake inhibitor, in volunteers. J Psychopharmacol. 1999;13(Suppl A):A26. [Google Scholar]
- 30.King DJ, Devaney N. Clinical pharmacology of sibutramine hydrochloride (BTS-54524), a new antidepressant, in healthy volunteers. Br J Clin Pharmacol. 1988;26:607–611. doi: 10.1111/j.1365-2125.1988.tb05303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Everitt BS. The analysis of repeated measures: a practical review with examples. Statistician. 1995;44:113–135. [Google Scholar]


