Abstract
Aims
To investigate the pharmacokinetics of morphine, morphine-6-glucuronide (M6G) and morphine-3-glucuronide (M3G) in healthy volunteers after the administration of morphine by subcutaneous bolus injection (s.c.b.) and subcutaneous infusion (s.c.i.) over 4 h, and to compare the results with the intravenous bolus (i.v.) administration of morphine.
Methods
Six healthy volunteers each received 5 mg morphine sulphate by i.v., s.c.b. and short s.c.i. over 4 h, on three separate occasions, in random order, each separated by at least 1 week. Plasma samples were assayed for morphine, M6G and M3G.
Results
After i.v. morphine, the concentrations of morphine, M6G and M3G and their pharmacokinetic parameters were similar to those we have observed previously, in other healthy volunteers (when standardized to nmol l−1, for a 10 mg dose to a 70 kg subject). After s.c.b. morphine, similar results were obtained except that the median tmax values for morphine and M3G were significantly longer than after i.v. morphine (P < 0.001 and P < 0.05, respectively), with a trend to a longer tmax for M6G (P = 0.09). The appearance half-lives after s.c.b. morphine for M6G and M3G were also significantly longer than after i.v. morphine (P = 0.03 and P < 0.05, respectively). Comparison of log-transformed AUC values indicated that i.v. and s.c.b. administration of morphine were bioequivalent with respect to morphine, M6G and M3G. In comparison with i.v. morphine, morphine by s.c.i. was associated with significantly longer median tmax values for morphine (P < 0.001), M6G (P < 0.001) and M3G (P < 0.05), and the mean standardized Cmax values significantly lower than after both i.v. and s.c.b. morphine (morphine P < 0.001, M6G P < 0.001 and M3G P < 0.01 for each comparison). Comparison of log-transformed AUC values after i.v. and s.c.i. morphine indicated that the two routes were not bioequivalent for morphine (log-transformed AUC ratio 0.78, 90% CI 0.66–0.93), M6G (0.72, 90% CI 0.63–0.82), or M3G (0.65, 90% CI 0.54–0.78). A small stability study indicated no evidence of adsorptive losses from morphine infused over 4 h using the infusion devices from the study.
Conclusions
Although bioequivalence was demonstrated between the s.c.b. and i.v. routes of morphine administration, the bioavailabilities of morphine, M6G and M3G after s.c.i. were significantly lower than after i.v. administration. However, despite this, the study demonstrates that the subcutaneous route is an effective method for the parenteral administration of morphine.
Keywords: infusion, morphine-3-glucuronide (M3G), morphine-6-glucuronide (M6G), morphine, pharmacokinetics, subcutaneous
Introduction
Pain is one of the commonest symptoms in patients with advanced cancer and more than 70% of patients with advanced cancer experience pain at some stage of their disease. Pain is usually treated according to recommendations made by the World Health Organization [1], which, in 1986 proposed an analgesic ladder for the treatment of pain associated with advanced cancer. Morphine is the drug of choice in the treatment of patients with moderate or severe pain associated with their malignancy. Morphine is usually given orally, either in solution as an immediate release preparation, or in tablet form as a controlled-release preparation. However, in those patients unable to swallow, it can also be given parenterally. In recent years, the subcutaneous (s.c.) route has been demonstrated to be an effective and well tolerated method of parenteral administration of morphine. When given subcutaneously, it may either be given by 4 hourly s.c. bolus (s.c.b.) injection through an indwelling needle placed subcutaneously or as a s.c. infusion (s.c.i.) over 4 h or longer, delivered by an external infusion pump. We have shown previously that the s.c. administration of morphine may be associated with a reduction in nausea and vomiting compared with oral administration [2]. S.c. morphine can usually be administered by relatives and carers, enabling terminally ill patients to be looked after at home rather than being hospitalized, offering another advantage to patients with advanced cancer.
Following oral administration, morphine is absorbed in the upper bowel and metabolized extensively in the liver. The two principal metabolites are morphine-6-glucuronide (M6G) and morphine-3-glucuronide (M3G). In animal models M6G has been shown to produce potent antinociception [3, 4], while a phase I study of M6G in 19 cancer patients noted analgesia in 17 assessable patients [5]. Subsequent studies have confirmed this analgesic activity, with the suggestion that M6G may be less toxic than morphine at equi-analgesic doses [6, 7]. In contrast, M3G appears to have little or no µ-opioid agonist properties [8] and no analgesic activity [9], and recent evidence from animal studies has suggested that M3G may functionally antagonize the analgesic and respiratory depressant effects of morphine [10, 11] and M6G [10].
Our group has previously studied the pharmacokinetics of morphine and these two glucuronide metabolites after sublingual, buccal, oral and i.v. administration of morphine [12]. This work clearly demonstrated the importance of the two glucuronide metabolites of morphine, particularly M6G. In view of the increasing use of s.c. morphine, we have extended our previous examination of morphine and its glucuronide metabolites to the s.c. administration of morphine, both by s.c.b. and short s.c.i. over 4 h.
Methods
Six healthy volunteers participated in this study. Their mean age was 25.8 years (range 20–40 years), their mean weight 71.4 kg (range 49.2–102.1 kg) and three were male. Participation in the study required that each volunteer had a normal full blood count and normal hepatic and renal function, was not taking any regular medications and gave written informed consent. The volunteers received no financial incentive to participate. The study was approved by the Research and Ethics Committee of Westmead Hospital, Sydney, Australia.
Each volunteer agreed to receive morphine sulphate (5 mg) administered on three different occasions either by i.v. bolus injection, by s.c.b. or by s.c.i. over 4 h. The infusion time of 4 h for the s.c.i. was chosen to allow comparability between the doses examined. Each administration was separated by a washout period of at least 1 week. The order of the method of administration was randomised for each volunteer.
When morphine was administered s.c., either by s.c.b. or s.c.i., it was given into the s.c. tissues of the upper arm through a butterfly needle (Terumo 25G, 9 cm tube) placed subcutaneously. For the s.c.i., 5 mg morphine were diluted in normal saline to a total volume of 10 ml and placed in a syringe in an external syringe driver (Infusis Medis, Italy), programmed to deliver the 10 ml of the resulting solution over a 4 h period. The syringe driver was connected to the butterfly needle placed subcutaneously in the upper forearm. The 9 cm lead of the butterfly needle was primed with a solution of morphine, prepared in an identical manner to that placed in the syringe driver.
Blood samples (10 ml) were collected into lithium heparin tubes through an i.v. cannula placed in the upper forearm at 0 h (immediately prior to dosing), and then at 0.08, 0.17, 0.25, 0.50, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 5.0, 6.0, 8.0, 10.0 and 12.0 h following each administration of morphine. Samples were taken from the opposite forearm to that used for the administration of morphine. After each sample was taken the cannula was locked with 1 ml of heparinized saline. For each blood sample withdrawn, the first 2 ml of blood withdrawn was taken into a separate syringe and discarded.
Thirty minutes after collection, blood samples were separated by centrifugation and plasma stored at −40 °C. The frozen samples were subsequently air freighted in a refrigerated container to London, for analysis. Plasma concentrations of morphine, M6G and M3G were determined using an h.p.l.c. procedure involving solid phase extraction, followed by reverse phase ion-paired chromatography with electrochemical and fluorescence detection [13]. Briefly, 750 µl of plasma were buffered with 2.25 ml 500 mm ammonium sulphate, pH 9.3, and applied to a 100 mg C8 Bond-Elut cartridge (Anachem, Luton, UK), which had been preconditioned with methanol, eluant (10 mm sodium dihydrogen phosphate, pH 2.1, with 10% acetonitrile), and water. After washing with 5 mm ammonium sulphate, pH 9.3, compounds of interest were eluted with 800 µl eluant and 200 µl injected into the h.p.l.c. system. Separation was achieved by reverse phase ion-paired chromatography with electrochemical detection for morphine and M6G and fluorescence detection for M3G. Limits of quantification for this assay were 2 nmol l−1 for morphine and M6G, and 20 nmol l−1 for M3G. Reproducibility of quality control samples at three levels (low 20 nmol l−1 for morphine and M6G, 200 nmol l−1 for M3G; medium 100 nmol l−1 for morphine and M6G, 1000 nmol l−1 for M3G; high 200 nmol l−1 for morphine and M6G, 2000 nmol l−1 for M3G) analysed with the study samples was < 10% for morphine and < 12% for M3G at each level.
Morphine stability
A small stability study was conducted to investigate the stability of morphine in the infusion devices used. Briefly, morphine was made up to 0.5 mg ml−1 in saline, and drawn up into a 10 ml syringe, which was connected to a butterfly needle (Terumo 25G, 9 cm tube). At regular intervals, 0.5 ml of solution were dispensed from the syringe, immediately diluted down to a concentration of 200 ng ml−1 and morphine concentration quantified by h.p.l.c. This experiment was repeated in quadruplicate.
Pharmacokinetic analysis
Pharmacokinetic parameters were determined using PHARMKIT, an interactive pharmacokinetic analysis program [14]. The absortion half-life of morphine or the appearance half-life of M3G and M6G was determined from the initial slope. The area under the concentration-time curve (AUC) was determined by the trapezoidal method, and extrapolated out to infinity using the concentration at Ct and the elimination rate constant (λz). Half-life (t½,z) was calculated as 0.693 divided by λz. The apparent volume of distribution (Vd) was calculated as dose divided by the product of AUC(0,∞) and λz and apparent clearance (CL) as dose divided by AUC(0,∞). Pharmacokinetic parameters were standardized to a dose of 10 mg/70 kg, to facilitate comparison with published data.
Statistical analysis
All statistical analyses were performed using Minitab for Windows (version 10, Minitab Inc, State College, PA, USA). Comparison of pharmacokinetic parameters between treatments was made by analysis of variance, controlling for subjects. For data that were not normally distributed (determined using the Shapiro-Wilk test on the analysis of variance residuals), a nonparametric analysis of variance (Friedman's test) was used. Where these analyses indicated significant differences (P < 0.05) between treatments, individual comparisons were made using the paired t-test or Wilcoxon Signed-Ranks test for parametric and nonparametric data, respectively.
The bioavailabilities of morphine, M6G and M3G after s.c. administration were analysed according to recommendations for bioequivalence studies [15]. AUC values were log transformed, and the ratio (and 90% confidence intervals [CI]) of s.c.b. to i.v., and s.c.i. to i.v. calculated. Limits of acceptance for bioequivalence were 0.80–1.25. This apparent asymmetry is because the data were log-transformed.
Results
I.v. bolus injection of morphine
Mean plasma concentrations of morphine, M6G and M3G after i.v. administration of morphine are shown Figure 1 and pharmacokinetic parameters for morphine, M6G and M3G in Tables 1, 2 and 3, respectively.
Figure 1.
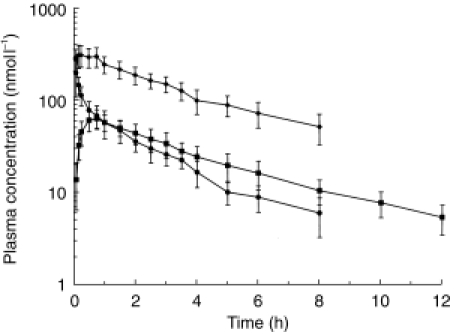
Mean plasma concentrations of morphine (•), M6G (▪) and M3G (✦) after i.v. administration of morphine to six healthy volunteers. Error bars indicate standard deviation (s.d.). (Corrected to a dose of 10 mg/70 kg. Data shown are the mean of at least 3 values).
Table 1.
Pharmacokinetic parameters for morphine after i.v., s.c.b. or s.c.i. administration of morphine to healthy volunteers.
| i.v. | s.c.b. | s.c.i. | |
|---|---|---|---|
| †Appearance t½ (h) | – | 0.08 ± 0.03 | 1.53 ± 0.74 ** |
| Elimination t½ (h) | 2.2 ± 1.0 | 2.1 ± 0.4 | 2.2 ± 0.8 |
| Apparent CL (ml min−1) | 1584 ± 408 | 1385 ± 259 | 2125 ± 860 |
| Apparent Vd (l) | 276 ± 72 | 248 ± 74 | 406 ± 229 |
| Cmax (nmol l−1) | 283 ± 74 | 262 ± 49 | 46 ± 8***### |
| tmax (h) | 0.08 (0.08-0.08) | 0.25 (0.17-0.25)*** | 4.0 (3.5-5.0)***### |
| AUC(0,t) (nmol l−1 h) | 269 ± 62 | 303 ± 55 | 198 ± 55 # |
| AUC(0,∞) (nmol l−1 h) | 290 ± 67 | 323 ± 60 | 225 ± 49 # |
| Bioavailability (%) | 100 | 113.6 ± 16.9 | 83.2 ± 34.6 |
Data standardized to 10 mg dose to 70 kg subject.
Results are mean±s.d. except tmax which is median (range).
i.v. = intravenous; s.c.b. = subcutaneous bolus; s.c.i. = subcutaneous infusion
Equivalent to absorption half-life for the parent drug
*P < 0.05 compared with i.v.
P < 0.01 compared with i.v.
P < 0.001 compared with i.v.
= P < 0.05 compared with s.c. b.
= P < 0.01 compared with s.c. b
= P < 0.001 compared with s.c.b.
N.B. Paired t-tests used throughout, except for appearance t½ and tmax for M3G for which the Wilcoxon Signed-Ranks test was used as the data were not normally distributed.
Table 2.
Pharmacokinetic parameters for M6G after i.v., s.c.b. and s.c.i. administration of morphine to healthy volunteers.
| i.v. | s.c.b. | s.c.i. | |
|---|---|---|---|
| Appearance t½ (h) | 0.18 ± 0.02 | 0.28 ± 0.09 * | 1.21 ± 0.61 **# |
| Elimination t½ (h) | 3.6 ± 1.1 | 4.1 ± 1.3 | 3.5 ± 1.8 |
| Cmax (nmol l−1) | 66.7 ± 14.8 | 62.2 ± 17.5 | 30.1 ± 1.9**## |
| tmax (h) | 0.63 (0.5-0.75) | 1.25 (0.25-1.5) | 5.25 (5.0-6.0)***### |
| AUC(0,t) (nmol l−1 h) | 259 ± 60 | 252 ± 42 | 171 ± 15*# |
| AUC(0,∞) (nmol l−1 h) | 291 ± 66 | 290 ± 59 | 171 ± 15*# |
| Bioavailability (%) | 100 | 102.0 ± 17.4 | 74.5 ± 22.2*# |
| M6G:M AUC(0,∞) | 1.33 ± 0.25 | 1.19 ± 0.25 | 1.28 ± 0.48*# |
See legend to Table 1 for details.
Table 3.
Pharmacokinetic parameters for M3G after i.v., s.c.b and s.c.i administration of morphine to healthy volunteers.
| i.v. | s.c.b | s.c.i | |
|---|---|---|---|
| Appearance t½ (h) | 0.07 ± 0.04 | 0.44 ± 0.67* | 0.92 ± 0.54* |
| Elimination t½ (h) | 3.1 ± 0.7 | 2.8 ± 0.5 | 3.3 ± 1.4 |
| Cmax (nmol l−1) | 334 ± 75 | 280 ± 65 | 135 ± 47** |
| tmax (h) | 0.25 (0.17-0.75) | 1.0 (0.5-2.0)* | 5.25 (2.5-6.0)* |
| AUC(0,t) (nmol l−1 h) | 1022 ± 256 | 1085 ± 308 | 645 ± 329 |
| AUC(0,∞) (nmol l−1 h) | 1293 ± 288 | 1308 ± 345 | 848 ± 208* |
| Bioavailability (%) | 100 | 104.1 ± 32.5 | 69.5 ± 26.0 |
| M3G:M AUC(0,∞) | 5.9 ± 0.2 | 5.4 ± 1.3 | 5.0 ± 1.6 |
| M3G:M6G AUC(0,∞) | 4.5 ± 0.9 | 4.5 ± 0.4 | 4.1 ± 0.9 |
See legend to Table 1 for details.
Plasma morphine concentrations declined throughout the study and were detectable in four subjects up to 6 h, in two up to 8 h, but in only one up to 10 h following administration. As expected, in all subjects the maximum plasma morphine concentration was in the first postdose sample (0.08 h), with a maximum concentration (Cmax) of 283 ± 74 nmol l−1. In contrast, the median tmax for M6G was 0.63 h (0.5 - 0.75), with a Cmax of 66.7 ± 14.8 nmol l−1, and for M3G was 0.25 h (0.17-0.25), with a Cmax of 334 ± 75 nmol l−1.
S.c. bolus s.c.b. injection of morphine
Mean plasma concentrations of morphine, M6G and M3G after s.c.b. administration of morphine are shown in Figure 2 and pharmacokinetic parameters for morphine, M6G and M3G in Tables 1, 2, and 3, respectively.
Figure 2.
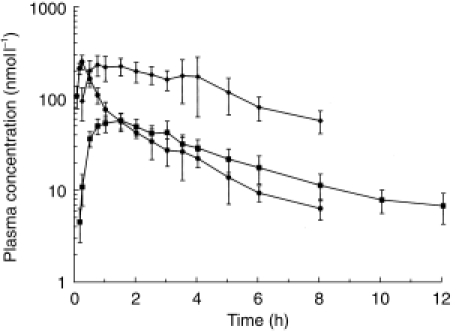
Mean plasma concentrations of morphine (•), M6G (▪) and M3G (✦) after s.c.b. administration of morphine to six healthy volunteers. Error bars indicate standard deviation (s.d.).(Corrected to a dose of 10 mg/70 kg. Data shown are themean of at least three values).
After s.c.b. dosing, morphine was detected in the plasma of five subjects at 0.08 h, and up to 6 h in all subjects, up to 8 h in five subjects, but in none thereafter. The mean values for Cmax, AUC, CL and Vd after s.c.b. were very similar to the respective parameters for i.v. administration, although the median tmax after s.c.b. morphine was significantly longer than after i.v. morphine (0.25 vs 0.08 h, P < 0.001). Nevertheless, this difference was relatively small and may not be significant clinically.
Whilst morphine was detected 0.08 h after s.c.b., M6G was first detected at 0.17 h in three subjects, and not until 0.25 h in all six subjects. Pharmacokinetic parameters for M6G after s.c.b were similar to those after i.v. morphine, but with a significantly longer appearance half-life (t½) (P = 0.03), and with a trend to a longer tmax (1.25 vs 0.63 h, P = 0.09).
M3G was only detected in one volunteer at 0.17 h, in five at 0.25 h, but not until 0.5 h in all volunteers. M3G could still be detected in all volunteers at 6 h, but in only four at 8 h, in one at 10 h, and in none by the end of sampling at 12 h. The mean AUC and Cmax were again similar to i.v. dosing, but with a significantly longer appearance t½,z (P < 0.05) and tmax (1.00 vs 0.25 h, P < 0.05).
Comparison of log-transformed AUC values after i.v. and s.c.b. dosing indicated that the two routes were bioequivalent with regard to morphine (log-transformed AUC ratio 1.13, 90% CI 1.05–1.21), M6G (1.01, 90% CI 0.93–1.09) and M3G (1.00, 90% CI 0.88–1.15).
S.c. infusion (s.c.i.) of morphine
Mean plasma concentrations of morphine, M6G and M3G after s.c.i. administration of morphine are shown in Figure 3 and pharmacokinetic parameters for morphine, M6G and M3G in Tables 1, 2 and 3, respectively.
Figure 3.
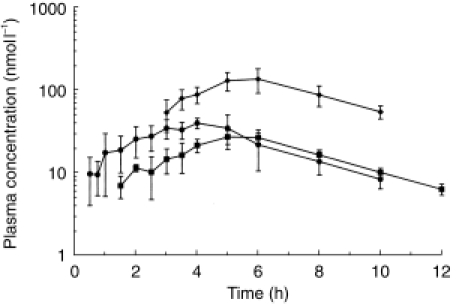
Mean plasma concentrations of morphine (•), M6G (▪) and M3G (✦) after s.c.i. administration of morphine to six healthy volunteers. Error bars indicate standard deviation (s.d.). (Corrected to a dose of 10 mg/70 kg. Data shown are the mean of at least three values).
Morphine was first detected at 0.17 h in two volunteers, but was not detected in all six volunteers until 2 h. However, morphine could still be detected in all six volunteers at 6 h and was still detectable in two at the end of sampling at 12 h. The median tmax for s.c.i. morphine (4.0 h) was significantly longer than after i.v. morphine (0.08 h, P < 0.001) and s.c.b. morphine (0.25 h, P < 0.001), and the standardized Cmax after s.c.i. morphine (46 ± 8 nmol l−1) was significantly lower than after both i.v. (283 ± 74 nmol l−1, P < 0.001) and s.c.b. administration (262 ± 49 nmol l−1, P < 0.001).
M6G was first detected in two volunteers at 1 h, but not in all six volunteers until 2.5 h. However, M6G remained detectable in all six volunteers until the end of sampling at 12 h. The median tmax for M6G after s.c.i. administration (5.25 h) was significantly longer than after i.v. (0.63 h, P < 0.001) or s.c.b. morphine (1.25 h, P < 0.001). Similarly, the Cmax for M6G after s.c.i. (30.1 ± 1.9 nmol l−1) was significantly lower than after i.v. (66.7 ± 14.8 nmol l−1, P < 0.001) or s.c.b. morphine (62.2 ± 17.5 nmol l−1, P < 0.001).
Although M3G was detected in one volunteer as early as 0.25 h, it was not detected in the remaining five until 3 h. The median tmax for M3G after s.c.i. (5.25 h) was again significantly longer than after i.v. morphine (0.25 h, P < 0.05), with a lower Cmax (135 ± 47 nmol l−1) than after i.v. (334 ± 75 nmol l−1, P < 0.01) or s.c.b. morphine (280 ± 65 nmol l−1, P < 0.01).
Comparison of log-transformed AUC values after i.v. and s.c.i. dosing indicated that the two routes were not bioequivalent with regard to morphine (log-transformed AUC ratio 0.78, 90% CI 0.66–0.93), M6G (0.72, 90% CI 0.63–0.82) or M3G (0.65, 90% CI 0.54–0.78). In view of this finding, a further experiment of s.c.i. was performed in vitro, using the original equipment.
Morphine stability
The limited stability study (n = 4) indicated that there was no adsorption of morphine onto the surface of the infusion lines, with measured morphine concentra-tion (0.5 mg ml−1) collected at the needle of 0.50 ± 0.01 mg ml−1 at 2 h, 0.49 ± 0.01 mg ml−1 at 4 h, 0.51 ± 0.01 mg ml−1 at 24 h.
Adverse effects of morphine
None of the subjects involved in this study had received morphine previously. All noted some adverse effects associated with the administration of morphine. All six noted a feeling of pressure in the occipital region of the skull, accompanied by some difficulty in concentration. One experienced severe drowsiness. Two complained of nausea and one of these also experienced vomiting. In general, these side-effects were more severe after morphine given i.v. than by s.c.b. or s.c.i.. However, the administration of morphine both by s.c.b. and s.c.i. was accompanied by pain at the injection site, reported by all volunteers. For the s.c.i., brief stinging pain was noted at the injection site shortly after each operation of the syringe driver. Although four of the six volunteers were clinically experienced in the administration of morphine to patients with advanced cancer, none recalled hearing patients complain of pain at the injection site after the s.c. administration of morphine.
Discussion
There have been previous studies of the pharmacokinetics of morphine, M6G and M3G after administration of morphine by various different routes, either in healthy volunteers [12] or in patients [16–20]. However, despite the widespread use of the s.c. route for the parenteral administration of morphine, the current study is the first which has examined the concentrations of morphine and its glucuronide metabolites and their pharmacokinetic parameters after i.v., s.c.b. and short s.c.i. of morphine in the same group of healthy volunteers. Although the principal aim of this study was to investigate the pharmacokinetics of morphine given either by s.c.b. or short s.c.i., the i.v. route was included to enable comparison with previous data, and to permit the determination of absolute bioavailability.
The log-transformed data indicated that the s.c.b. route of morphine administration was bioequivalent to the i.v. route. The mean Cmax values for morphine (Table 1), M6G and M3G, and the mean standardized AUCs for each were similar to those achieved after i.v. morphine, indicating that absorption from the administration site was both rapid and complete, and that the parent drug was metabolized in the same way as after i.v. dosing. As anticipated, the median tmax values for morphine and M3G were significantly longer than after i.v. morphine (with a trend to a longer tmax for M6G).
When comparing s.c.i. with i.v. administration, log-transformed data indicated that the two routes were not bioequivalent for morphine, M6G or M3G, although for morphine this was not apparent from the measured, untransformed AUC and bioavailability values. As would be anticipated, the mean Cmax values for morphine, M6G and M3G were significantly lower and the median tmax values for each significantly longer than after i.v. morphine, differences also apparent when comparing s.c.i. and s.c.b.. For measured AUC, there was also a significant difference between s.c.i. and s.c.b..
The results obtained for i.v. morphine in the current study (after conversion to nmol l−1, for a 10 mg dose administered to a 70 kg volunteer) are very similar to those we have published previously [12], and are in agreement with data from studies in cancer patients. In cancer patients given i.v. morphine 1 week after abdominal surgery Sawe et al. [21] reported values of 4.0 ± 2.3 l kg−1 for volume of distribution, 28.0 ± 5.6 ml min−1 kg−1 for total plasma clearance and 1.7 ± 0.8 h for elimination half-life. Respective values in the current study after i.v. morphine were 4.0 ± 1.3 l kg−1 for volume of distribution, 22.6 ± 5.3 ml min−1 kg−1 for clearance and 2.1 ± 1.0 h for half-life. In a study investigating subcutaneous morphine infusions in cancer pain, Bruera et al. [22] reported a total plasma clearance of 1600 ± 300 ml min−1, compared with 1383 ± 257 ml min−1 in the current study, when corrected for bioavailability.
This is the first formal demonstration of the bio-equivalence of s.c.b. and i.v. morphine with regard to morphine, M6G and M3G, in healthy subjects. The data suggest that the two routes should be equivalent with regard to their pharmacodynamic effects, although it is likely that circulating morphine concentrations would have been higher immediately after the i.v. bolus was completed than at the first sampling point at 0.08 h, and these early high concentrations may influence penetration into the brain.
However, the data suggest that the bioavailability of morphine after s.c.i. was less than after i.v. or s.c.b. administration, and this was unexpected. This could be due to failure to deliver the full dose, or reduced absorption of administered morphine when given by prolonged infusion. It is unlikely that these observations were due to assay errors as the decrease in morphine AUC was also apparent for M6G and M3G, again suggesting that less morphine reached the systemic circulation after s.c.i.. Although it is possible that the 4 h infusion may have resulted in protracted absorption from the administration site, this was not apparent from the morphine tmax which was 4.0 h. A previous study which compared i.v. and s.c. infusions of hydromorphone reported an estimated bioavailability of 78% with s.c. administration [23], but a similar study with continuous i.v. and s.c. infusions of morphine found no difference in serum morphine concentrations at 6, 12, 18, or 24 h [24]. Although bioavailability was significantly reduced after s.c.i. in the current study, it is noteworthy that variability in bioavailability was much greater after this route than after s.c.b. (35% vs 17%). Bioavailability of morphine after s.c.i. was between 52% and 67% in four subjects, but in the remaining two subjects was 115% and 138%. It is possible that, in studies with small numbers of subjects, that differences in infusion site characteristics (e.g. the amount of fat or vascularity, etc.) may result in differences in bioavailability in some studies and not others.
If the difference in bioavailability cannot be explained biologically, it remains possible that less morphine was delivered by s.c.i. than by i.v. or s.c.b. administration, although there is no obvious explanation for this. Although it is conceivable that the syringe driver delivered less than the full volume of solution contained in the syringes, this was not noted during the study. Other studies have reported that morphine is stable for several days [25], weeks [26] and even months [27] when stored in plastic syringes and/or PCA devices, and our own stability study indicated no loss of drug from absorption onto plastic during a 24 h incubation period.
Whatever the reason, it should be emphasized that the majority of the volunteers involved in this study were experienced in the use of morphine given subcutaneously to patients with moderate or severe pain from advanced cancer. Therefore, if the full dose of the morphine was not infused, it is possible that similar errors may occur when s.c. infusions of morphine are used clinically in the management of patients. Although statistically different from i.v. and s.c.b. morphine, the bioavailability of around 80% for morphine and around 70% for M6G and M3G after s.c.i. morphine may not be important clinically, when one considers that the bioavailability of oral morphine is only around 25% [12]. Overall therefore this study confirms that the s.c. administration of morphine is an effective method for the parenteral administration of morphine.
References
- 1.World Health Organization. Cancer pain relief program. Geneva, Switzerland: WHO; 1986. [Google Scholar]
- 2.McDonald P, Graham P, Clayton M, Buhagiar A, Stuart-harris R. Regular subcutaneous bolus morphine via an indwelling cannula for pain from advanced cancer. Pall Med. 1991;5:323–329. [Google Scholar]
- 3.Yoshimura H, Ida S, Oguri K, Tsukamoto H. Biochemical basis for analgesic activity of morphine-6-glucuronide. I. Penetration of morphine-6-glucuronide in the brain of rats. Biochem Pharmacol. 1973;22:1423–1430. doi: 10.1016/0006-2952(73)90320-1. [DOI] [PubMed] [Google Scholar]
- 4.Abbott FV, Palmour RM. Morphine-6-glucuronide: analgesic effects and receptor binding profile in rats. Life Sci. 1988;43:1685–1695. doi: 10.1016/0024-3205(88)90479-1. [DOI] [PubMed] [Google Scholar]
- 5.Osborne R, Thompson P, Joel S, Trew D, Patel N, Slevin M. The analgesic effects of morphine-6-glucuronide. Br J Clin Pharmacol. 1992;34:130–138. doi: 10.1111/j.1365-2125.1992.tb04121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peat SJ, Hanna MH, Woodham M, Knibb AA, Ponte J. Morphine-6-glucuronide: effects on ventilation in normal volunteers. Pain. 1991;45:101–104. doi: 10.1016/0304-3959(91)90170-3. [DOI] [PubMed] [Google Scholar]
- 7.Thompson PI, Joel SP, John L, Wedzicha JA, Maclean M, Slevin ML. Respiratory depression following morphine and morphine-6-glucuronide in normal subjects. Br J Clin Pharmacol. 1995;40:145–152. [PMC free article] [PubMed] [Google Scholar]
- 8.Pasternak GW, Bodnar RJ, Clark JA, Inturrisi CE. Morphine-6-glucuronide, a potent mu agonist. Life Sci. 1987;41:2845–2849. doi: 10.1016/0024-3205(87)90431-0. [DOI] [PubMed] [Google Scholar]
- 9.Shimomura K, Katama O, Ueki S, Ida S, Oguri K. Analgesic effect of morphine glucuronides. Tohoku J Exp Med. 1971;105:45–52. doi: 10.1620/tjem.105.45. [DOI] [PubMed] [Google Scholar]
- 10.Gong QL, Hedner J, Bjorkman Hedner T. Morphine-3-glucuronide may functionally antagonize morphine-6-glucuronide induced antinociception and ventilatory depression in the rat. Pain. 1992;48:249–255. doi: 10.1016/0304-3959(92)90065-J. [DOI] [PubMed] [Google Scholar]
- 11.Smith MT, Watt JA, Cramond T. Morphine-3-glucuronide – a potent antagonist of morphine analgesia. Life Sci. 1990;47:579–585. doi: 10.1016/0024-3205(90)90619-3. [DOI] [PubMed] [Google Scholar]
- 12.Osborne R, Joel S, Trew D, Slevin M. Morphine and metabolite behavior after different routes of morphine administration: Demonstration of the importance of the active metabolite morphine-6-glucuronide. Pharmacol Ther. 1990;47:12–19. doi: 10.1038/clpt.1990.2. [DOI] [PubMed] [Google Scholar]
- 13.Joel SP, Osborne R, Slevin M. An improved method for the simultaneous determination of morphine and its principal glucuronide metabolites. J Chromatogr. 1988;430:394–399. doi: 10.1016/s0378-4347(00)83176-x. [DOI] [PubMed] [Google Scholar]
- 14.Johnston A, Woollard RC. STRIPE. an interactive computer program for the analysis of drug pharmacokinetics. J Pharmacol Methods. 1983;9:193–199. doi: 10.1016/0160-5402(83)90038-4. [DOI] [PubMed] [Google Scholar]
- 15.Pabst G, Jaeger H. Review of methods and criteria for the evaluation of bioequivalence studies. Eur J Clin Pharmacol. 1990;38:5–10. doi: 10.1007/BF00314794. [DOI] [PubMed] [Google Scholar]
- 16.Peterson GM, Randall CT, Paterson J. Plasma levels of morphine and morphine glucuronides in the treatment of cancer pain: relationship to renal function and route of administration. Eur J Clin Pharmacol. 1990;38:121–124. doi: 10.1007/BF00265969. [DOI] [PubMed] [Google Scholar]
- 17.Breda M, Bianchi M, Ripamonti C, Zecca E, Ventafridda V, Panerai AE. Plasma morphine and morphine-6-glucuronide patterns in cancer patients after oral, subcutaneous, sublabial and rectal short-term administration. Int J Clin Pharmacol Res. 1991;11:93–97. [PubMed] [Google Scholar]
- 18.Wolff T, Samuelsson H, Hedner T. Concentrations of morphine and morphine metabolites in CSF and plasma during continuous subcutaneous morphine administration in cancer pain patients. Pain. 1996;68:209–216. doi: 10.1016/s0304-3959(96)03102-8. [DOI] [PubMed] [Google Scholar]
- 19.Ashby M, Fleming B, Wood M, Somogyi A. Plasma morphine and glucuronide (M3G and M6G) concentrations in hospice inpatients. J Pain Symptom Manage. 1997;14:157–167. doi: 10.1016/S0885-3924(97)00020-1. [DOI] [PubMed] [Google Scholar]
- 20.Vermeire A, Remon JP, Rosseel MT, Belpaire F, Devulder J, Bogaert MG. Variability of morphine disposition during long-term subcutaneous infusion in terminally ill cancer patients. Eur J Clin Pharmacol. 1998;53:325–330. doi: 10.1007/s002280050387. [DOI] [PubMed] [Google Scholar]
- 21.Sawe J, Odar-cederlof I. Kinetics of morphine in patients with renal failure. Eur J Clin Pharmacol. 1987;32:377–382. doi: 10.1007/BF00543973. [DOI] [PubMed] [Google Scholar]
- 22.Bruera E, Fainsinger R, Spachynski K, Babul N, Harsanyi Z, Darke AC. Steady-state pharmacokinetic evaluation of a novel, controlled-release morphine suppository and subcutaneous morphine in cancer pain. J Clin Pharmacol. 1995;35:666–672. doi: 10.1002/j.1552-4604.1995.tb04106.x. [DOI] [PubMed] [Google Scholar]
- 23.Moulin DE, Kreeft JH, Murray-parsons N, Bouquillon AI. Comparison of continuous subcutaneous and intravenous morphine infusions for the management of cancer pain. Lancet. 1991;337:465–468. doi: 10.1016/0140-6736(91)93401-t. [DOI] [PubMed] [Google Scholar]
- 24.Waldmann CS, Eason JR, Rambohul E, Hanson GC. Serum morphine levels. A comparison between continuous subcutaneous and continuous intravenous infusion in postoperative patients. Anaesthesia. 1984;39:768–771. doi: 10.1111/j.1365-2044.1984.tb06520.x. [DOI] [PubMed] [Google Scholar]
- 25.Bray RJ, Davies PA, Seviour JA. The stability of preservative-free morphine in plastic syringes. Anaesthesia. 1986;41:294–295. doi: 10.1111/j.1365-2044.1986.tb12791.x. [DOI] [PubMed] [Google Scholar]
- 26.Strong ML, Scaaf LJ, Pankaskie MC, Robinson DH. Shelf-lives and factors affecting the stability of morphine sulphate and meperidine (pethidine) hydrochloride in plastic syringes for use in patient- controlled analgesic devices. J Clin Pharm Ther. 1994;19:361–369. doi: 10.1111/j.1365-2710.1994.tb00695.x. [DOI] [PubMed] [Google Scholar]
- 27.Roos PJ, Glerum JH, Meilink JW. Stability of morphine hydrochloride in a portable pump reservoir. Pharm Weekbl Sci. 1992;14:23–26. doi: 10.1007/BF01989221. [DOI] [PubMed] [Google Scholar]


