Abstract
Aims
To establish whether gender or N-acetyltransferase 2 (NAT2) genotype influence the urinary 17 U+17X/137X ratio after dosing with caffeine.
Methods
Ninety-two nonsmoking individuals underwent caffeine phenotyping. NAT2 genotype was determined by the polymerase chain reaction followed by a restriction digest (PCR-RFLP).
Results
The median ratio for urinary 17 U+17X/137X was 6.7 (range 1.45–18.65). 55% of subjects were slow acetylators. Gender did not affect the metabolic ratio or NAT2 genotype. Mean 17 U+17X/137X ratio differed between fast (6.75) and slow (8.69) acetylators (95% CI for the difference, 0.32–3.56).
Conclusions
The findings are further evidence that the 17 U+17X/137X urinary ratio is not a robust measure of CYP1A2 activity. A possible mechanism by which the ratio might be influenced by NAT2 genotype is suggested.
Keywords: caffeine, CYP1A2, NAT2
Introduction
CYP1A2 is a cytochrome P450 enzyme that is expressed primarily in the liver and is important in the metabolism of many drugs as well as procarcinogens such as aromatic amines. A number of methods for determining CYP1A2 activity in vivo have been developed that rely on the central role of CYP1A2 in caffeine metabolism (Figure 1). Wide interindividual variation in CYP1A2 activity has been reported and this is an important source of variability in response to drugs metabolized by CYP1A2. Some evidence has been presented suggesting that a polymorphism in CYP1A2 activity may account for a part of the variability and may be important in determining susceptibility to colorectal cancer [1]. The role of gender in determining CYP1A2 activity has been unclear because the effect of the oral contraceptive pill and differences in smoking rate between men and women have not always been controlled adequately [2, 3]. Mathematical modelling has suggested that the 17 U+17X/137X ratio could theoretically be influenced by urine flow rate or by polymorphism in acetylation of caffeine metabolites encoded by the NAT2 locus, although this has not been examined experimentally [4].
Figure 1.
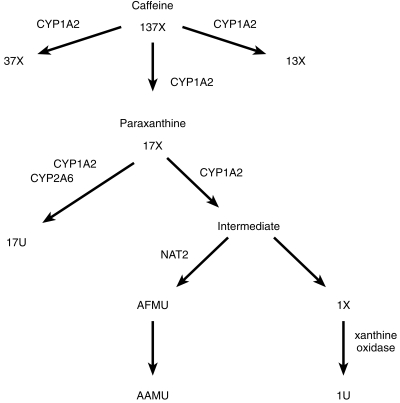
The major biotransformation pathways for caffeine. The predominant pathways for caffeine metabolism are shown with thick arrows and the minor ones with thin arrows. The enzymes responsible for the individual transformations are indicated with symbols. Abbreviations: 17U 1,7-dimethyluric acid; 17X 1,7-dimethylxanthine; 137X caffeine; AFMU 5-acetylamino-6-formylamino-3-methyluracil; AAMU 5-acetylamino-6-amino-3-methyluracil; 13X 1,3-dimethylxanthine; 37X 3,7-dimethylxanthine; 1X 1-methylxanthine; 1U 1-methyluric acid.
The aim of this study was to determine the effect of polymorphism at the NAT2 locus and gender on CYP1A2 activity.
Methods
Ninety-two healthy nonsmoking individuals (49 women) employed at the University or hospital and aged 18–50 years performed caffeine phenotyping as described in detail elsewhere and had blood taken for DNA extraction [5]. Subjects were excluded if they were taking any medication at all, including the oral contraceptive pill, if they had recently smoked tobacco or if they were known to have any significant impairment of hepatic or renal function. Foods and beverages containing caffeine were stopped for 12 h before the study and until the end of the study. After an overnight fast, subjects consumed two strong cups of instant coffee at 08.00 h, approximately equivalent to 200 mg caffeine and similar to the 3 mg kg−1 given by Butler et al. [6]. We did not attempt to demonstrate caffeine absence by urine analysis before caffeine dosing and we did not record urine volumes. Specimen storage and preparation and the performance of h.p.l.c. were as described previously [5]. The reproducibility of the analytical technique used was tested by comparing the results from paired samples using the t-test for paired samples. There was a high degree of between-test reproducibility with a correlation of 0.945, P < 0.0001 and the mean difference between the paired samples was 0.038 (P = 0.784).
NAT2 genotype was detected as previously described by a PCR-RFLP method with primers Nat-Hu-14 and Nat-Hu-16 followed by digest with KpnI, TaqI and BamHI [7]. KpnI detects the T481C polymorphism, present in both the NAT2*5A and NAT2*5B alleles, TaqI detects the presence of the G590A polymorphism which is the key inactivating polymorphism found in alleles NAT2*6A, B C and D, and BamHI detects the G857A polymorphism found in alleles NAT2*7A and NAT2*7B [8]. Based on knowledge of allele frequency, this method should identify correctly the genotype in more than 95% of cases in Caucasian populations and correlates well with phenotype, as measured with sulphamethazine [9].
All comparisons between subgroups were performed by t-test on log-transformed data. The proportions of men and women in different NAT2 genotypes were compared using c2. The relationship between gender and 17 U+17X/137X ratio has been published previously [5] but is included here because it was essential to demonstrate that there was no interaction between gender and NAT2 genotype.
The study was approved by the institutional ethics committee and all subjects gave informed written consent.
Results
As described in a previous paper, the range for urinary 17 U+17X/137X in the 92 subjects was from 1.45 to 18.65 with a median ratio of 6.7 and a mean of 7.78 and the ratio was log-normally distributed [5]. The mean urinary 17 U+17X/137X ratio in men was 7.56 (s.d. 3.88) and in women was 7.99 (s.d. 3.97) (2-tailed P = 0.57). Genotyping for NAT2 was available for 90 of the subjects and 49 (55%) were slow acetylators. There was no difference in the proportion of men (58%) and women (51%) who were slow acetylators (odds ratio = 0.74[95% confidence intervals 0.29–1.85]). The fast acetylators had a mean urinary 17 U+17X/137X ratio of 6.75 (s.d. 3.68) whereas the slow acetylators had a mean ratio of 8.69 (s.d. 3.99) (95% confidence intervals for the difference 0.32–3.56, P = 0.006) (Figure 2).
Figure 2.
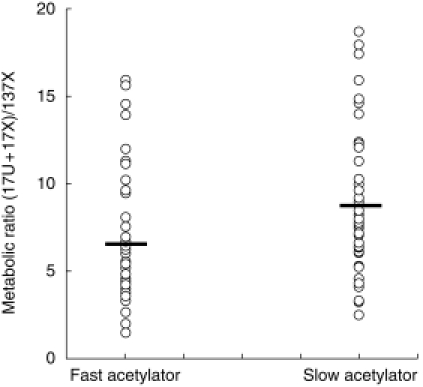
The relationship between the urinary caffeine 17U+17X/137X ratio in fast and slow acetylators. The urinary 17U+17X/137X ratio is shown for individuals genotyped as fast or slow acetylators at the NAT2 locus. Each individual is represented by an open circle and the horizontal bars represent the mean in each group.
Discussion
In the present study it was found that NAT2 genotype does influence the urinary 17X+17 U/137X ratio and that the metabolic ratio was lower in fast acetylators. The NAT2 polymorphism might influence the 17 U+17X/137X ratio if NAT2 activity had some influence, either directly or indirectly, on whether paraxanthine (17X) was metabolized down the demethylation pathway (to AAMU/AFMU and 1X) or via hydroxylation to 17 U (see Figure 1). The latter reaction is catalysed by CYP1A2 and CYP2A6 and not influenced by NAT2. It then remains to be determined whether NAT2 might affect the rate of demethylation of 17X. The chemistry of the demethylation of 17X has not been characterised completely but it is thought that the first step is the formation of an unstable ring-opened compound that is then stabilized by acetylation. However, CYP1A2 is believed to be the enzyme responsible for initial demethylation and subsequently NAT2 is responsible for the acetylation reaction, so a direct effect of NAT2 on the rate of 17X demethylation has been thought to be unlikely [4]. One possible mechanism by which NAT2 might influence the 17 U+17X/137 ratio is product inhibition of demethylation by the ring-opened intermediate metabolite. In fast acetylators the intermediate would be rapidly metabolized to AFMU with the dual effect of lowering 17X and (indirectly) 17 U concentrations and thereby lowering the urinary 17 U+17X/137X ratio. The opposite would occur in slow acetylators. This explanation certainly is compatible with our experimental findings.
We have recently reported that the 17 U+17X/137X ratio is log-normally distributed and not bimodally distributed and that there is no significant polymorphism in the CYP1A2 gene [5]. It may seem surprising that the distribution of the urinary 17 U+17X/137X ratio was found to be log-normal when there were differences in the mean ratio between fast and slow acetylators. However, the effect of NAT2 genotype on the urinary 17 U+17X/137X ratio is modest (means of 6.75, range 2.45–18.6 and 8.69, range 1.45–15.9) but the standard deviation of the CYP1A2 ratio is quite high (3.99 in slow acetylators and 3.68 in fast acetylators). Log-normal distributions often arise from the multiplicative effects of several variables [10] and acetylator type is only one influence on the urinary 17 U+17X/137X ratio.
A number of studies have demonstrated a lower CYP1A2 activity in women taking the oral contraceptive pill than in other women [2, 3], but this has not been confirmed in all studies. The effect of gender on CYP1A2 activity has been obscured by the effect of the oral contraceptive pill and differential smoking rates between men and women. In the present study the effects of the oral contraceptive pill and smoking were excluded at the study design stage rather than being accounted for by statistical manipulations and we found no evidence that gender affects basal CYP1A2 activity. This study therefore contributes to evidence that CYP1A2 activity is not influenced by gender.
The use of the urinary caffeine metabolite ratio has been suggested to be polymorphic, suggesting a polymorphism in CYP1A2 activity and it has been suggested that this polymorphism contributes to susceptibility to colorectal cancer. Increasing evidence suggests that the use of urinary ratios to study CYP1A2 activity is not as reliable as the use of salivary ratios [4], which are also easy to perform [11]. In this study we have shown that the NAT2 genotype is another variable that influences the urinary 17 U+17X/137X ratio and in a previous study we were not able to confirm polymorphism in CYP1A2 activity [5]. It may be preferable to use salivary caffeine metabolite measurements to assess in vivo CYP1A2 activity [4].
Acknowledgments
This research was sponsored by the North of England Cancer Research Campaign and the Northern Regional Health Authority.
References
- 1.Lang NP, Butler MA, Massengill J, et al. Rapid metabolic phenotypes for acetyltransferase and cytochrome P4501A2 and putative exposure to food-borne heterocyclic amines increase the risk for colorectal cancer and polyps. Cancer Epidemiol Biomarkers Prevention. 1994;3:675–682. [PubMed] [Google Scholar]
- 2.Catteau A, Bechtel YC, Poisson N, Bechtel PR, Bonaiti-Pellie C. A population and family study of CYP1A2 using caffeine urinary metabolites. Eur J Clin Pharmacol. 1995;47:423–430. doi: 10.1007/BF00196856. [DOI] [PubMed] [Google Scholar]
- 3.Nakajima M, Yokoi T, Mizutani M, Shin S, Kadlubar FF, Kamataki T. Phenotyping of CYP1A2 in Japanese population by analysis of caffeine urinary metabolites: absence of mutation prescribing the phenotype in the CYP1A2 gene. Cancer Epidemiol Biomarkers Prevention. 1994;3:413–421. [PubMed] [Google Scholar]
- 4.Rostami-Hodjegan A, Nurminen S, Jackson PR, Tucker GT. Caffeine urinary metabolite ratios as markers of enzyme activity: a theoretical assessment. Pharmacogenetics. 1996;6:121–149. doi: 10.1097/00008571-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Welfare MR, Aitkin M, Bassendine MF, Daly AK. Detailed modelling of caffeine metabolism and examination of the CYP1A2 gene: lack of a polymorphism in CYP1A2 in Caucasians. Pharmacogenetics. 1999;9:367–375. doi: 10.1097/00008571-199906000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Butler MA, Lang NP, Young JF, et al. Determination of CYP1A2 and NAT2 phenotypes in human populations by analysis of caffeine urinary metabolites. Pharmacogenetics. 1992;2:116–127. doi: 10.1097/00008571-199206000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Welfare MR, Cooper J, Bassendine MF, Daly AK. Relationship between acetylator status, smoking, diet and colorectal cancer risk in the north-east of England. Carcinogenesis. 1997;18:1351–1354. doi: 10.1093/carcin/18.7.1351. [DOI] [PubMed] [Google Scholar]
- 8.NAT. nomenclature website at: http://www.louisville.edu/medschool/pharmacology/NAT.html.
- 9.Hickman D, Sim E. N-acetyltransferase polymorphism. Comparison of phenotype and genotype in humans . Biochem Pharmacol. 1991;42:1015–1019. doi: 10.1016/0006-2952(91)90282-a. [DOI] [PubMed] [Google Scholar]
- 10.Daly LE, Bourke GJ, McGilvray J, editors. Interpretations, uses of medical statistics. Oxford: Blackwell; 1991. [Google Scholar]
- 11.Fuhr U, Rost KL. Simple and reliable CYP1A2 phenotyping by the paraxanthine/caffeine ratio in plasma and in saliva. Pharmacogenetics. 1994;4:116. doi: 10.1097/00008571-199406000-00001. [DOI] [PubMed] [Google Scholar]


