Abstract
Aims
Grapefruit juice increases blood concentrations of many drugs metabolized by CYP3A. Amiodarone is metabolized by CYP3A to N-desethylamiodarone (N-DEA). The aim of this study was to determine amiodarone kinetics when administrated with and without grapefruit juice.
Methods
Eleven healthy adult volunteers took part in a single sequence, repeated-measures design study. Each subject, who had been evaluated 6 months previously for amiodarone pharmacokinetics, was given a single oral dose of amiodarone (17 mg kg−1) with three glasses of 300 ml of grapefruit juice on the same day.
Results
Grapefruit juice completely inhibited the production of N-DEA, the major metabolite of amiodarone, in all subjects and increased the area-under-the-curve (AUC) and maximum concentration of amiodarone (Cmax) by 50% and 84%, respectively, as compared with the control period during which water had been administrated instead of grapefruit juice (AUC: 35.9 ± 14.3 vs 23.9 ± 11.2 µg ml−1 h, P < 0.005 and Cmax: 3.45 ± 1.7 vs 1.87 ± 0.6 µg ml−1, P < 0. 02, respectively) (means ± s.d.). This inhibition of N-DEA production led to a decrease in the alterations caused by amiodarone on PR and QTc intervals.
Conclusions
Grapefruit juice dramatically alters the metabolism of amiodarone with complete inhibition of N-DEA production. These results are in agreement with in vitro data pointing to the involvement of CYP3 A in the metabolism of amiodarone and suggests that this interaction should be taken into account when prescribing this antiarrhythmic drug.
Keywords: amiodarone, grapefruit juice, interaction, metabolism, pharmacokinetics
Introduction
Amiodarone is an antiarrhythmic with predominantly class III (Vaughan – William's classification) effects [1]. The major metabolite of amiodarone, N-desethylamiodarone (N-DEA), has been identified in humans as a consequence of hepatic and possibly intestinal mucosa N-dealkylation [2–4]. Both amiodarone and N-DEA are characterized by long elimination half lives with an average of 50 days for the parent drug and 60 days for the metabolite [5–7]. Formation of N-DEA is mediated by cytochrome P4503 A activity in both rodent and human microsomes [8–10].
There is marked intersubject variation in amiodarone elimination which can be explained, at least in part, by differences in CYP3A4 and formation of N-DEA. Since grapefruit juice has been shown to inhibit CYP3A4 activity significantly, in both the intestine and the liver, thus reducing first-pass metabolism, the bioavailability of various orally administered drugs is markedly increased. Examples are dihydropyridine calcium-channel blockers, cyclosporin, the benzodiazepines midazolam and triazolam and the antihistamine terfenadine [11–17]. A better understanding of the origin of the variations of N-DEA production is important and might lead to some guidelines for limiting the use of concurrently prescribed treatments or beverage intake.
It has been suggested in experimental protocols that N-DEA could have clinically relevant activities: for example, N-DEA has been shown to produce larger increases than amiodarone in QRS duration, and in atrial and ventricular refractory periods in anaesthetized dogs as a consequence of its relatively greater effect on the fast sodium channel [18]. Zhou et al. [19] have also recently demonstrated that N-DEA increases ventricular defibrillation threshold to a greater extent than its parent drug in a randomized single blind study in anaesthetized pigs when using clinically relevant dosages of either amiodarone or N-DEA.
Given the putative therapeutic consequences [18–22] of variations of N-DEA production, we decided to study the effects of grapefruit juice on the pharmacokinetic properties of amiodarone.
Methods
Study population
Eleven nonsmoking, non obese healthy male volunteers with a mean age of 24 years (19–40 years) and an average weight of 72.6 kg (61–81 kg) were included in the study after local ethics committee approval and informed consent were obtained. All subjects had been thoroughly evaluated 6–8 months earlier for their ability to metabolize amiodarone. This first evaluation of amiodarone pharmacokinetics was performed with the same study protocol as that used in the present evaluation (see study protocol). The long washout period following the first evaluation was justified by the long half-life of the parent drug and its main metabolite [4–7, 23]. The subjects were required to have no history of iodine intolerance, thyroid disorder or any other clinical, biological or electrocardiographic contraindications to amiodarone prescription.
Subjects excluded from the study included those currently using medications (including over-the-counter treatments), those who drank alcoholic, cola or caffeine-containing beverages, and those who took any food or drink known to contain significant amount of flavonoids within 48 h before the beginning of the pharmacokinetic evaluation. In addition, subjects were required to fast for 14 h preceding the evaluation with a view to avoiding any xenobiotic interaction. A clinical examination was carried out and blood samples taken for thyroid hormone and amiodarone concentration measurements were carried out before inclusion in the study.
Study protocol
Each subject was given one oral dose of amiodarone in 300 ml of grapefruit juice (as opposed to the water used in the first evaluation). The grapefruit juice was commercially prepared (REA jus de fruits, Sarre-Union, France) and was all from the same preparation batch with a calibrated amount of flavonoids (nariturine: 135 mg l−1; hesperidine: 5.5 mg l−1; naringine: 419.1 mg l−1; neoheperidine: 8.2 mg l−1; dydimine: 7.1 mg l−1 and poncirine:19.8 mg l−1). The dose of amiodarone was about 17 mg kg−1. This loading dose (5–7 × 200 mg tablets according to the body weights of each individual of the studied population; Sanofi Pharmaceutical Inc.) is in accordance with the mean loading dose recommended by the U.S. and European Drug Regulatory Agencies [24]. In contrast with the first evaluation, subjects were also given 300 ml grapefruit juice 3 h and 9 h following amiodarone administration. ECG monitoring was then done over a 24 h period. No other oral intake was permitted during this period, save for a light calibrated lunch and supper lacking in flavonoids which were given, respectively, 5 and 12 h later. After 24 h of monitoring, subjects were allowed to leave the clinical unit and return home. Five follow up visits were scheduled 48 h, 1 week, 1 month, 3 months and 6 months after drug intake. Blood pressure and ECG data were recorded every 3 h during the first 24 h, at 48th hour, and at days 7, 30, and 90. The study design was justified by the risk of an unforseeable occurrence of major electrocardiographic alterations which could happen in individuals after an amiodarone loading dose given with grapefruit juice and was decided upon ethics committee advice which suggested the non inclusion of subjects who had major ECG alteration after the first amiodarone intake. This design was also justified given the very long wash-out period needed by the long elimination half-lives of amiodarone and its main metabolite: if a randomized cross-over design had been used, this period (at least 6 months) could not have allowed the use of the same preparation batch of grapefruit juice. On the contrary, by performing the entire grapefruit juice period in less than 2 months, our design allowed the use of the same batch of grapefruit juice, thus avoiding disparities in the various components of this fruit juice such as flavonoids which could be season dependent.
Analysis of electrocardiograms
All 12-lead electrocardiograms were obtained by means of a paper ECG recorder (EK53 Helige cardiotest recorder) and were performed by the same research nurse throughout the study. ECG intervals were coded and then measured randomly by a single blinded observer without knowledge of the randomization data. The QTc was calculated from the Bazett formula [25] after R-R and QT intervals were measured on V2 lead.
Measurement of drug and metabolite concentrations
A total of 24 blood samples (at each evaluation) were taken to measure serum amiodarone and N-DEA levels. Blood samples were obtained at 0, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 5.5, 6, 7, 8, 9, 10, 11, 12, 24, 48 h and 7, 30, and 90 days after the loading dose.
The concentrations of amiodarone and its principal metabolite N-DEA were measured by high-pressure liquid chromatography (h.p.l.c.) with u.v. detection at 242 nm, using the method described by Trivier et al. [26] with a slight modification to analyse serum amiodarone and N-DEA. The samples were analysed after acetonitrile deproteinization on a C18 reversed-phase 5 µm ODS Hypersil column (150 × 4.6 mm I.D. – Touzart & Matignon, Vitry-sur-Seine, France) using a mobile phase consisting of methanol-water-58% ammonium hydroxide (88 : 10 : 2, v/v/v) delivered at a flow-rate of 1.8 ml min−1. All assays were performed at room temperature.
All manipulations were carried out in amber glass-capped tubes following our observation that, in the presence of light, deiodinated compounds were formed (monoiodoamiodarone and bis-desiodoamiodarone), the retention times of which could interfere with those of N-DEA and bis-desethylamiodarone, respectively.
The limit of quantification was 0.1 µg ml−1 and the limit of detection was 0.05 µg ml−1 for both amiodarone and N-DEA in serum. When the concentrations were below the quantification limit, the samples were concentrated by a factor 2 or 3. From the analysis of 10 serum samples using concentrations of 0.2 µg ml−1 for amiodarone and N-DEA, the intra-assay precision was 7.43 and 7.71%, respectively, for amiodarone and N-DEA and the interassay precision for these two compounds were 9.5 and 10.39%, respectively.
All analytical procedures were blindly done by the same laboratory.
Statistical analysis
Pharmacokinetic parameters were determined by using a standard monocompartmental method. Area-under-the-curve (AUC) was computed by the linear trapezoidal rule for the measured concentrations during the dosing interval [27]. Other pharmacokinetic parameters determined were: observed maximum concentration (Cmax), time at which maximum concentration was observed (tmax), and initial elimination half lives (t½) for both amiodarone and N-DEA which were estimated from the elimination rate constant calculated as the negative slope of the initial log-linear regression of the elimination using a monocompartmental model. The choice of the initial elimination half-life instead of the terminal half-life was made because the terminal phases of amiodarone and its metabolite are very long and follow an asymptotic curve leading to failure in precise evaluation of these terminal half-lives.
ECG data recorded 6 h after amiodarone intake were selected for statistical analysis. This time of recording was chosen because it was the nearest to the tmax and showed the greatest changes in ECG.
Analysis of amiodarone and N-DEA concentrations was first done using an anova for repeated measures to test the main effect and the interactions between both studied periods (control and grapefruit juice) and time. The anova was performed on ranks, according to the method of Conover & Iman [28] as a result of the limited number of subjects included (n = 11) and because the studied parameters could not be assumed to have a Gaussian distribution.
Comparison of AUC, Cmax, tmax and t½ for amiodarone and N-DEA was by the Wilcoxon paired test [29] to test the two periods (control and grapefruit juice periods) (Figures 1 and 2). Electrocardiographic data at 6 h were also compared using the Wilcoxon paired test [29].
Figure 1.
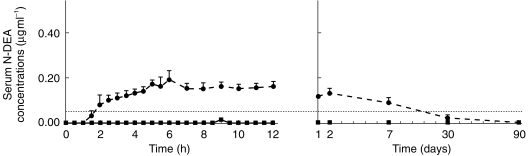
Mean serum concentration vs time graph for N-DEA in 11 subjects receiving amiodarone with grapefruit juice (solid line andfull squares) or with water (broken line and full circles). Each point is mean ± s.e.mean Throughout grapefruit juice administration, N-DEA concentrations remained below 0.05 µg ml−1 (limit of detection: dotted line).
Figure 2.
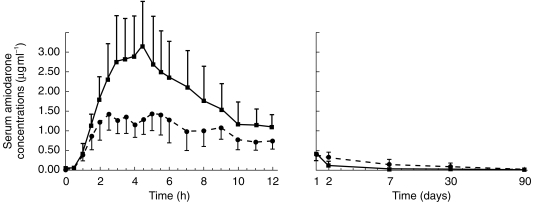
Mean serum concentration vs time graph for amiodarone in 11 subjects receiving amiodarone with grapefruit juice (solid line and full squares) or with water (broken line and full circles). Each point is mean ± s.e.mean.
A value of P < 0.05 was required to reject the null hypothesis.
Results were expressed as mean value ± s.d..
Results
Grapefruit juice totally inhibited the production of N-DEA in all subjects, with no significant trace of N-DEA being found in any of the samples (Figure 1). As compared with the control administration, grapefruit juice also significantly increased the AUC of amiodarone (Figure 2) by 50% (35.9 ± 14.3 vs 23.9 ± 11.2 µg ml−1 h, mean ± s.d.; P < 0.005; 95% CI on mean difference 6.14–17.84). Cmax increased by 84% (3.45 ± 1.7 vs 1.87 ± 0.6 µg ml−1, mean ± s.d.; P < 0.02; 95% CI on mean difference 0.67 vs 2.42) (Table 1).
Table 1.
Pharmacokinetic data of amiodarone (mean ± s.d.).
| Period 1 (water) | Period 2 (grapefruit juice) | 95% CI on mean difference | P | |
|---|---|---|---|---|
| Cmax (µg ml−1) | 1.87 ± 0.6 | 3.45 ± 1.7 | 0.67, 2.42 | < 0.02 |
| tmax (h) | 4.08 ± 1.66 | 3.63 ± 0.96 | −1.79, 0.89 | NS |
| AUC (µg ml−1 h) | 23.9 ± 11.2 | 35.9 ± 14.3 | 6.14, 17.84 | < 0.005 |
| t½ (h) | 11.56 ± 8.0 | 9.53 ± 5.5 | −5.21, −1.18 | NS |
During the water period, the ratio N-DEA/amioda-rone, which was about 0.17 6 h after amiodarone intake, reached a value of 1.00 on the 7th day.
Mean amiodarone tmax and t½ were not significantly altered (3.63 ± 0.96 vs 4.08 ± 1.66 and 9.53 ± 5.50 vs 11.56 ± 8.00 h, respectively).
No difference was observed between the two baseline periods before amiodarone intake (with water or grapefruit juice) for ECG parameters and arterial pressure data.
The greatest ECG changes were seen at 6 h postdose: during the water period, amiodarone intake caused ECG variations in all subjects with an mean increase of PR and QTc intervals by + 17.9% ± 6.6 (P < 0.001) and + 11.3% ± 8.0 (P < 0.001), respectively (Table 2). During the grapefruit juice period, however, these variations were only + 10.2% ± 8.8 (P < 0.01) and + 3.3% ± 4.5 (NS), respectively.
Table 2.
ECG alterations during period 1 (water) and period 2 (grapefruit juice) at 6 h postdose (mean ± s.d.).
| Period 1 | Period 2 | |||||
|---|---|---|---|---|---|---|
| Baseline | Amiodarone and water | % of increase | Baseline | Amiodarone and grapefruit | % of increase | |
| PR (ms) | 166.2 ± 14.2 | 196.3 ± 23.7 | 17.9 P < 0.001 | 168.2 ± 14.8 | 186.1 ± 28.8 | 10.2 P < 0.01 |
| RR (s) | 0.89 ± 0.17 | 0.895 ± 0.12 | 2.86 NS | 0.876 ± 0.15 | 0.930 ± 0.10 | 8.70 NS |
| QTc | 0.38 ± 0.03 | 0.42 ± 0.03 | 11.28 P < 0.001 | 0.39 ± 0.03 | 0.40 ± 0.02 | 3.33 NS |
For both periods, RR intervals and QRS durations were not statistically altered and systolic arterial pressure decreased slightly (−6%, NS). It should be noted nevertheless that for some of the subjects the decrease in systolic arterial pressure went as far as 25% without any clear relation to the type of amiodarone administration.
Discussion
These results show for the first time that grapefruit juice given simultaneously with amiodarone dramatically alters N-DEA production from the parent drug, with complete inhibition of the formation of this major metabolite. Because it is not possible to control every CYP3A4 modulation from xenobiotic factors, we cannot quantify the influence of such factors on intrasubject variability. Nevertheless, the use of calibrated lunches and of a 14 h fasting period preceding the evaluations should have reduced such variability.
As grapefruit juice is already known to inhibit CYP3A4 activity, these results are in agreement with previous experimental data which have suggested that N-DEA is produced mainly by the CYP3A4 in humans [9, 10]. The exact mechanism of inhibition of this isoenzyme activity remains unclear: it has been suggested that flavonoids present in grapefruit juice could be involved in this alteration [11–15, 30], but other inhibitors like furanocoumarins have also been cited [31–35]. Metabolism alteration by inhibitors, such as those found in grapefruit or citrus juices and probably in many fruit and vegetable juices including red wine [36], could well explain unexpected variations of amiodarone bioavailibility. The pronounced interindividual variability of naringin and naringenin production by intestinal bacteria after grapefruit juice intake could also provide a possible explanation for the wide range of amiodarone metabolism [37].
Since the gut wall contains CYP3A4, it is highly probable that these inhibitors act, at least in part, on the prehepatic amiodarone biotransformation [2–7]. These induced alterations of amiodarone metabolism could have clinical consequences: like amiodarone, N-DEA has class III antiarrhythmic properties, but it also has some electrophysiological properties which differ from the parent drug, notably by a higher fast sodium channel blockade and lower class IV effects [38–43]. Experimental data have shown a correlation between N-DEA plasma concentration and antiarrhythmic properties of amioda-rone [18, 19]. During chronic treatment, it has also been shown that the ratio of metabolite to parent compound is ≈ 1 at the plasma level, but go up to 4.8 at the myo-cardial level [20, 22]. These ratios could be significantly dependent on flavonoids or other CYP3 A inhibitor intake.
In spite of the lack of statistically significant arterial pressure alterations and although the extent of the ECG changes observed in these healthy volunteers was not of concern, these changes could be important in patients suffering from cardiomyopathy and/or from conduction or rhythmic disorders. As it has been shown that during the grapefruit juice period amiodarone concentrations reached values exceeding therapeutic concentrations [44, 45], this could result in significant electrophysiologic and clinical consequences linked to the parent drug. Furthermore, inhibition of N-DEA production could decrease the antiarrhythmic action of amiodarone as evoked by experimental data showing the effect of N-DEA on ventricular defibrillation threshold [18] but this could conversely decrease the arrhythmogenic effects linked to QT prolongation and thus lead to beneficial effects. These consequences are worth further investigation.
References
- 1.Singh BN, Vaughan Williams EM. The effect of amiodarone, a new antianginal drug, on cardiac muscle. Br J Pharmacol. 1970;39:657–667. doi: 10.1111/j.1476-5381.1970.tb09891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young RA, Mehendale HM. In vitro metabolism of amiodarone by rabbit and rat liver and small intestine. Drug Metab Dispos. 1986;14:423–429. [PubMed] [Google Scholar]
- 3.Flanagan RJ, Storey GCA, Holt DW, Farmer PB. Identification and measurement of desethylamiodarone in blood plasma specimens from amiodarone-treated patients. J Pharm Pharmacol. 1982;34:638–643. doi: 10.1111/j.2042-7158.1982.tb04692.x. [DOI] [PubMed] [Google Scholar]
- 4.Zipes DP, Pristowsky EN, Heger JJ. Amiodarone: electrophysiologic actions, pharmacokinetics and clinical effects. J Am Coll Cardiol. 1984;3:1059–1071. doi: 10.1016/s0735-1097(84)80367-8. [DOI] [PubMed] [Google Scholar]
- 5.Harris L, McKenna W, Rowland DE, et al. Plasma amiodarone and desethylamiodarone levels in chronic oral therapy. Circulation. 1981;64(SupplIV):263. [Google Scholar]
- 6.Holt DW, Tucker GT, Jackson PR, Storey GCA. Amiodarone pharmacokinetics. Am Heart J. 1983;106:840–847. doi: 10.1016/0002-8703(83)90006-6. [DOI] [PubMed] [Google Scholar]
- 7.Marchiset D, Bruno R, Djiane P, et al. Amiodarone and desethylamiodarone elimination kinetics following withdrawal of long-term amiodarone maintenance therapy. Biopharm Drug Dispos. 1985;6:209–215. doi: 10.1002/bdd.2510060211. [DOI] [PubMed] [Google Scholar]
- 8.Fruncillo RJ, Bernhard R, Swanson BN, Vlasses PH, Fergusson RK. Effect of phenobarbitone on the pharmacokinetics and tissue levels of amiodarone in rat. J Pharm Pharmacol. 1985;37:729–731. doi: 10.1111/j.2042-7158.1985.tb04953.x. [DOI] [PubMed] [Google Scholar]
- 9.Trivier JM, Libersa C, Belloc C, Lhermitte M. Amiodarone N-desethylamiodarone in human liver microsomes: involvement of cytochrome P450, 3A enzymes (first report) Life Sci. 1993;52:PL91–96. doi: 10.1016/0024-3205(93)90523-6. [DOI] [PubMed] [Google Scholar]
- 10.Fabre G, Julian B, Saint-auber B, Joyeux H, Berger Y. Evidence of CYP3A-mediated N-deethylation of amiodarone in human liver microsomal fractions. Drug Metab Dispos. 1993;21:978–985. [PubMed] [Google Scholar]
- 11.Bailey DG, Spence JD, Munoz C, Arnold JMO. Interaction of citrus juices with felodipine and nifedipine. Lancet. 1991;337:268–269. doi: 10.1016/0140-6736(91)90872-m. [DOI] [PubMed] [Google Scholar]
- 12.Yee GC, Stanley DL, Pessa LJ, et al. Effect of grapefruit juice on blood cyclosporin concentration. Lancet. 1995;345:955–956. doi: 10.1016/s0140-6736(95)90700-9. [DOI] [PubMed] [Google Scholar]
- 13.Ducharme MP, Provenzano R, Dehoorne-smith M, Edwards DJ. Trough concentrations of cyclosporin in blood following administration with grapefruit juice. Br J Clin Pharmacol. 1993;36:457–459. doi: 10.1111/j.1365-2125.1993.tb00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ha HR, Chen J, Leuenberger PM, Freiburghaus AU, Follath F. In vitro inhibition of midazolam and quinidine metabolism by flavonoids. Eur J Clin Pharmacol. 1995;48:367–371. doi: 10.1007/BF00194952. [DOI] [PubMed] [Google Scholar]
- 15.Honig PK, Wortham DC, Lazarev A, Cantilena LR. Grapefruit juice alters the systemic bioavailibility and cardiac repolarization of terfenadine in poor metabolizers of terfenadine. J Clin Pharmacol. 1996;36:345–351. doi: 10.1002/j.1552-4604.1996.tb04210.x. [DOI] [PubMed] [Google Scholar]
- 16.Ameer B, Weintraub RA. Drug interactions with grapefruit juice. Clin Pharmacokinet. 1997;33:103–121. doi: 10.2165/00003088-199733020-00003. [DOI] [PubMed] [Google Scholar]
- 17.Runkel M, Bourian M, Tegmeier M, et al. The role of naringin in the interaction of drugs with juices or grapefruit juices [abstract] Naunyn Schmiedeberg's Arch Pharmacol. 1997;355(Suppl 4):R123. [Google Scholar]
- 18.Talajic M, DeRodde MR, Nattel S. Comparative electrophysiologic effects of intravenous amiodarone and desethylamiodarone in dogs: evidence for clinically relevant activity of the metabolite. Circulation. 1987;75:265–271. doi: 10.1161/01.cir.75.1.265. [DOI] [PubMed] [Google Scholar]
- 19.Zhou L, Chen BP, Klugger J, Fan C, Chow MS. Effects of amiodarone and its metabolite desethylamiodarone on the ventricular defibrillation threshold. J Am Coll Cardiol. 1998;31:1672–1678. doi: 10.1016/s0735-1097(98)00160-0. [DOI] [PubMed] [Google Scholar]
- 20.Nattel S, Talajic M. Recent advances in understanding the pharmacology of amiodarone. Drugs. 1988;36:121–131. doi: 10.2165/00003495-198836020-00001. [DOI] [PubMed] [Google Scholar]
- 21.Tieleman RG, Gosselink AT, Crijns HJ, Van-gelder IC, Van--berg MP, DeKam PJ. Efficacy, safety, and determinants of conversion of atrial fibrillation and flutter with oral amiodarone. Am J Cardiol. 1997;79:53–57. doi: 10.1016/s0002-9149(96)00675-3. [DOI] [PubMed] [Google Scholar]
- 22.Plomp TA, Hauer RNW, Robles de Medina EO. Amiodarone and desethylamiodarone concentrations in plasma and tissues of surgically treated patients on long-term oral amiodarone treatment. In Vivo. 1990;4:97–100. [PubMed] [Google Scholar]
- 23.Singh BN. Antiarrhythmic actions of amiodarone: a profile of a paradoxical agent. Am J Cardiol. 1996;78(Suppl 4A):41–53. doi: 10.1016/s0002-9149(96)00452-3. [DOI] [PubMed] [Google Scholar]
- 24.Montvale: Medical Economics Company; 1997. Physician's Desk Reference. [Google Scholar]
- 25.Bazett HC. An analysis of the times relations of electrocardiograms. Heart. 1920;7:353–370. [Google Scholar]
- 26.Trivier JM, Pomery J, Libersa C, Caron J, Lhermitte M. High performance liquid chromatography assay for amiodarone N-deethylation in microsomes of rat liver. J Chromatogr. 1992;579:269–276. doi: 10.1016/0378-4347(92)80391-3. [DOI] [PubMed] [Google Scholar]
- 27.Gibaldi M. Biopharmaceutics and Clinical Pharmacokinetics. Philadelphia: Lea and Febiger; 1984. [Google Scholar]
- 28.Conover WJ, Iman RN. Analysis of covariance using the rank transformation. Biometrics. 1982;38:715–724. [PubMed] [Google Scholar]
- 29.Bethea RM, Duran BS, Bouillon TL. Statistical methods for Engineers and Scientists. 2. New York: Marcel Dekker Ed; 1985. [Google Scholar]
- 30.Moochhala SM, Loke KH, Das NP. Spectral perturbation of human microsomal cytochrome P 450 by flavonoid binding. Biochem Int. 1988;17:755–762. [PubMed] [Google Scholar]
- 31.Edwards DJ, Bernier SM. Naringin and naringenin are not the primary CYP3A inhibitors in grapefruit juice. Life Sci. 1996;59:1025–1030. doi: 10.1016/0024-3205(96)00417-1. [DOI] [PubMed] [Google Scholar]
- 32.Edwards DJ, Bellevue Fh 3rd, Woster PM. Identification of 6′-7′-dihydroxybergamottin, a cytochrome P450 inhibitor, in grapefruit juice. Drug Metabol Dispos. 1996;24:1287–1290. [PubMed] [Google Scholar]
- 33.Schmiedlin-ren P, Edwards DJ, Fitzsimmons ME, et al. Mechanisms of enhanced oral availability of CYP3A4 substrates by grapefruit constituents. Decreased enterocyte CYP3A4 concentration and mechanism-based inactivation by furanocoumarins. Drug Metab Dispos. 1997;25:1228–1233. [PubMed] [Google Scholar]
- 34.Fukuda K, Ohta T, Yamazoe Y. Grapefruit component interacting with rat and human. P450 CYP3A: possible involvement of non-flavonoid components in drug interaction. Biol Pharm Bull. 1997;20:560–564. doi: 10.1248/bpb.20.560. [DOI] [PubMed] [Google Scholar]
- 35.He K, Iyer KR, Hayes RN, Sinz MW, Woolf TF. Inactivation of cytochrome P450 3A4 by bergamottin, a component of grapefruit juice. Chem Res Toxicol. 1998;11:252–269. doi: 10.1021/tx970192k. [DOI] [PubMed] [Google Scholar]
- 36.Chan WK, Nguyen LT, Miller VP, Harris RZ. Mechanism-based inactivation of human cytochrome P450 3A4 by grapefruit juice and red wine. Life Sci. 1998;62:PL135–PL142. doi: 10.1016/s0024-3205(98)00013-7. [DOI] [PubMed] [Google Scholar]
- 37.Fuhr U, Kummert AL. The fate of naringin in humans: a key to grapefruit juice–drug interactions? Clin Pharmacol Ther. 1995;58:365–373. doi: 10.1016/0009-9236(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 38.Wellens HJJ, Brugada H, Abdollah H, Dassen WRI. A comparison of the electrophysiologic effects of intravenous and oral amiodarone in the same patient. Circulation. 1984;69:120–124. doi: 10.1161/01.cir.69.1.120. [DOI] [PubMed] [Google Scholar]
- 39.Morady F, DiCarlo LA, Krol RB, et al. Acute and chronic effects of amiodarone on ventricular refractoriness intraventricular conduction and ventricular tachycardia induction. J Am Coll Cardiol. 1986;7:148–157. doi: 10.1016/s0735-1097(86)80273-x. [DOI] [PubMed] [Google Scholar]
- 40.Shenasa M, Denker S, Mahmud R, et al. Effect of amiodarone on conduction and refractoriness of the His-Purkinje system in the human heart. J Am Coll Cardiol. 1984;4:105–110. doi: 10.1016/s0735-1097(84)80326-5. [DOI] [PubMed] [Google Scholar]
- 41.Torres V, Tepper D, Flowers D, et al. QT prolongation and the antiarrhythmic efficacy of amiodarone. J Am Coll Cardiol. 1986;7:142–147. doi: 10.1016/s0735-1097(86)80272-8. [DOI] [PubMed] [Google Scholar]
- 42.Connolly SJ, Latini R, Kates RE. Pharmacodynamics of intravenous amiodarone in the dog. J Cardiovasc Pharmacol. 1984;6:531–535. doi: 10.1097/00005344-198405000-00023. [DOI] [PubMed] [Google Scholar]
- 43.Ikeda T, Nadamanee K, Kannan R, Singh BN. Electrophysiologic effects of amiodarone: experimental and clinical observation relative to serum and tissue drug concentration. Am Heart J. 1984;108:890–898. doi: 10.1016/0002-8703(84)90451-4. [DOI] [PubMed] [Google Scholar]
- 44.Staubli M, Bircher J, Galeazzi RL, Remund H, Stunder H. Serum concentrations of amiodarone during long term therapy: relation to dose, efficacy and toxicity. Eur J Clin Pharmacol. 1983;24:485–494. doi: 10.1007/BF00609891. [DOI] [PubMed] [Google Scholar]
- 45.Rotmensch HH, Belhassen B, Swanson BN, et al. Steady-state serum amiodarone concentrations: relationships with antiarrhythmic efficacy and toxicity. Ann Intern Med. 1984;101:462–469. doi: 10.7326/0003-4819-101-4-462. [DOI] [PubMed] [Google Scholar]


