Abstract
Aims
The objective was to explore differences in lipid-lowering drug (LLD) prescribing in Italy and Denmark.
Methods
We used two geographical areas with computerized drug prescription records in defined populations, one in Funen, Denmark with 500000 inhabitants, the other in Bologna, Italy with 400000 inhabitants. Prescriptions for patients who had purchased a LLD from 1994 until 1996 were retrieved as well as coprescriptions of antidiabetic and cardiovascular drugs as markers for diabetes and cardiovascular disease. Only patients surviving and remaining in the area were included. Compliance was defined as percentage of DDDs purchased divided by the number of days within the time window. The limit between good and poor compliance was set at 82%.
Results
In Bologna, LLD consumption measured in DDD increased by 41% and in Funen by 129%. Annual prevalence increased from 36.9 to 46.3 users/1000 inhabitants from 1994 to 1996 and from 3.2 to 6.6 users/1000 inhabitants in Bologna and Funen, respectively. From 1995 to 1996, the incidence of use decreased slightly in Bologna from 19.3 to 18.8/1000 inhabitants/year, whereas in Funen the incidence increased from 1.8 to 2.3/1000 inhabitants/year. In Bologna 48% and in Funen 91% of users persisted with treatment for 2 years or longer. In Bologna, 7% and in Funen 45% were good compliers. In Bologna, 61% and in Funen, 72% received other drugs indicating cardiovascular or diabetic comorbidity.
Conclusions
Patterns of use differed substantially between the two areas. In contrast with Funen, where long-term use was common, Bologna LLD use was sporadic. Based on a higher rate of coprescription, LLDs seemed to be used for secondary prevention to a higher extent in Funen than in Bologna. In Funen it appeared that the correct patients, but an insufficient number of them, were being treated adequately according to guidelines. The higher discontinuation rate of lipid lowering drugs in the Bologna area indicates that a large proportion of patients use these drugs for too short a period of time to benefit from treatment. Since society’s health care resources are limited it is difficult to justify public funding of these medications without at the same time giving appropriate attention to these problems.
Keywords: individualized prescription databases, international comparison, lipid-lowering drug utilization, persistence
Introduction
The rising costs of pharmaceuticals is of great concern and different methods have been used to combat this development. With a limited health budget, priority decisions have to be made by politicians. The aim of health care professionals is to make sure that the resources provided are used in the most effective way. Medications are no exception in this regard. New lipid lowering drugs (LLD) – HMG CoA reductase inhibitors, also referred to as statins – were introduced in the late 1980s and constituted a breakthrough in the management of hypercholesterolaemia. In a previous comparison of the use of LLDs in five countries in 1990–94, it was found that usage was double in Australia and Italy compared with three of the Nordic countries [1]. Additionally, it was found that a restriction in the reimbursement status in Italy in 1994 almost halved the use in that year. The pattern of use in Italy and Sweden was very different, with much higher use in Italian women than in men, with a reverse pattern in Sweden. At that time key studies such as 4S (secondary prevention of myocardial infarction) [2] and West of Scotland (primary prevention) [3] had not yet influenced the prescribing of LLDs. Several studies outside Europe have demonstrated failure to continue long-term treatment with LLDs [4–6]. In one study a high 1 year discontinuation rate was found, and in the other, about half of the surviving cohort of patients older than 65 years had stopped using LLDs after 5 years [5, 6].
The primary purpose of the present study was to explore differences in the prescribing of LLDs demonstrated in the previous paper [1] by analysing on an individual level the prescribing patterns in two population-based prescription databases, one in Funen, Denmark representing a Nordic country (with an age and sex pattern of use similar to the Swedish as such data were not available in Sweden), the other one in Bologna, Italy.
We were specifically interested in how LLDs were prescribed in the two defined populations with regard to prevalence and incidence, in different age and sex strata, according to different risk profiles (comorbidity with diabetes and cardiovascular diseases), and if there were any differences with regard to persistence and continuity of treatment with these medications.
Methods
Setting
Drug prescription data were retrieved from the Odense Pharmacoepidemiologic Database (Denmark) [7] and the Emilia Romagna Health Authority Database (Italy) [8]. Both databases provide the following information for each reimbursed prescription: identification of the dispensed product according to the ATC-classification [9], number of packages and number of Defined Daily Doses (DDD) dispensed, code of the prescriber, anonymous code of the patient and date of prescription. In both databases, the patient code allows the reconstruction of each individual’s drug history without identification of the individual.
In the present study, we retrieved the 1994–96 data on filled prescriptions from the County of Funen, Denmark (467695 inhabitants in 1995 [10]), and from the town of Bologna, Italy (386935 inhabitants in 1995 [11]).
The study population was restricted to individuals permanently living in the areas; those who had moved or died during the study period were excluded from the analyses. The study cohort comprised 420731 persons in Funen and 334935 persons in Bologna. In the following sections, figures from Funen and Bologna refer to these selected cohorts.
Data on overall LLD utilization in previous years and in other countries were retrieved from the previous study [1], from other sources [12–14] and from our prescription databases.
Ethics
Information from both regions consisted exclusively of anonymised data. Therefore, the project needed no approval by the regional scientific ethical committees. For the Danish part, the Danish Registry Board approved the project and the regional scientific ethics committee was notified.
Reimbursement
In Italy, several restrictions in reimbursement rules have been made since the early nineties; in 1993 this lead to a decrease in LLD consumption [1]. In 1994, a reimbursement list was issued where almost all drugs (including LLDs) were without any copayment by the patient apart from a fixed fee of Euro 1.55 per prescription. Owing to this, the average copayment fell from 20 to 25% to 10% of the gross drug bill of the Italian National Health Service (NHS). LLDs were, however, free-of-charge only for patients with familial hyperlipidaemias with high blood levels of cholesterol or triglycerides, and fully paid for by the others. Only by the end of 1997, full reimbursement of simvastatin and pravastatin was extended to the secondary prevention of myocardial infarction in patients with a total cholesterol of greater than 5.4 mmol l−1.
In Denmark the reimbursement rules remained unchanged throughout the study period. LLDs were reimbursed to the extent of 75% of the cost after individual application from the patient’s physician to the Danish National Board of Health under the following conditions: (1) known ischaemic heart disease, e.g. former myocardial infarction, angina pectoris and serum cholesterol above 5.4 mmol l−1 in spite of diet; (2) inherited hyperlipidaemia; and (3) high serum cholesterol and concurrent important risk factors for developing ischaemic heart disease (family disposition or diabetes mellitus). Furthermore, following publication of the 4S study [2] it was pointed out by the Danish National Board of Health in a letter to all physicians in January 1995 that reimbursement could not be obtained for the purpose of primary prevention.
Age and sex breakdown of drug use
The extent of LLD use (ATC code C10, formerly B04) was analysed as the number of DDD/1000 inhabitants/day, according to the ATC/DDD methodology [9], and as the annual prevalence of use (number of individuals receiving at least one prescription in 1 year per 1000 inhabitants). Data were broken down by sex and age classes in ways that ensured comparability with previous studies.
Waiting-time distribution and incidence
The waiting time distribution as described by Hallas et al. [15] charts for drug users their first prescription presented within a specified time window. For drugs used for chronic treatment, most current users will be captured at the beginning of the window. After some months (the run-in period), incident users will dominate the graph. In the present study, the waiting-time distributions were generated for LLD users according to sex and age below or above 65 years of age on the basis of both Italian and Danish data. It was used mainly for determining the length of the run-in period.
Incidence was calculated as the number of new users per 1000 inhabitants per year after having determined the run-in period. Data from Bologna from July 1995 concerning new users were missing. Therefore, the sex-and age specific incidence was for this month estimated as the average incidence for the rest of 1995.
Co-prescription of other drugs as indicator of risk factors
The presence of cardiovascular disease or risk factors was assessed by identifying the LLD-treated individuals who also received in the same year at least one prescription of one or more drugs belonging to the following ATC groups: A10 (insulins and oral antidiabetics) as a marker for diabetes and B01 (antithrombotics), C01 (cardiac glycosides, antiarrhythmics, nitrates), C02 (antihypertensives), C03 (diuretics), C07 (beta-blockers), C08 (calcium antagonists) and C09 (ACE inhibitors) as markers for cardiovascular disease.
Persistence and continuity of lipid-lowering drugs
Persistence of LLD use during 1994–95–1996 was assessed by identifying all individuals treated in 1994 who were still on a lipid-lowering drug in 1996. In a more detailed analysis, the total number of DDDs received by each individual in 1 year was evaluated. This number reflects both persistence of treatment and within-treatment adherence to the recommended regimen (continuity).
Continuity was further investigated under the assumption that 1 DDD represents an average day of treatment. A fully compliant long-term user should grossly receive 365 DDDs in 1 year. The limit between good and poor compliance has previously been defined as 80% [5]. For practical purposes, we put the limit at 300 DDD/year (i.e. 82%). This analysis was performed in the subgroups of individuals who received one or more prescriptions of LLDs in the first month (January) of each year. This was done in order to capture prevalent users in three complete 1 year periods.
Statistical methods
Univariate analyses were used to estimate the percentage and 95% confidence intervals of LLD users in 1994 still under treatment in 1996 according to sex, age group and comedication. The Chi-square test was used to evaluate differences between categories. A multivariate model was used to describe the relative influence of the variables on each other. STATA version 6.0 was used for all of the analyses mentioned.
Results
Overall use
In the Emilia Romagna Region (Italy), consumption increased by 37% from 6.7 DDD per 1000 inhabitants per day (TID) in 1994 to 9.2 DDD/TID in 1996. Statins made up 72% of the use, an increase of 5% since 1994. In Bologna (part of the Emilia Romagna Region), use was generally about 30% higher than in Italy as a whole. In Funen, Denmark, consumption increased from 2.1 to 4.8 DDD/TID. The share of statin use increased from 81.8% to 91.3%. In 1996, use was slightly higher in Funen compared with the Danish average [13].
In Bologna and in Funen use of LLDs increased during the study period by 41% and 129%, respectively. Bologna LLD prescribing exceeded Funen by a factor of 3–4 in all 3 years. This difference was found to be more pronounced in patients over the age of 65 years and in females.
Table 1 shows the number and percentage of users for each region specified by LLD group as average of the 3 years and the dose officially recommended in Denmark [16].
Table 1.
Average number and percentage of users over the years 1994–96 specified by LLD group in Bologna, Italy and Funen, Denmark. Doses recommended in official drug catalogues and corresponding number of DDD as of 1998 in Denmark [9, 16].
| Bologna | Funen | Recommended dose | |||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | mg | DDD | ||
| C10AA01 | Simvastatin | 6721 | 43.4 | 915 | 42.8 | 10 | 0.67 |
| 10–40* | 0.67–2.67* | ||||||
| C10AA02 | Lovastatin | 517 | 24.2 | 30 | 1.00 | ||
| C10AA03 | Pravastatin | 2383 | 15.4 | 314 | 14.7 | 15–20 | 0.75–1.00 |
| C10AA04 | Fluvastatin | 593 | 3.7 | 57 | 2.7 | 40 | 1.00 |
| C10AA | STATINS | 9696 | 62.5 | 1803 | 84.4 | ||
| C10AB01 | Clofibrate | 7 | 0.3 | 2000 | 1.00 | ||
| C10AB02 | Bezafibrate | 1696 | 10.9 | 95 | 4.4 | 600 | 1.00 |
| C10AB04 | Gemfibrozil | 3022 | 19.6 | 96 | 4.5 | 1200 | 1.00 |
| C10AB05 | Fenofibrate | 388 | 2.5 | 300* | 1.00** | ||
| C10AB06 | Simfibrate | 1 | 0.0 | 750–1500* | 0.75–1.50** | ||
| C10AB | FIBRATES | 5107 | 33.0 | 198 | 9.3 | ||
| C10AC01 | Colestyramin | 488 | 3.2 | 99 | 4.6 | 16000 | 1.14 |
| C10AC02 | Colestipol | 22 | 1.0 | 20000 | 1.00 | ||
| C10AC03 | Detaxtran | 181 | 1.2 | 2000–3000* | 0.80–1.20** | ||
| C10AC | RESINS | 669 | 4.4 | 121 | 5.6 | ||
| C10AD05 | Nicotinyl alcohol | 4 | 0.2 | 900 | 0.96 | ||
| C10AD06 | Acipimox | 10 | 0.5 | 500 | 1.00 | ||
| C10AD | OTHERS | 14 | 0.7 | ||||
| C10 | 15473 | 100.0 | 2136 | 100.0 |
Italian recommendations.
DDD created by DURG-Italia, as no information was available from the WHO.
Annual prevalence
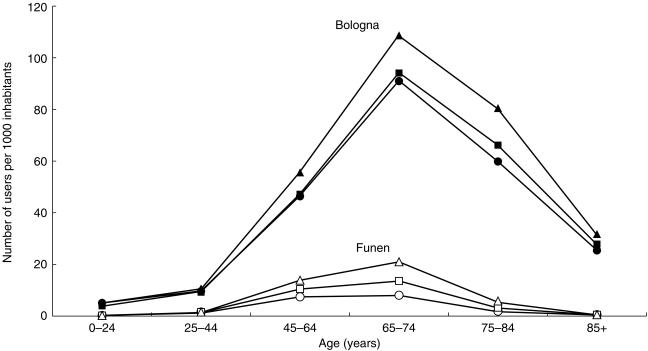
In Bologna, the annual prevalence of LLD treatment was on average about eight times higher than in Funen (36.9 vs 3.2 users/1000 inhabitants/year in 1994, 38.7 vs 4.7 in 1995 and 46.3 vs 6.6 in 1996). Figure 1 shows the development in age specific 1 year prevalence for the two regions. Trends were similar for females and males in both countries (data not shown).
Figure 1.
Age specific 1 year prevalence of LLD use in Bologna (closed symbols) and Funen (open symbols) 1994–1996. •, ○ 1994; ▪, □ 1995; ▵, ▴, 1996.
In Funen, for all 3 years analysed, prevalence turned out to be highest in the 65 - 74 year age group for both males and females, with the exception of 1994 where prevalence for males was marginally higher in the 45 - 64 year age group. The prevalence in those below 65 years of age increased from 5.6 to 13.2 users per 1000 inhabitants. The overall female:male ratio was 0.7–0.8. In Bologna, the 1 year prevalence was also higher in the 65 + age group (78.7–104.3 users per 1000 inhabitants) in all 3 years, being highest in the 65 - 74 year age group. In contrast to Funen, Bologna had a higher female:male ratio of 1.4–1.5.
In Bologna, a considerable percentage of people treated were 75 years or older and some were even over 85 years old, whereas in Funen, the number of people treated over the age of 75 years was very low, while no patients were over 85 years old.
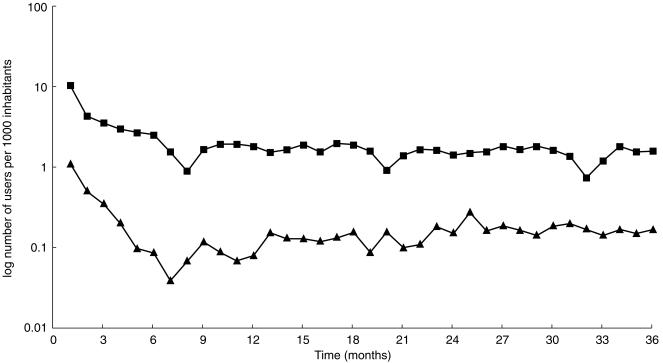
Incidence
The waiting time distribution, showing similar patterns for the sex and age groups described, justifies the use of a run-in period of 12 months before a steady level of new users per month is reached (Figure 2). In Bologna the incidence decreased slightly from 19.3 new users/1000 inhabitants/year in 1995 to 18.3 in 1996, the figure being higher for females (21.5–19.9) than for males (16.8–16.5), and higher for the age group above 65 (33.8–29.4) than for the lower age group (14.7–14.8). In Funen, the overall incidence increased from 1.8 new users/1000 inhabitants/year in 1995 to 2.3 in 1996, the figure being higher for males (2.2–2.6) than for females (1.4–2.0). For the age group below 65 years, incidence increased from 1.4 to 1.7, and for the age group above 65 years incidence increased from 4.0 to 5.0 new users/1000 inhabitants/year.
Figure 2.
Waiting time distribution for LLD users, Funen (▴) and Bologna (▪) 1994–1996. Users were counted the first time they appeared in the time window. In the first 6 months, the curve represents prevalent users, and after 12 months, it represents incident users. Note that the scale for Funen figures is lower than that for Bologna figures by a factor of 10.
Co-prescription
In Bologna, the number of persons who were cotreated with medications indicating cardiovascular or diabetic comorbidity increased from 57.5% to 62.4% during the study period. Of these, about 62% were females and 74% were greater than 65 years old. In Funen, the number of cotreated persons increased from 68.5% to 76.4% during the period and of these 46% were females and 85% were equal to or greater than 65 years of age.
Persistence and continuity of LLD use
In Bologna, only 47.5% of those who obtained a LLD in 1994 were still receiving this medication in 1996. In Funen, 90.8% of people receiving LLDs in 1994 still had at least one prescription in 1996. When stratifying by age, sex and comedications as predictors of cardiovascular risk factors, none of the subgroups were below 88% in Funen, but all subgroups were below 53% in Bologna (data not shown). In both areas there was a significantly higher degree of persistence of LLD usage in people receiving coprescription of antidiabetic and/or cardiovascular drugs (chi-square P < 0.001 in Bologna, P < 0.05 in Funen) compared with those without such comedications. In Bologna, but not in Funen, a significantly (chi-square P < 0.001) higher degree of persistence of LLD usage was observed in women compared with men and in the over 65 year old vs younger patients. After stratification of the Danish data into narrower age groups, age was also shown to have an effect on persistent usage: in age groups between 45 and 75 years, persistence was high (92%) whereas only 81% of patients below 45 years and above 75 years of age were persistent users. A subgroup consisting of males over the age of 75 years had the lowest persistence (60%). In a multivariate analysis of the Danish data, the effect of comedication and age (the narrower age groups) remained statistically significant even after adjusting each variable for each other and for sex. In a similar analysis of the Italian data, the youngest (11.4%) and the oldest females (43.0%) turned out to be least persistent and in males, the lowest percentage of persistent users (21.8%) was found in the youngest age group. Multivariate analysis of the Italian data showed that sex as well as age (below or above 65 years or narrower age groups) and comedication remained significant, and that neither of these factors could alone account for the pattern seen.
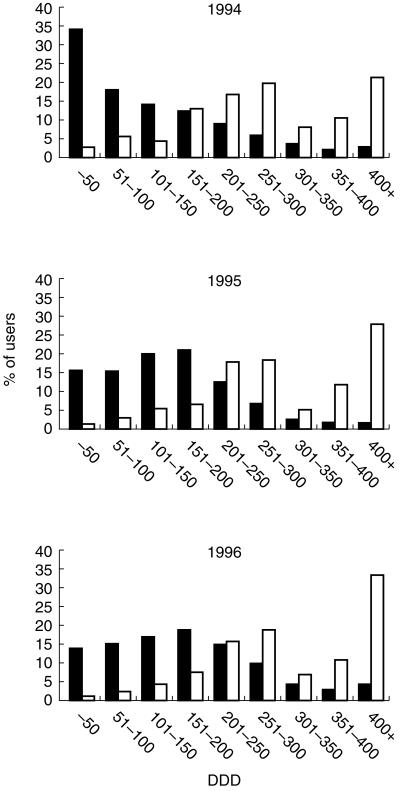
The average number of DDD per treated person in Bologna was 87 in 1994, 104 in 1995 and 105 in 1996, whereas in Funen the corresponding figures were 254, 261 and 290, respectively, indicating that continuity of treatment was better in Funen compared with Bologna. Figure 3 shows the proportion of users for both areas according to their use in DDD/year. In all 3 years the number of users peaked at 200–300 (average 250) and 400 + DDD/year in Funen. In Bologna the number of users peaked at 50 or less DDD in 1994 and at 151–200 DDD in 1995 and 1996. According to our definition only 7.3% of users in Bologna in the 3 years considered could be categorized as being good compliers (i.e. 82% continuity or better), while in Funen, 45.2% of users were good compliers. For patients aged 65 years or more, the figures were 7.6% and 38.8%, respectively.
Figure 3.
Distribution of annual LLD use (DDD/user/year) for selected populations prescribed a LLD in January for each of the years 1994–95–1996 in Bologna (▪) (n = 3550–3434–4280) and Funen (□) (n = 517–635–1024).
Discussion
Use of LLDs in Denmark according to the age and sex profile corresponded to the pattern seen earlier in Sweden [1], while the pattern in Bologna remained the same with a higher usage in women, particularly in those above 65 years of age when compared with Denmark/Sweden.
In Bologna, consumption and prevalence increased, while incidence (using a 1 year run-in period) decreased during the study period, indicating that an increasing proportion of LLDs is consumed by prevalent users. This suggests better compliance over time, or that only the most motivated patients remain on therapy. In Funen, consumption and prevalence as well as incidence of use increased threefold from a very low level compared with other countries. Despite this, the number of patients being treated was still only about 10% of the expected. In 1996, Danish cardiologists estimated that the prevalence of patients who needed LLD treatment was 5% of the total population [17]. In the present study, we found that only 0.5% were actually being treated, in spite of the fact that the 4S study had a notable effect on overall use in Denmark [18]. Future research should explore why so few patients actually start LLD use in Denmark and why knowledge from the controlled trials is not implemented.
The distribution of users according to individual use in DDD/year (Figure 3) and the results concerning persistent usage (Table 2) indicate that there is a higher degree of sporadic use in Bologna compared with Funen. According to our definition, very few LLD users in Bologna were good compliers (7.3%), while in Funen 45.2% were good compliers. In a study from Australia (top LLD using country [1]), 60% of the patients failed to collect prescription refills over 12 months [4]. The predominant reasons for discontinuation were: a) patient unconvinced about need for treatment, b) poor efficacy and c) adverse events. The reason for the apparent worse compliance in Italy is presently unknown, but it is possible that more patients were prescribed a LLD without proper justification and in many of those the drug was stopped fairly soon. As a speculative explanation, we could invoke an effect of the verbal promotional messages given by pharmaceutical representatives in the recent past to doctors according to which the best regimen was as represented by 3 month courses of treatment in patients with a cholesterol level just above 5.2 mmol l−1.
Table 2.
Persistence with lipid lowering drugs 1994–96 in Funen and Bologna in total and in selected strata. Co-prescription with antidiabetic or cardiovascular drugs.
| Funen | Bologna | |||||
|---|---|---|---|---|---|---|
| Persistent | Total | Persistent | Total | |||
| Variable | n | n | % Persistent (95% CI) | n | n | % Persistent (95% CI) |
| Total | 1204 | 1326 | 90,8 (89,1–92,3) | 5870 | 12360 | 47,5 (46,6–48,4) |
| Females | 552 | 604 | 91,4 (88,9–93,5) | 3861 | 7844 | 49,2 (48,1–50,3) |
| Males | 652 | 722 | 90,3 (87,9–92,4) | 2009 | 4616 | 43,5 (42,1–45,0) |
| 0–44 years | 122 | 151 | 80,8 (73,6–86,7) | 200 | 1162 | 17,2 (15,1–19,5) |
| 45–64 years | 750 | 809 | 92,7 (90,7–94,4) | 2356 | 4848 | 48,6 (47,2–50,0) |
| 65–74 years | 298 | 324 | 92,0 (88,5–94,7) | 2618 | 4680 | 55,9 (54,5–57,4) |
| 75 + years | 34 | 42 | 81,0 (65,9–91,4) | 696 | 1670 | 41,7 (39,3–44,1) |
| −Co-prescription | 462 | 524 | 88,2 (85,1–90,8) | 2200 | 5259 | 41,8 (40,5–43,2) |
| + Co-prescription | 742 | 802 | 92,5 (90,5–94,2) | 3670 | 7101 | 51,7 (50,5–52,9) |
The use of individualized prescription databases as opposed to wholesale statistics enabled us to describe LLD utilization in large populations in a natural setting. We were able to describe duration of treatments as well as the amount of drug collected by each individual. In both these databases the amount of drugs purchased by the patients was expressed as the number of Defined Daily Doses (DDD), a useful technical unit for comparative drug utilization studies [1, 19]. A disadvantage of our prescription databases is that they do not hold information on prescribed daily dose (PDD), which measure together with a count of the number of doses ingested by patients within a particular time frame would be the ideal for compliance studies. Since such data are not available, we have to rely on approximations.
A weakness of using DDD as a measure in compliance calculations is that one DDD may be a poor reflection of the actual daily use of a certain drug [20]. In Bologna as well as in Funen, 43% of LLD users used a statin assigned 0.67 DDD as the lowest suggested daily dose (Table 1). Thus, having used one DDD in calculations of continuity, we have clearly underestimated compliance in both regions. The variations in DDD for the various drugs cannot, however, completely account for the six-fold higher compliance in Funen. If serum cholesterol was generally lower in Bologna this would probably lead to prescribing of lower than recommended doses. However, as the mortality rate from cardiovascular diseases is slightly higher in Bologna than in Funen (5.51 vs 4.82/1000 inhabitants/year) this is unlikely to be the reason.
A more relevant measure of daily use for statins may be one tablet, as tablets come in different strengths. For example, the recommended daily dose of simvastatin in Denmark is 10 mg [16], which corresponds to 0.67 DDD. According to this, 100% compliance would imply an annual use of 245 DDD. Using this method, 27.7% (CI95%: 21.5–35.9) of Italians and 81.0% (CI95%: 75.2–84.9) of Danes would be good compliers. While these figures may better reflect the compliance in these regions, it does not change the fact that there are marked differences between the regions.
Not surprisingly, the status of reimbursement regimens tends to influence consumption of LLDs to a considerable extent. In Italy after changing full reimbursement (up until 1993) to copayment for prescriptions exceeding 16 per year, consumption decreased from 11.5 to 10.4 DDD/1000 inhabitants/day [1]. Again, after restricting the fully reimbursed part of LLDs to the indication of familial hyperlipidemia with high levels of cholesterol or triglycerides and full patient’s payment for other indications, consumption further decreased to 6.7 DDD/1000 inhabitants/day. The influence of the restrictions was, however, rather transient, as consumption increased again to reach 9.2 (12.5 in Bologna) DDD/1000 inhabitants/day in 1996 without any change in the reimbursement rules. In Denmark, consumption has been low compared with other countries. Reimbursement (75%) – remaining unchanged until December 1998 – has been restricted to secondary prevention of ischaemic heart disease, failure of dietary intervention and only after individual application from the patient’s physician. In Italy, restrictions with reimbursement rules showed only temporary impact on consumption, a pattern also seen in other countries [21]. It is possible that the low Danish consumption is due to the rather restrictive reimbursement rules, and also that consumption could increase if the restrictions were eased. As of mid December 1998, reimbursement rules have been eased in Denmark, so that no individual application is needed for the use of LLDs for secondary prevention, although it is still needed for primary prevention. In the years, to come consumption of LLDs will be followed in order to monitor the effect of this change.
As to persistence of usage, our follow-up period was 2 years, i.e. 3 years shorter than in the North American study [6]. In that study approximately 50% of patients aged over 65 years were still on the drugs after 5 years. The Italian cohort of similar age had already reached this level after 2 years with 52.2% persistent users, whereas from the Danish cohort, 90.7% were still on the drugs. Compared with the North American study, the percentage of continuous users in Italy was extremely low (7.6% vs 34.1% in New Jersey and 38.9% in Quebec), while the Danish figures were similar (38.8%).
According to our definition of the study cohort, only patients staying alive during the whole study period were included. This selection may have biased our results leading to overestimation of compliance and underestimation of prevalence as patients treated with LLDs are supposed to live longer. Considering this fact, the Italian results may differ even more from the North American results.
In the previously published international comparison of LLD use, Sweden was one of the participating countries [1]. The level of LLD use in Sweden (12.6 DDD/TID) was almost three times the use in Funen, Denmark (4.8 DDD/TID), and corresponds to the use in Bologna, Italy (12.7 DDD/TID). Whether the adherence among Swedish patients is as low as in Bologna, or similar to that reported from Australia [4] is, however, not known as corresponding individual prescription data are lacking.
In conclusion, the present study found the same differences in age-and sex-specific prescription patterns between the Emilia Romagna Region and Funen as previously published for another Nordic country [1]. The Danish LLD utilization pattern was comparable to the previously described Swedish pattern, although Danish consumption in DDD/TID was just about 1/3 of the Swedish consumption. Contrary to the use in Denmark, Italian use was characterized by high consumption, prevalence and incidence (although decreasing). Female use dominated in Italy. Two-year persistence with LLD use was only about half the Danish level, lowest in the youngest age groups. Causes for the rather sporadic use in Italy are unclear. Compared with North American results, persistence and continuity of LLD use in Funen was similar, while in Bologna, figures showed considerably lower values. The higher discontinuation rate of lipid lowering drugs in the Bologna area indicates that a large proportion of patients use these drugs for too short a period of time to benefit from the treatment, a pattern also seen in Australia [4]. Additionally, patients may have been prescribed a lipid-lowering drug without proper justification. Since society’s health care resources are limited, it is difficult to justify public funding of these medications without at the same time giving appropriate attention to these problems.
Acknowledgments
The authors wish to thank Cristina Castelvetri and Rosa Rizzo for the computer analysis of Italian data and Penelope North-Lewis for proof-reading of the manuscript. The Italian part of the study was supported by a grant of the Italian Ministry of University and the Danish part by The Danish Medical Research Council (grants no. 9501767 and 9700814) and by the Danish Ministry of Research (grant no. 115–1996).
References
- 1.Magrini N, Einarson T, Vaccheri A, McManus P, Montanaro N, Bergman U. Use of lipid-lowering drugs from. 1990 to 1994: an international comparison among Australia, Finland, Italy (Emilia Romagna Region), Norway and Sweden. Eur J Clin Pharmacol. 1997;53:185–189. doi: 10.1007/s002280050360. [DOI] [PubMed] [Google Scholar]
- 2.Scandinavian Simvastatin Survival Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 3.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 4.Simons LA, Levis G, Simons J. Apparent discontinuation rates in patients prescribed lipid-lowering drugs. Med J Aust. 1996;164:208–211. doi: 10.5694/j.1326-5377.1996.tb94138.x. [DOI] [PubMed] [Google Scholar]
- 5.Andrade SE, Walker A, Gottlieb LK, et al. Discontinuation of antihyperlipidemic drugs – do rates reported in clinical trials reflect rates in primary care settings? N Engl J Med. 1995;332:1125–1131. doi: 10.1056/NEJM199504273321703. [DOI] [PubMed] [Google Scholar]
- 6.Avorn J, Monette J, Lacour A, et al. Persistence of use of lipid-lowering medications: a cross-national study. JAMA. 1998;279:1458–1462. doi: 10.1001/jama.279.18.1458. [DOI] [PubMed] [Google Scholar]
- 7.Gaist D, Sorensen HT, Hallas J. The Danish prescription registries. Dan Med Bull. 1997;44:445–448. [PubMed] [Google Scholar]
- 8.Montanaro N, Vaccheri A, Magrini N, Battilana M. FARMAGUIDA. a databank for the analysis of the Italian drug market and drug utilization in general practice. Eur J Clin Pharmacol. 1992;42:395–399. doi: 10.1007/BF00280125. [DOI] [PubMed] [Google Scholar]
- 9. Atc index with DDDs.WHO Collaborating Centre for Drug Statistics Methodology, Oslo, Norway,
- 10.Danmarks Statistik. Population in municipalities. Copenhagen 1995,
- 11.Web site of the Regional Government of Emilia Romagna by February. 1999. www.regione.emilia-romagna.it/sas/statistica/
- 12.Nordic Council on Medicines. Nordic Statistics on Medicines, Uppsala, Sweden. Web site by February 1999: www.nln.se.
- 13.Lægemiddelstatistik Danmark 1994–1996. Lægemiddelstyrelsen, Copenhagen 1995–1997.
- 14.Commonwealth Department of Human Services, Health. Australian Statistics on Medicines 1996. Canberra: Government Publishing Service; 1997. [Google Scholar]
- 15.Hallas J, Gaist D, Bjerrum L. The waiting time distribution as a graphical approach to epidemiologic measures of drug utilization. Epidemiology. 1997;8:666–670. doi: 10.1097/00001648-199710000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Aldershvile J, Hansen MS, Kampmann JP, Vej-hansen B. 1998. Laegeforeningens Medicinfortegnelse 1998. Copenhagen 213. [Google Scholar]
- 17.Medicintilskudsnævnets ekspertgruppe vedr. kolesterolsænkende lægemidler, København1996.
- 18.Larsen J, Andersen M, Kragstrup J, Gram LF. Lipid lowering drugs. Consumption and compliance 1993 - 1998 in Funen County, Denmark. (manuscript).
- 19.Einarson T, Bergman U, Wiholm B-E. Principles and practice of pharmacoepidemiology. In: Speight T, Holford N, editors. Drug treatment. 4th. Auckland: Adis International Ltd: 1997; 371–392.; [Google Scholar]
- 20.Illingworth DR, Erkelens DW, Keller U, Thompson GR, Tikkanen MJ. Defined daily doses in relation to hypolipidaemic efficacy of lovastatin, pravastatin, and simvastatin. Lancet. 1994;343:1554–1555. doi: 10.1016/s0140-6736(94)92945-9. [DOI] [PubMed] [Google Scholar]
- 21.Martikainen J, Klaukka T, Reunanen A, Peura S, Wahlroos H. Recent trends in the consumption of lipid-lowering drugs in Finland. J Clin Epidemiol. 1996;49:1453–1457. doi: 10.1016/s0895-4356(96)00267-3. [DOI] [PubMed] [Google Scholar]