Abstract
Aims
To assess the sensitivity of 103 Plasmodium falciparum isolates to a combination of lumefantrine (benflumetol) and artemether (CGP 56697), with the objective of determining a correlation between in vitro drug sensitivity and therapeutic outcome.
Methods
Patients suffered from uncomplicated falciparum malaria and came from areas of Thailand affected by multidrug resistance. CGP 56697 was given in the form of tablets containing 20 mg artemether and 120 mg lumefantrine. The standard dose regimen, 4 doses of 4 tablets over 48 h, was compared with two lower dose regimens (4 × 2 tablets and 3 × 4 tablets).
Results
The parasites showed high resistance to chloroquine, fairly advanced resistance to mefloquine and compromised sensitivity to quinine. Sensitivity to artemisinin and lumefantrine prior to treatment was similar in all treatment groups. The 4 × 4 tablet regimen was more effective than the other regimens in coping with infections with relatively low sensitivity to artemisinin and/or lumefantrine. The EC90 for artemisinin is an important determinant of treatment success. Parasite density at the start of treatment was identified as another critical predictor of treatment outcome.
Conclusions
The results indicate that parasite exposure to the drugs may have been inadequate and/or too short in the cases of treatment failure, particularly marked in the lower dose regimens. This could probably be remedied by expanding the dose regimen in areas affected by multidrug resistance and in the case of relatively high parasitaemia.
Keywords: artemether, artemisinin, benflumetol, drug response, lumefantrine, Plasmodium falciparum
Introduction
Artemether is the semisynthetic methyl ether of artemisinin (Figure 1a), a natural sesquiterpene lactone extracted from the annual composite plant Artemisia annua L [1]. The parent compound was found to possess high and rapid blood schizontocidal activity, but its bioavailability via the oral route is low and its formulation as an injectable solution was not feasible. Artemether proved to be readily soluble in injectable oils. It was therefore used for the production of a formulation for intramuscular injection. Subsequently, the oral administration of artemether was found to yield good clinical results in the treatment of falciparum malaria [2]. Neurotoxicity occurred in dogs treated with excessive doses of i.m. artemether in oily solution [3]. However, to date there is no report of neurotoxicity in association with the oral administration of artemether.
Figure 1.
a) Artemisinin and b) lumefantrine (benflumetol).
Lumefantrine (benflumetol), a fluorene (benzindene) derivative (Figure 1b), synthesized in the 1970s by the Academy of Military Medical Sciences, Beijing, has marked blood schizontocidal activity against a wide range of plasmodia. Its antimalarial activity is slower as compared with that of the artemisinin-based drugs, but at the recommended dose regimen the rescrudescence rate with lumefantrine is lower [4].
Artemether and lumefantrine were found to have synergistic activity and both have been used together in China for the treatment of falciparum malaria [4, 5]. A combined formulation of artemether and lumefantrine (CGP 56697) has been developed for the treatment of falciparum malaria in areas where Plasmodium falciparum is resistant to chloroquine and antifolates [6]. The formulation has been registered in Switzerland (RIAMET®, Novartis). Clinical trials have been conducted in tropical Africa [7–9], China [10], Thailand [11–13] and Europe [14].
This study was carried out between May and December 1995 in the framework of a clinical dose finding trial of the combined formulation of artemether and lumefantrine (CGP 56697). It was approved by the Ethics Committee of Mahidol University and conducted in accordance with the stipulations of the Declaration of Helsinki. The study was initiated at the Unit of Clinical Pharmacology, Faculty of Tropical Medicine, Mahidol University, Bangkok, and continued at the clinical facility at Mae Sot in north-western Thailand. The patients came from areas with low communal immunity to malaria. In Thailand P. falciparum is highly resistant to 4-aminoquinolines and antifolates. Resistance to quinine and mefloquine is known to occur on the borders to Cambodia and Myanmar. The investigation was conducted with the objective of assessing the sensitivity of P. falciparum to lumefantrine and artemisinin and several standard antimalarials, and to analyse the data in relation to clinical outcome. Detailed results of the clinical studies were reported earlier [15].
Methods
Parasite isolates
The source of the P. falciparum isolates used for sensitivity testing were patients recruited into the clinical dose finding trial. As these patients had to fulfil the inclusion criteria, all isolates had a parasitaemia of at least 1000 asexual forms of P. falciparum per µl blood. This is also the minimum requirement for drug sensitivity testing in vivo and in vitro. In total 103 fresh isolates of P. falciparum were tested.
Drug sensitivity testing
Heparinized blood samples were drawn by puncture from the cubital vein and used for sensitivity testing, employing a modification of the method of Richards & Maples [16]. The fresh blood samples were centrifuged at 1500 rev min−1 for 7 min. After decanting the plasma, the tenfold volume of complete RPMI-1640, without serum, was added to the cell concentrate. After resuspending the cells, the cell-medium-mixture was centrifuged at 1500 rev min−1 for 7 min. After decanting the supernatant medium, washing was repeated once more. From the final cell concentrate a thin blood film was prepared for the determination of preincubation parasite density. The cell concentrate was then diluted with 19 parts complete RPMI-1640 medium with 10% AB serum from donors who had neither a history of malaria nor malaria antibodies in the blood.
After resuspending the cell layer, the cell-medium-mixture (CMM) was added in 50 µl aliquots to the wells of microtitre plates (Falcon 3070, Becton Dickinson) which were predosed with the scheduled drugs. The microtitre plates were closed with the appropriate lids (Falcon 3071, Becton Dickinson) and placed into a candle jar. After closing the jar and the extinction of the candle, the jar was placed in an incubator and held at 37.5 ° C [17]. Incubation lasted for 48 h, with a renewal of the gas phase after 24 h.
After incubation, the microtitre plates were removed from the candle jar and thin films prepared from the cell sediment of the wells for subsequent staining and reading.
Pre-dosed microtitre plates
Wells A of the microtitre plates served as drug-free controls, wells B – H contained the following drug quantities:
lumefantrine plates well B-H 0.15–150 pmol corresponding to 3–3000 nmol l−1 CMM;
artemisinin plates well B-H 0.15–150 pmol corresponding to 3–3000 nmol l−1 CMM;
mefloquine plates well B-H 2–128 pmol corresponding to 40–2560 nmol l−1 CMM;
quinine plates well B-H 4–256 pmol corresponding to 80–5120 nmol l−1 CMM;
chloroquine plates well B-H 1–64 pmol corresponding to 20–1280 nmol l−1 CMM.
The microtitre plates predosed with mefloquine, quinine and chloroquine were obtained from the WHO test kit production unit through the WHO Regional Office for the Western Pacific, Manila. The plates predosed with artemisinin and lumefantrine were prepared at the Institute of Specific Prophylaxis and Tropical Medicine, University of Vienna, Austria.
Although the trial drug contains artemether, artemisinin was used for the sensitivity tests. Artemether is irreversibly absorbed into the plastic material and thus not suitable for dosing plastic plates. Dihydro-artemisinin, the main metabolite of artemether is unstable on the plates. However, in observations outside this study, artemisinin and its active analogues showed a highly significant correlation (Pearson) between the parameters of their (in vitro) antimalarial activity with P = 0.0000054 for artemisinin and artemether, and P = 0.0000056 for artemisinin and dihydro-artemisinin. Artemisinin was the most appropriate choice for representative sensitivity tests as it is the most stable among the potential candidates.
Parasite counts
The cell films obtained prior to and after incubation were fixed with methanol and stained with diluted Giemsa stain at pH 7.2. They were read by counting the number of asexual parasites per 10 000 red blood cells. In the calculation of growth inhibition the parasite count in the control well (A) was considered 100%.
Statistical evaluation
The method of Litchfield & Wilcoxon [18] was used for the calculation of response parameters of individual isolates and of grouped isolates standardized for control growth. This method employs log-concentration/response probit analysis and is based on the least squares procedure. Student’s t-test was used in the statistical comparison of continuous data. Correlation analysis followed the standard procedures [19]. Statistical significance has been accepted at a level of 95% probability (P < 0.05).
Treatment
The clinical part of the study was carried out as a dose-finding trial in adult symptomatic patients with uncomplicated falciparum malaria (mono-infections), using three dose regimens of CGP 56697. The drug was available in the form of tablets containing 20 mg β-artemether and 120 mg lumefantrine. Group A received 4 doses of 4 tablets each at 0, 12, 24 and 48 h, group B received 4 doses of 2 tablets each, with the same schedule, whereas group C received 3 doses of 4 tablets each at 0, 12 and 24 h.
Results
All patients experienced rapid fever and parasite clearance. On day 7 after the start of treatment none of the patients showed symptoms of malaria or asexual forms of P. falciparum in the peripheral blood.
Sensitivity tests were run with a total of 103 fresh P. falciparum isolates 79 of which were taken prior to treatment only, and 10 after treatment only. The remaining 14 were examined at admission and after recrudescence. In total 297 individual tests were performed, 105 with artemisinin, 101 with lumefantrine, 43 with quinine, 41 with mefloquine, and 10 with chloroquine. Paired tests for sensitivity at admission and after recrudescence were available for 12 isolates with lumefantrine and 9 isolates with artemisinin.
Basic drug response prior to treatment
All 10 P. falciparum isolates tested with chloroquine were resistant to the drug. The mean cut-off point, i.e. the concentration at which a complete inhibition of parasite growth had occurred, was at 485 nmol l−1 CMM. This was nearly 10 times higher than the critical concentration indicative of resistance in this test system (50 nmol l−1 CMM). Only 14 isolates out of 34 tested with mefloquine showed a sensitive or borderline response, the other 20 had cut-off points indicative of resistance. The mean cut-off concentration was 614 nmol l−1, i.e. well beyond the critical level of 250 nmol l−1. The frequency of isolates with a resistant in vitro response is consistent with the in vivo failure rate of 50–60% observed in the trial area. Sensitivity to quinine showed a borderline response, with a mean cut-off concentration of 2503 nmol l−1.
The majority of isolates (58/83) showed complete inhibition at an artemisinin concentration of 300 nmol l−1, all were completely inhibited at 3000 nmol l−1. The mean cut-off point was 367 nmol l−1. This is higher than the mean cut-off concentration we observed in East Africa and lower than that seen in Hainan, P.R.China.
The majority of P. falciparum isolates (65/80) was fully inhibited at a lumefantrine concentration of 300 nmol l−1, and the highest cut-off concentration was 1000 nmol l−1. The mean cut-off concentration was 255 nmol l−1. This was higher than the mean cut-off concentrations we observed in East Africa [20] and China.
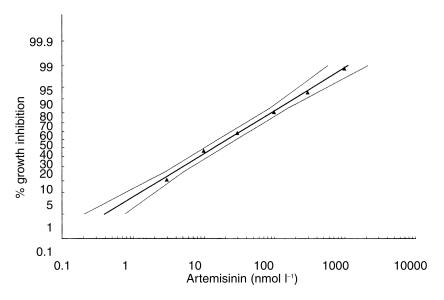
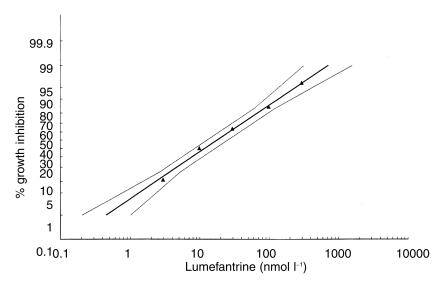
All log-concentration/response probit regressions had correlation coefficients > 0.975 and therefore showed low χ2 values for heterogeneity and a good fit of the mathematically derived regression lines to the observed data points. The effective concentrations required for 50% and 90% growth inhibition, i.e. EC50 and EC90, for chloroquine, mefloquine, quinine, artemisinin and lumefantrine are summarised in Table 1. The regressions for artemisinin and lumefantrine are shown in Figure 2 and Figure 3, respectively.
Table 1.
In vitro drug response of Plasmodium falciparum isolates tested prior to treatment with CGP 56697 or after recrudescence. Mean EC50 and EC90 (and 95% confidence intervals) in nmol l−1 cell medium mixture.
| Timing in relation to treatment | Drug | n | EC50 | EC90 |
|---|---|---|---|---|
| Prior | Chloroquine | 10 | 101.0 (213.0–410.2) | 436.7 (177.2–1076.2) |
| Mefloquine | 34 | 117.3 (88.3–155.8) | 445.4 (291.9–679.5) | |
| Quinine | 34 | 295.6 (213.0–410.2) | 1285.9 (768.1–2152.7) | |
| Artemisinin | 83 | 21.0 (15.5–28.3) | 187.4 (120.4–291.6) | |
| Lumefantrine | 80 | 18.1 (12.8–25.5) | 137.3 (80.9–233.0) | |
| Post-recrudescence | Mefloquine | 71 | 46.0 (73.7–289.2) | 626.7 (193.0–2034.6) |
| Quinine | 93 | 53.7 (182.2–686.4) | 1756.8 (584.9–5277.3) | |
| Artemisinin | 22 | 19.3 (11.1–33.9) | 224.1 (91.9–546.5) | |
| Lumefantrine | 21 | 14.9 (8.2–26.9) | 180.4 (70.2–463.3) |
Figure 2.
In vitro response of Plasmodium falciparum to artemisinin prior to treatment (n = 83).
Figure 3.
In vitro response of Plasmodium falciparum to lumefantrine (benflumetol) prior to treatment (n = 80).
Response after recrudescence
The isolates tested after recrudescence showed higher EC90 values for artemisinin, lumefantrine, mefloquine and quinine as compared to the isolates tested prior to treatment (Table 1).
Paired tests before treatment and after recrudescence
Paired isolates from the same patients prior to treatment and after recrudescence came almost exclusively from patients in groups B and C as recrudescences in these groups were significantly more frequent as compared with group A (Table 2).
Table 2.
Treatment results with CGP 56697.
| Group | Treatment | Number of patients | Number cured | % cured | Mean day of recrudescence |
|---|---|---|---|---|---|
| A | 4 × 4 tablets | 78 | 62 | 79 | 20 |
| B | 4 × 2 tablets | 75 | 41 | 55 | 18 |
| C | 3 × 4 tablets | 76 | 42 | 55 | 18 |
| Total | 229 | 145 | 63 |
The mean EC90 s for artemisinin and benflumetol have increased after exposure to these drugs, with artemisinin more than with benflumetol (Table 3).
Table 3.
Mean EC50 and EC90 and their 95% confidence intervals (nmol l−1 cell medium mixture) for artemisinin and lumefantrine in paired isolates tested before treatment and after recrudescence.
| Drug | EC | n | Before treatment | After recrudescence |
|---|---|---|---|---|
| Artemisinin | EC50 | 9 | 23.5 (10.6–51.9) | 22.2 (8.8–55.8) |
| EC90 | 9 | 112.5 (34.7–364.2) | 206.3 (52.1–817.6) | |
| Lumefantrine | EC50 | 12 | 17.5 (8.1–37.7) | 15.9 (6.5–39.1) |
| EC90 | 12 | 100.9 (33.9–300.6) | 123.1 (34.3–442.1) |
Group-specific sensitivity data
The group-specific sensitivity data (EC50 and EC90) for artemisinin and benflumetol prior to treatment are shown in Table 4. There were only small intergroup differences, unlikely to have influenced treatment outcome.
Table 4.
Mean EC50 and EC90 values and 95% confidence intervals (in nmol l−1 cell medium mixture) for artemisinin and lumefantrine prior to treatment, by treatment group and P for comparison of group mean with total mean.
| Drug | Group A | Group B | Group C |
|---|---|---|---|
| Artemisinin | |||
| n | 23 | 29 | 31 |
| EC50 | 22.3 (12.3–40.4) | 20.2 (12.3–33.1) | 25.1 (15.7–40.2) |
| P | >0.80 | >0.80 | >0.50 |
| EC90 | 222.8 (90.1–550.7) | 171.4 (83.5–351.8) | 207.4 (104.8–410.6) |
| P | >0.7 | >0.80 | >0.80 |
| Lumefantrine | |||
| n | 23 | 29 | 28 |
| EC50 | 15.5 (8.3–29.1) | 17.1 (9.7–30.2) | 21.9 (13.6–35.1) |
| P | >0.60 | >0.80 | >0.50 |
| EC90 | 112.2 (43.5–289.4) | 127.8 (53.7–304.2) | 164.7 (79.8–340.1) |
| P | >0.70 | >0.80 | >0.80 |
When the mean EC90 values for artemisinin of isolates from subsequently cured patients were compared, a significant difference was seen between group A (259 nmol l−1 CMM) and group B (163 nmol l−1 CMM), and between group A and group C (180 nmol l−1 CMM), but none between groups B and C.
The EC90 values for artemisinin (prior to treatment) of isolates from successfully treated patients and from subsequent treatment failures showed no difference in group B (4 × 2 tablets). However, there was a significant difference in group C: the sensitivity of the isolates from successfully treated patients was higher than that of isolates from patients who later produced recrudescences.
A similar difference was seen with regard to baseline sensitivity to lumefantrine between isolates from successfully treated patients and treatment failures in groups B and C, but it did not reach statistical significance. For group A no such comparison was possible due to the paucity of isolates from patients who showed recrudescence.
Pre-treatment parasite density and treatment outcome
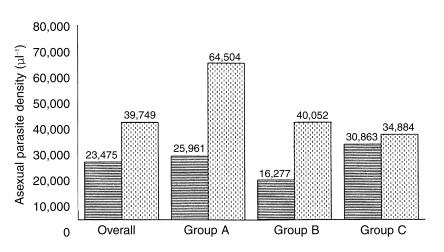
The role of parasite density prior to treatment in determining treatment outcome was already suggested from the overall analysis of the pretreatment routine parasite counts. However, these counts are less precise than those from the preculture slides. Based on the results of the preculture slides it could be shown conclusively that the geometric mean parasite density in patients with subsequent radical cure was significantly lower as compared to that observed in patients experiencing recrudescence (23 475 µl−1 vs 39 749 µl−1; t = 2.1645; P < 0.05). This phenomenon was most marked in groups A (4 × 4 tablets) and B (4 × 2 tablets) and almost absent in group C (3 × 4 tablets) (Figure 4).
Figure 4.
Geometric mean asexual parasite density (µl−1) prior to treatment, comparing patients who were cured (S, line shading) and patients who experienced recrudescence (R, dotted shading) after treatment with CGP 56697. Group A (4 × 4 tablets), Group B (4 × 2 tablets), Group C (3 × 4 tablets).
Inter-drug correlations
Correlations between the individual EC50 values of paired tests were evaluated for all possible drug pairs of artemisinin, benflumetol, mefloquine and quinine. Using the quadratic fit, statistically significant correlations were observed with all drug pairs. With benflumetol, mefloquine and quinine this was to be expected since earlier studies indicated that benflumetol belongs to the class 2 blood schizontocidal drugs. It was not expected with artemisinin.
Discussion and conclusions
In the interpretation of the results obtained with the common in vitro test systems for Plasmodium falciparum it should be borne in mind that they are mostly based on the use of blood-medium-mixture containing 5–6% plasma [21] or CMM with 10% serum [16]. Selective uptake by asexual parasites is known to influence the critical inhibitory concentration of chloroquine, but also that of mefloquine, a compound bound to plasma proteins at > 98%. While EC99 levels indicating in vivo resistance to chloroquine and mefloquine in nonimmunes have been defined [21], this has not yet been attempted with lumefantrine or artemisinin and its derivatives. Such studies require the parallel performance of in vivo and in vitro tests, complemented by the assessment of individual drug concentration profiles.
The P. falciparum isolates tested in this study showed a high degree of resistance to chloroquine, fairly advanced resistance to mefloquine, and compromised sensitivity to quinine. They were less sensitive to artemisinin than isolates from East Africa, but more sensitive than isolates from southern China. Their sensitivity to lumefantrine was less than that observed in isolates from East Africa [20], West Central Africa [22] and southern China, but higher than in Senegal [23].
The response parameters for lumefantrine and artemisinin prior to treatment were similar in all treatment groups (A, B, C) and far from any statistically significant intergroup difference. Thus, there was obviously no sensitivity difference that could have influenced treatment outcome in the three groups.
Parasite isolates obtained after recrudescence showed diminished sensitivity to artemisinin and lumefantrine as compared to the status prior to treatment. This was more marked with artemisinin.
The clinical-parasitological results obtained with the standard 4 × 4 tablet regimen are similar to those reported from two studies in Thailand [11, 12], but they are inferior to those observed in tropical Africa [7–9] where the standard drug regimen with CGP 56 697 (4 × 4 tablets) yielded satisfactory cure rates. Since the clinical studies in Africa covered young children, the difference appears to be related to the relative sensitivity of the parasites rather than the immune status of the patients.
The drug sensitivity tests gave a clear indication that the sensitivity to artemisinin, at the therapeutically relevant EC90, is one of the determinants of clinical-parasitological response. Thus, the mean EC90 of isolates from subsequently cured patients was significantly higher in the 4 × 4 tablet group as compared to the lower dose groups. The critical role of parasite sensitivity to artemisinin was most obvious in the group that received the short treatment course.
The combined use of two blood schizontocidal drugs with vastly different half-lives – like lumefantrine and artemether or, even more so, mefloquine and artesunate – obviously contradicts the conventional concept where matching half-lives are considered to be an essential prerequisite of optimum activity. This is plausible for interacting partners inhibiting enzymes and interfering with particular metabolic pathways. However, artemisinin and its derivatives exert oxidative activity leading to ultrastructural damage, especially at outer and organellar parasite membranes. They therefore act like disinfectants rather than enzyme inhibitors. The combination of mefloquine and artesunate or artemether proved to be highly effective in the treatment of falciparum malaria despite manifest resistance to mefloquine alone [24]. This has been explained by the independent mechanism of action of the partner drugs, the artemisinin derivatives securing a rapid reduction of the parasite biomass, the longer acting partner having the task of eliminating residual parasites. This principle is the basis for the strong postulation of combining the artemisinin drugs with suitable partners in order to secure complete parasitological cure and, in consequence, to extend the useful life-span of the drugs by delaying the advent of resistance [25].
The therapeutic results with the 4 × 4 tablet regimen (group A) were significantly better than those obtained with courses of 4 × 2 tablets (group B) or 3 × 4 tablets (group C). Despite the lower total dose in group B the cure rate equalled that of group C. These findings may, in part, be explained by the relative impact of artemether on the biomass of P. falciparum, the underlying principle in the effect of artemisinin and its derivatives [25, 26]. The observations in groups B and C suggest that for such an impact the number of pulses seems to be at least as important as the dose level. The other determinant in the activity of the combination drug is the pharmacokinetic profile of lumefantrine, the component that is meant to consolidate the effect of artemether and to ensure radical cure. For this purpose, the lumefantrine concentrations need to be maintained above the minimum inhibitory concentration (MIC, generally considered equivalent to the EC99) for the span of 3–4 blood schizogony cycles (tmin) in analogy to other class 1 and 2 antimalarial drugs [27]. The MIC depends on the relative sensitivity of the parasites. The maintenance of adequate concentrations is a function of the pharmacokinetic properties of the drug. Benflumetol has a terminal half-life between 36 and 60 h [6]. The last dose was given 24 h after the first in group C, or 48 h after the first dose in groups A and B. Under these circumstances the drug concentrations may have dropped below the MIC before tmin had been reached. This would obviously have affected group C more than group A.
Asexual parasite density, i.e. relative and absolute biomass of parasites, at the onset of treatment was found to be an important determinant of the therapeutic outcome with mefloquine in falciparum malaria [28]. Our results indicate the same for the combination of artemether and benflumetol.
The overall results indicate that parasite sensitivity and biomass are critical determinants of treatment outcome. The observations relate to the treatment of falciparum malaria in an area affected by severe multidrug resistance. They suggest that treatment results with CGP 56 697 in such areas could be considerably improved by increasing the number of doses and extending treatment, the more so as the drug has a good safety and tolerability record. Clinical experience justifies such a therapeutic modification [13]. It should also be considered in the treatment of nonsevere falciparum malaria with relatively high parasitaemia, regardless of the parasite’s sensitivity status.
Acknowledgments
The study was conducted with support from the Pharmaceutical Division of Ciba-Geigy AG (now Novartis Pharma AG), Basel, Switzerland.
References
- 1.China Cooperative Research Group on Qinghaosu and its Derivatives as Antimalarials. The chemistry and synthesis of qinghaosu derivatives. J Tradit Chin Med. 1982;2:9–16. [PubMed] [Google Scholar]
- 2.Karbwang J, Na Bangchang K, Thanavibul A, et al. Comparison of oral artemether and mefloquine in acute uncomplicated falciparum malaria. Lancet. 1992;340:1245–1248. doi: 10.1016/0140-6736(92)92947-e. [DOI] [PubMed] [Google Scholar]
- 3.Brewer TG, Peggins JO, Grate SJ, et al. Neurotoxicity in animals due to arteether and artemether. Trans R Soc Trop Med Hyg. 1994;88(Suppl 1):33–36. doi: 10.1016/0035-9203(94)90469-3. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization Practical chemotherapy of malaria. WHO Technical Report Series No. 805, Geneva, WHO. [PubMed]
- 5.Alin MH, Björkman A, Wernsdorfer WH. Synergism of benflumetol and artemether in Plasmodium falciparum. Am J Trop Med Hyg. 1999;61:439–445. doi: 10.4269/ajtmh.1999.61.439. [DOI] [PubMed] [Google Scholar]
- 6.Novartis. Meeting the challenge: CGP 56697, a novel antimalarial combination. London, Current Medical Literature Ltd.
- 7.von Seidlein L, Jaffar S, Pinder M, et al. Treatment of African children with uncomplicated falciparum malaria with a new antimalarial drug, CGP 56697. J Infect Dis. 1997;176:1113–1116. doi: 10.1086/516524. [DOI] [PubMed] [Google Scholar]
- 8.von Seidlein L, Bojang K, Jones P, et al. Randomized controlled trial of artemether/benflumetol, a new antimalarial and pyrimethamine/sulfadoxine in the treatment of uncomplicated falciparum malaria in African children. Am J Trop Med Hyg. 1998;58:638–644. doi: 10.4269/ajtmh.1998.58.638. [DOI] [PubMed] [Google Scholar]
- 9.Hatz C, Abdulla S, Mull R, et al. Efficacy and safety of CGP 56697 (artemether and benflumetol) compared with chloroquine to treat acute falciparum malaria in Tanzanian children aged 1–5 years. Trop Med Internat Hlth. 1998;3:498–504. doi: 10.1046/j.1365-3156.1998.00250.x. [DOI] [PubMed] [Google Scholar]
- 10.Jiao X, Liu GY, Shan CO, et al. Phase II trial in China of a new, rapidly-acting and effective oral antimalarial, CGP 56697, for the treatment of Plasmodium falciparum malaria. Southeast Asian J Trop Med Publ Hlth. 1997;28:476–481. [PubMed] [Google Scholar]
- 11.Van Vugt M, Brockman A, Gemperli B, et al. Randomized comparison of artemether-benflumetol and artesunate-mefloquine in treatment of multidrug-resistant falciparum malaria. Antimicrob Agents Chemother. 1998;42:135–139. doi: 10.1128/aac.42.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Looareesuwan S, Wilairatana P, Chokejindachai W, et al. A randomized, double-blind, comparative trial of a new oral combination of artemether and benflumetol (CGP 56697) with mefloquine in the treatment of acute Plasmodium falciparum malaria in Thailand. Am J Trop Med Hyg. 1999;60:238–243. doi: 10.4269/ajtmh.1999.60.238. [DOI] [PubMed] [Google Scholar]
- 13.Vugt MV, Wilairatana P, Gemperli B, et al. Efficacy of six doses of artemether-lumefantrine (benflumetol) in multidrug-resistant Plasmodium falciparum malaria. Am J Trop Med Hyg. 1999;60:936–942. doi: 10.4269/ajtmh.1999.60.936. [DOI] [PubMed] [Google Scholar]
- 14.van Agtmael MA, Bouchaud O, Malvy D, et al. The comparative efficacy and tolerability of CGP 56697 (artemether and lumefantrine) versus halofantrine in the treatment of uncomplicated falciparum malaria in travellers returning from the tropics to The Netherlands and France. Int J Antimicrob Agents. 1999;12:159–169. doi: 10.1016/s0924-8579(99)00070-9. [DOI] [PubMed] [Google Scholar]
- 15.Ezzet F, Mull R, Karbwang J. Population pharmacokinetics and therapeutic response of CGP 56697 (artemether + benflumetol) in malaria patients. Br J Clin Pharmacol. 1998;46:553–561. doi: 10.1046/j.1365-2125.1998.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richards WHG, Maples BK. Studies on Plasmodium falciparum in continuous cultivation. I. The effect of chloroquine and pyrimethamine on parasite growth and viability. Ann Trop Med Parasitol. 1979;73:99–108. [PubMed] [Google Scholar]
- 17.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 18.Litchfield JT, Wilcoxon F. A simplified method for evaluating dose-effect experiments. J Pharmacol Exp Ther. 1949;96:99–113. [PubMed] [Google Scholar]
- 19.Daniel WW. Biostatistics: a Foundation for Analysis in the Health Sciences. 5th. New York.: John Wiley & Sons; 1991. [Google Scholar]
- 20.Wernsdorfer WH, Landgraf B, Kilimali VA, et al. Activity of benflumetol and its enantiomers in fresh isolates of Plasmodium falciparum from East Africa. Acta Tropica. 1998;70:9–15. doi: 10.1016/s0001-706x(97)00141-1. [DOI] [PubMed] [Google Scholar]
- 21.Wernsdorfer WH, Payne D. Drug sensitivity tests in malaria parasites. In: Wernsdorfer WH, McGregor IA, editors. Malaria, Principles and Practice of Malariology. Edinburgh: Churchill Livingstone; 1988. pp. 1765–1800. [Google Scholar]
- 22.Basco LK, Bickii J, Ringwald P. In vitro activity of lumefantrine (benflumetol) against clinical isolates of Plasmodium falciparum in Yaoundé, Cameroon. Antimcrob Agents Chemother. 1998;42:2347–2351. doi: 10.1128/aac.42.9.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pradines B, Tall A, Fusai T, et al. In vitro activities of benflumetol against 158 Senegalese isolates of Plasmodium falciparum in comparison with those of standard antimalarial drugs. Antimicrob Agents Chemother. 1999;43:418–420. doi: 10.1128/aac.43.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White NJ. Preventing antimalarial drug resistance through combinations. Drug Resis Update. 1998;1:3–9. doi: 10.1016/s1368-7646(98)80208-2. [DOI] [PubMed] [Google Scholar]
- 25.White NJ, Nosten F, Looareesuwan S, et al. Averting a malaria disaster. Lancet. 1999;353:1965–1967. doi: 10.1016/s0140-6736(98)07367-x. [DOI] [PubMed] [Google Scholar]
- 26.White NJ, van Vugt M, Ezzet F. Clinical pharmacokinetics and pharmacodynamics of artemether-lumefantrine. Clin Pharmacokinet. 1999;37:105–125. doi: 10.2165/00003088-199937020-00002. [DOI] [PubMed] [Google Scholar]
- 27.Wernsdorfer WH. Epidemiology of drug resistance in malaria. Acta Tropica. 1994;56:143–156. doi: 10.1016/0001-706x(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 28.ter Kuile FO, Luxemburger F, Nosten F, et al. Predictors of mefloquine treatment failure: a prospective study in 1590 patients with uncomplicated falciparum malaria. Trans R Soc Trop Med Hyg. 1995;89:660–664. doi: 10.1016/0035-9203(95)90435-2. [DOI] [PubMed] [Google Scholar]