Abstract
Aims
5-hydroxytryptamine3 receptor antagonists act antiemetically and slow colonic transit. This study evaluated effects of the high-affinity 5-HT3 antagonist, cilansetron, on fasting, meal-and anticholinesterase-stimulated phasic contractile activity of the human sigmoid colon as well as on bowel habits and stool consistency.
Methods
Five female and seven male healthy volunteers received, during three 7 day periods separated by 7 day wash-out periods, 4 mg cilansetron, 8 mg cilansetron or placebo three times daily orally under random, double-blind, crossover conditions. On day 8 of each treatment period, motility 20–40 cm from the anal verge was recorded using five pressure sensors spaced at 5 cm intervals. After a basal 30 min, subjects swallowed a further dose of the scheduled treatment; 60 min later, blood was taken for the determination of plasma cilansetron levels. Thereafter, subjects ingested a 4200 kJ meal and 250 ml sweetened mallow tea (166 kJ); 90 min after meal onset, 1 mg neostigmine was administered intramuscularly and motility recording was continued for 60 min
Results
Phasic contractile activity and intraluminal base-line pressure increased postprandially and more so after neostigmine. With cilansetron, the area under the pressure curve as the primary outcome variable and the number of contractions were significantly greater than with placebo (P = 0.005), amplitude and duration of contractions and base-line pressure were not affected. The effects of the two cilansetron dosages did not differ. With cilansetron, stool tended to become firmer. No adverse effects were observed. Plasma levels were highest with 8 mg cilansetron.
Conclusions
Cilansetron slightly augments meal-stimulated and markedly neostigmine-stimulated phasic motility of the sigmoid colon. When administered over 7 days, it tends to increase stool consistency and is well tolerated.
Keywords: 5-hydroxytryptamine3 receptor antagonist, cilansetron, colonic motility, double-blind crossover trial, meal stimulation, neostigmine, phasic contractile activity
Introduction
Serotonin type 3 (5-HT3) receptor antagonists have an established role in the treatment and prevention of nausea and vomiting induced by chemotherapy, irradiation and drugs [1–3]. Side-effects are scarce but constipation has been reported to occur in 4–8% of patients [1, 2, 4]. After oral doses of ondansetron [5–7] and alosetron [8], a firmer stool consistency has been noted in controlled studies on healthy subjects [5, 8], patients with irritable bowel syndrome (IBS) [5, 8] and patients with diarrhoea-predominant IBS [6, 7]. Patients with carcinoid syndrome have a decreased frequency of bowel movements after ondansetron [9–11] and tropisetron [11–13]. These observations are consonant with findings of a prolonged colonic transit of chyme after ondansetron [6, 14, 15], alosetron [16, 17] and cilansetron [18] in healthy volunteers [14–16, 18] as well as in patients with IBS [6, 17]. However, there is little information on the effects of 5-HT3 antagonists on phasic colonic motor activity. In healthy young men, the number and amplitude of contractions in the sigmoid colon as well as the area under the pressure curve are significantly greater after tropisetron than after placebo [19]. In five patients with carcinoid diarrhoea, the postprandial increase in phasic contractile activity of the descending colon was not affected by ondansetron compared with placebo, although the postcibal increment in colonic tone was significantly smaller with ondansetron [20]. In a parallel group study, six healthy volunteers exhibited no fasting and postprandial colonic phasic activity after i.v. ondansetron, whereas the postprandial rise in tone was significantly smaller with ondansetron than with placebo [21]. Thus, information on the effects of 5-HT3 antagonists on colonic motor activity are somewhat contradictory. The present study aimed to determine, in healthy subjects, the effects of two dosages of the high-affinity 5-HT3 receptor antagonist, cilansetron (Solvay Pharmaceuticals, Hannover, Germany) [22, 23], on the phasic contractile activity of the sigmoid colon in the fasting state, after a standard meal and after cholinergic stimulation by intramuscular (i.m.) administration of the anticholinesterase agent neostigmine. The study further aimed at assessing the effects of 1 week oral administration of cilansetron on bowel habits, stool consistency and possible adverse effects.
Methods
Study design
The study was a randomized, double-blind crossover trial comparing the effects of two dosages of cilansetron and placebo. For the detection of a difference between the effect of a cilansetron dosage and placebo on the primary outcome measure, i.e. the area under the colonic pressure curve, the target sample size was projected, using the paired two-tailed t-test, on the basis of an earlier study [19]. In that study, the interindividual standard deviation (s.d.) of the mean area was about 50 units. As the intraindividual s.d. would be expected to be half the interindividual s.d., 12 cases were assumed to be necessary to detect a difference of > 23 units between the effects of cilansetron and placebo with a power of > 80%, an error probability < 5% and an intraindividual s.d. of 25 units.
Subjects were included if their weight was within the 20% limit according to the Broca Index (height in cm −100), their pulse rate after 5 min resting in supine position was > 50 beats min−1, a 12 lead electrocardiogram (ECG) showed no abnormalities and they had ≤3 stools per day and ≥3 stools per week. Subjects were not included if there was evidence, as revealed by history, physical examination or laboratory assessment, of any illness, if they had a history of gastrointestinal disease, drug sensitivity or food allergy, had undergone major abdominal surgery, had been treated with an investigational drug in the preceding 4 weeks, had a history of alcohol (>50 g ethanol per day) or drug abuse, or, for the past 2 years, of caffeine (> 6 cups of coffee per day) or tobacco (>10 cigarettes per day) use. Females of child-bearing potential were only included if they were using a medically acceptable method of birth control. Subjects were instructed not to take any concomitant medication during the study.
The randomization was performed in blocks. The treatment code was kept hidden from the investigators at Solvay Pharmaceuticals and was broken at the end of the study when all case record forms were completed. The investigators were provided, for each subject, with the code contained in sealed envelopes to be opened in case of emergency only and the medication in form of identically appearing cilansetron and placebo capsules. The investigation was carried out in accordance with the declaration of Helsinki as revised in 1996. It was approved by the Joint Ethical Committee of the Faculty of Medicine, University of Vienna, and The General Hospital of the City of Vienna and was monitored throughout by Solvay.
Subjects
Five healthy females and seven males participated. They were 24–35 (median, 29.5) years old and their body mass index ranged from 19.1 to 29.1 (median, 22.5) kg m−2. The subjects were given a full explanation of the meaning of the investigation, the procedures to be followed as well as a description of any foreseeable risks and discomforts. They were informed that they were free to discontinue their participation at any time. Each subject documented willingness to participate in writing.
Procedure
The subjects entered the study a few days after the initial assessment and were allocated code numbers in chronological order. Each took part in three 7 day treatment periods followed by recording sessions on days 8. The treatment periods were separated by 1 week wash-out periods. To each volunteer, 27 capsules were issued for each treatment period with instruction for them to be taken three times daily, i.e. in the morning, at noon and in the evening, one capsule with 200 ml water. They thus received either 8 mg cilansetron, 4 mg cilansetron or placebo three times daily The preparation’s duration of action is about 6 h [23]. In healthy subjects, oral doses of 4–64 mg cilansetron resulted in peak plasma concentrations after 1.0–1.5 h; with 4 and 8 mg three times daily orally for 6 days, the plasma elimination half-life was 1.6–1.9 h [23]. For the entire study period, the subjects were asked to record in diaries unusual feelings and the time of bowel movements, and to rate the consistency of each stool, on a visual analogue scale, from rock-hard to watery.
On the days following the 7 day treatment periods, subjects came to the laboratory for the colonic motility studies after fasting for at least 12 h. Where there was no spontaneous bowel movement after awakening, they were advised to cleanse their bowel using a sodium phosphate enema. On their arrival at 08.00 h, blood pressure, pulse rate and a 12 lead ECG were recorded and urine collected for urine analyses. Compliance was checked by questionning the subject and counting the capsules not taken. They were asked whether they had experienced any unusual feelings.
Recording and evaluation of colonic motor activity
The contractile activity was recorded manometrically. A round catheter fitted with five strain-gauge pressure sensors spaced at 5 cm intervals and orientated radially 90° apart (Konigsberg Instruments, Pasadena, CA) was inserted into the sigmoid colon via a disposable sigmoidoscope speculum (Kleen Spec, Welch Allyn, Skaneateles Falls, NY) with minimal air insufflation. The sensors were placed between 20 and 40 cm from the anal verge. To prevent the catheter from slipping out, it was strapped to the right buttock with adhesive tape. Thereafter, the subjects reclined in a semirecumbent supine position and refrained from unnecessary speaking and movements. The output of the pressure sensors and respiration, as monitored by a strain-gauge pneumograph fitted around the abdomen, were recorded using a computer-based system (PC-Polygraf, Synectics, Stockholm, Sweden).
Following an adaptation period of 30 min, colonic motility was recorded continuously for 240 min. After a 30 min basal period, the subjects received a further dose of the scheduled treatment. Sixty minutes later, blood was taken for the determination of cilansetron plasma levels and for biochemical and haematologic analyses. Thereafter they ingested a 4200 kJ meal together with 250 ml mallow tea sweetened with sugar (166 kJ) within about 10 min. The meal consisted of 40 g salami, 50 g cheese, 40 g rye bread, 10 g butter, 1 soft boiled egg, 30 g peanuts and 24 g milk chocolate. Ninety minutes after the start of meal ingestion, 1 mg neostigmine was administered i.m. and motility recording continued for another 60 min
The analysis of manometric recordings was computer assisted. For each of the 24 consecutive 10-min periods of every recording session, the mean end-expiratory base-line pressure, the mean number, amplitude and duration of contraction waves as well as the mean area enclosed by the pressure curve and the baseline pressure as an overall measure of motility were determined as averaged over the values recorded by the five sensors. All elevations > 5 mmHg above baseline were counted as contractions. Contraction amplitude was defined as the peak elevation above baseline pressure, contraction onset and end as the points at which lines extrapolated from the steepest pressure rise and fall intersected with baseline pressure. High amplitude propagated contractions were defined as pressure waves >50 mmHg in amplitude propagated over all five sensors, i.e. >20 cm. The analysis accounted for respiration as well as coughing, squeezing and movement artefacts appearing as rapid, simultaneous pressure changes at all sensors.
Assay of cilansetron
Blood samples were centrifuged for 10 min at 4° C and 300 rotations min−1 in a controlled temperature centrifuge. The plasma samples were stored at −20° C prior to shipment over dry ice. Cilansetron plasma levels were determined by Focus Clinical Drug Development (Neuss, Germany) using a validated method.
Statistical analysis
The analysis was performed on the differences between the means over the values recorded in the 30 min basal period and the respective means recorded over the consecutive below-defined periods. For the primary outcome variable, i.e. the area under the pressure curve, as well as the other variables characterizing colonic motility, analyses of variance for repeated measures [24] were carried out. The analysis accounted for influences of the fixed factors ‘treatment’ (placebo, 4 mg cilansetron, 8 mg cilansetron), ‘session’ (1–3), ‘period’ (the 60 min period after drug administration, the 90 min after meal onset and the 60 min after neostigmine), the interactions ‘treatment-period’ and ‘session-period’ as well as the random factors ‘subject’ (1–12) and ‘interaction subject-period’. The means recorded in the basal 30 min were used as covariables. In case a significant influence of the factor ‘treatment’ was revealed for a variable, orthogonal level α comparisons based on the analysis of variance were made (i) between the effects of the two cilansetron dosages and (ii) between the average effect of the two dosages and the effect of placebo.
Results
Compliance
The compliance was good; the difference between the number of capsules returned and the number expected to be returned, i.e. 6, was nil in nine subjects and only 1 in the remaining three. All subjects completed the study.
Basal values
For all variables characterizing motility, the values recorded in the basal 30 min of each session were in the same range after cilansetron and placebo (Figure 1, Table 1). With all treatments, colonic contractions occurred partly sporadically and partly in bursts. Nearly all contractions were propagated distally over 2–4 sensors and the remainder in the reverse direction over the same distances.
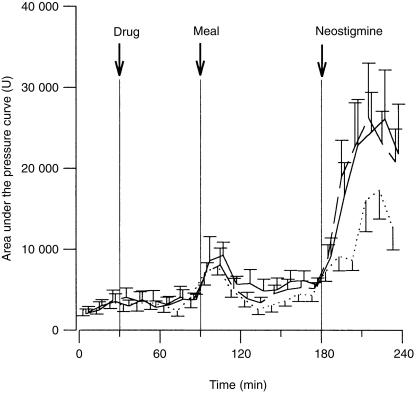
Figure 1.
Area under the pressure curve (U) in the basal 30 min, the 60 min following administration of 8 mg cilansetron (solid line), 4 mg cilansetron (broken line) and placebo (dotted line), the 90 min during and after meal ingestion and the 60 min following neostigmine (means and s.e. mean).
Table 1.
Intraluminal baseline pressure, number, amplitude and duration of colonic contractions in the basal 30 min (Basal), the 60 min following administration of placebo, 4 mg cilansetron (Cil 4 mg) and 8 mg cilansetron (Cil 8 mg; Drug), the 90 min during and after meal ingestion (Meal) and the 60 min following neostigmine (Neostigmine). Data represent means (s.e. mean).
| Variable | Period | Placebo | Cil 4 mg | Cil 8 mg |
|---|---|---|---|---|
| Baseline pressure | Basal | 0.9 (0.4) | 0.5 (0.2) | 0.7 (0.3) |
| (mmHg) | Drug | 1.3 (0.7) | 1.8 (0.3) | 1.7 (0.7) |
| Meal | 4.1 (1.1) | 4.3 (0.6) | 5.3 (0.9) | |
| Neostigmine | 4.5 (1.4) | 5.2 (1.0) | 5.2 (1.0) | |
| Number of contractions | Basal | 33.4 (7.4) | 30.3 (5.9) | 32.4 (6.9) |
| (number 10 min−1) | Drug | 34.5 (6.9) | 39.1 (6.7) | 35.2 (5.6) |
| Meal | 44.6 (6.4) | 54.4 (6.4) | 56.5 (8.2) | |
| Neostigmine | 80.8 (7.3) | 103.6 (10.3) | 94.2 (10.1) | |
| Amplitude of contractions | Basal | 12.8 (1.2) | 12.5 (1.0) | 14.8 (1.4) |
| (mmHg) | Drug | 13.9 (1.9) | 13.1 (1.2) | 14.1 (1.3) |
| Meal | 16.1 (1.4) | 15.6 (1.1) | 16.7 (1.1) | |
| Neostigmine | 22.4 (2.9) | 28.9 (3.9) | 31.2 (4.6) | |
| Duration of contractions | Basal | 9.7 (0.8) | 9.6 (0.7) | 10.0 (0.5) |
| (s) | Drug | 9.7 (0.8) | 10.1 (0.7) | 9.7 (0.6) |
| Meal | 10.7 (0.4) | 10.4 (0.6) | 10.4 (0.5) | |
| Neostigmine | 10.8 (0.6) | 12.1 (0.4) | 11.0 (0.4) |
Area under the pressure curve
In the 60 min following drug administration, the areas after the two cilansetron dosages were similar to those after placebo and there were no changes from basal values. In the first 20 min after meal onset, the area increased markedly and later decreased with all treatments. With placebo but not cilansetron the preprandial levels were restored. In response to neostigmine, the area increased sharply and markedly more after the two cilansetron dosages than after placebo (Figure 1). The increases with meal ingestion and neostigmine were highly significant (F [2,22] = 19.28, P = 0.0001), the treatment effects differed significantly (F [2, 59] = 4.98, P = 0.010). The values after cilansetron over the three periods differed from those after placebo as reflected by a significant interaction ‘treatment-period’ (F [4, 59] = 3.02, P = 0.025). The orthogonal comparisons revealed that the increases with 4 and 8 mg cilansetron did not differ, the estimated difference being 133 units (95% confidence interval [CI] −2281, 2547). The increases pooled over the two dosages were significantly more accentuated than after placebo, the estimated difference was 3288 units (95% CI, 1200–5376).
Intracolonic baseline pressures
Baseline pressures were slightly higher in the 60 min following drug administration than in the basal 30 min. In the 90 min after meal onset, pressures rose markedly and increased further following neostigmine (Table 1). These changes over time were highly significant (F [2, 22] = 32.39, P = 0.0001). The treatment effects did not differ (F [2, 59] = 1.06).
Number of contractions
The number of contractions in the 60 min after drug administration was as high as in the basal 30 min. After meal ingestion, the number increased markedly, the rises being higher with cilansetron than with placebo. In the period 21–50 min after meal onset, the number diminished but remained higher with the two cilansetron dosages than with placebo. Markedly more contractions occurred subsequent to neostigmine, peak levels being attained after 30–40 min (Table 1). The increases with eating and neostigmine were highly significant (F [2, 22] = 34.98, P = 0.0001) and the treatment effects differed significantly (F [2, 59] = 4.72, P = 0.013). The orthogonal comparisons showed that the increases pooled over the two cilansetron dosages were significantly greater than those with placebo; the estimated difference was 11.2 contractions per 10 min (95% CI 3.5,18.9). The effects of the two cilansetron dosages did not differ from each other, the estimated difference being −4.4 contractions per 10 min (95% CI −13.3,4.5).
Amplitude of contractions
The amplitudes in the 60 min after drug administration were as high as in the basal 30 min. During and following meal ingestion and more so with neostigmine, the amplitude increased markedly (F [2, 22] = 14.51, P = 0.0001; Table 1). The increments tended to be larger with the two cilansetron dosages than with placebo (interaction ‘treatment-period’, F [4, 59] = 2.15, P = 0.086), although the treatment effects did not differ significantly (F [2, 59] = 1.63, P = 0.204).
A high amplitude contraction was observed in only one of the 36 recording sessions on the 12 subjects. It occurred 82 min after the start of meal ingestion in a subject having received 4 mg cilansetron.
Duration of contractions
The duration of contractions in the 60 min following drug administration did not differ from the one in the basal 30 min. Following meal onset and in response to neostigmine, the duration increased significantly (F [2, 22] = 6.28, P = 0.007; Table 1). The treatment effects did not differ (F [2, 59] = 2.39, P = 0.101).
Plasma cilansetron levels
The levels varied considerably, i.e. from 2.38 to 71.85 (mean, 16.12 [s.e. mean 2.49]) ng ml−1 after 8 mg and from 0.92 to 26.53 (mean, 9.76 [6.11]) ng ml−1 after 4 mg cilansetron. After placebo, cilansetron levels were below the limit of quantification (0.89 ng ml−1) in 11 subjects and amounted to 1.74 ng ml−1 in subject 2. The latter could be ascribed to the measurement of a matrix interference peak.
Bowel movements
Frequency and consistency of stools during the 7 day treatment periods did not differ significantly, although there was a tendency towards firmer stools with the two cilansetron dosages (Table 2).
Table 2.
Number of stools per week and stool consistency as rated on a visual analogue scale during three times daily administration of placebo, 4 mg cilansetron and 8 mg cilansetron for 7 days (medians and ranges).
| Cilansetron | |||
|---|---|---|---|
| Variable | Placebo | 4 mg | 8 mg |
| Number of stools | 9.5 | 9.0 | 10.0 |
| (7–26) | (7–14) | (4–17) | |
| Stool consistency | 46.4 | 53.2 | 52.3 |
| (25.0–74.8) | (25.4–83.1) | (25.1–91.1) | |
Adverse effects
No serious adverse effects were observed or reported. Systolic and diastolic blood pressure as well as heart rate and ECG did not differ after the 7 day periods of cilansetron or placebo intake. Headache and other symptoms were reported no more frequently with cilansetron.
Discussion
The results of the present study show that 4 mg as well as 8 mg cilansetron elicited a slight but statistically significant augmentation of the phasic contractile activity of the sigmoid colon after eating [19, 25] and in particular after strong cholinergic stimulation with neostigmine [19, 26]. These observations are in accordance with findings of a stimulatory effect of cilansetron in rats, i.e. a reversal of the motor inhibition elicited by experimental colitis [27]. They are consonant also with the stimulatory effects of tropisetron on phasic colonic motility in healthy men [19]. Since increases in activity did not occur in the resting state but in states of stimulated motility, i.e. after meal ingestion and more so after neostigmine, this may indicate that 5-HT3 receptors are involved in the neural mechanisms respon-sible for the activation of phasic contractility. The enhancement could be brought about either by a facilitation of excitatory influences or an antagonism of inhibitory influences. The increase in phasic activity is, in parallel with the augmented phasic and diminished propagative activity prevailing in patients with idiopathic constipation [28] as well as after opioid administration [29–32], likely to impede transit and thus foster the desiccation of chyme. Prolonged colonic transit [6, 14–18] and as a consequence, firmer stools [5–7, 9–13, 33], have been described following 5-HT3 antagonists in healthy subjects [5, 14–18] as well as in patients with IBS [5–7, 17] and the carcinoid syndrome [9–13]. In a parallel group study in 27 patients with carcinoid diarrhoea, however, no significant overall effect of alosetron on gastrointestinal transit was found, although the agent reduced the emptying rate of the proximal colon and ‘appeared to change’ the diarrhoea score [34]. In 39 healthy volunteers [14] and 12 patients with IBS [17], the transit through the left colon and the rectosigmoid colon but not the proximal colon was found to be prolonged by ondansetron [15] and alosetron [17]. Both prolonged colonic transit and firm stools are characteristic of constipation, a well recognized side effect of 5-HT3 antagonists [1–5]. In the present study there was only a slight tendency towards firmer stools during the 7 day intake of cilansetron.
No effect of cilansetron was observable on intraluminal baseline pressure, which reflects the tension of the muscle wall of the colon at the sensor sites. The observation that the baseline pressure increased markedly with meal ingestion is consonant with findings of increased postprandial colonic tone as recorded by the barostat technique [21, 26, 35–38]. In contrast to an investigation in which ondansetron [21] inhibited the postprandial rise in tone, no effect on wall tension could be seen in the present study with either of the two cilansetron dosages.
The fact that of only one high amplitude propagated contraction was observed in the altogether 36 recording sessions may be explained by the reported scarcity of these events, i.e. on the mean 4.4 [39] to 6.1 [40] per 24 h. Further, these waves have been described to occur primarily after awakening in the morning and, more rarely, in the late postprandial phase [39, 40]. In our study, recordings took place neither in the period after awakening nor, as neostigmine was administered 90 min after meal onset, in the late postprandial phase. The occurrence of high amplitude propagated contractions may have been obscured by the vigorous high amplitude activity after neostigmine stimulation. In the latter condition, however, these contractions were propagated over 3–4, but not all sensors. The lack of adverse effects with either dose of cilansetron is consonant with earlier observations [23].
Cilansetron has also been reported to alter the perception of a visceral distending stimulus: the pressure in an intragastric barostat bag at which distension was first sensed as well as the threshold pressure for discomfort and pain were significantly higher after 8 mg cilansetron than after placebo [41]. Consonant changes were observed in a parallel group study in patients with IBS: the volumes at which the distension of a barostat bag in the left colon were first sensed and felt as painful, respectively, were significantly greater in groups of eight patients each having received 0.25 and 4 mg alosetron twice daily than in six patients having been on placebo [42]. No such effects were apparent in placebo-controlled double-blind crossover trials. In 12 healthy subjects, the perception of intragastric distension was not altered by alosetron 1 mg twice daily for 6 days [43] and in 10 healthy subjects and eight patients with nonulcer dyspepsia, gastric sensitivity was not changed by a single dose of 5 mg tropisetron [44]. In 10 healthy subjects and 12 community patients with diarrhoea-predominant IBS, the perception of barostat bag distension in the stomach and rectum after 0.15 mg kg−1 ondansetron i.v. did not differ from that after placebo [45]. Ondansetron, 16 mg three times daily for 2 weeks, also did not alter rectal sensitivity to balloon distension and electrical stimulation in 10 healthy subjects, although it tended to increase the sensation threshold to electrical stimulation and the maximum tolerated distension volume in five patients with IBS [46]. In a study carried out in blinded fashion, ondansetron did not alter rectal sensitivity to distension in healthy subjects or in patients with diarrhoea-predominant IBS [47]. The discrepancies between these results may stem from differences in study design [41-44, 46] or patients numbers [46]. Further studies adhering to a stringent design seem necessary.
In conclusion, cilansetron, in the dosages evaluated, augments slightly meal-stimulated and increases markedly neostigmine-stimulated phasic motor activity of the sigmoid colon. When administered over 7 days, cilansetron tends to increase stool consistency and is well tolerated. Whether these effects can be made use of in clinical settings remains to be assessed.
References
- 1.Tabona MV. An overview on the use of granisetron in the treatment of emesis associated with cytostatic chemotherapy. Eur J Cancer. 1990;26(Suppl 1):S37–S41. [PubMed] [Google Scholar]
- 2.Joss RA, Dott CS. Clinical studies with granisetron, a new 5-HT3 receptor antagonist, for the treatment of cancer chemotherapy-induced emesis. The Granisetron Study Group. Eur J Cancer. 1993;29A(Suppl 1):S22–S29. doi: 10.1016/s0959-8049(05)80256-4. [DOI] [PubMed] [Google Scholar]
- 3.Perez EA. Review of the preclinical pharmacology and comparative efficacy of 5-hydroxytryptamine-3 receptor antagonists for chemotherapy-induced emesis. J Clin Oncol. 1995;13:1036–1043. doi: 10.1200/JCO.1995.13.4.1036. [DOI] [PubMed] [Google Scholar]
- 4.Stewart A, McQuade B, Cronje JD, et al. Ondansetron compared with granisetron in the prophylaxis of cyclophosphamide-induced emesis in out-patients: a multicentre, double-blind, double-dummy, randomised, parallel-group study. Emesis Study Group for Ondansetron and Granisetron in Breast Cancer Patients. Oncology. 1995;52:202–210. doi: 10.1159/000227458. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg PA, Kamm MA, Setti-carraro P, van der Sijp JRM, Roth C. Modification of visceral sensitivity and pain in irritable bowel syndrome by 5-HT3 antagonism (ondansetron) Digestion. 1996;57:478–483. doi: 10.1159/000201377. [DOI] [PubMed] [Google Scholar]
- 6.Steadman CJ, Talley NJ, Phillips SF, Zinsmeister AR. Selective 5-hydroxytryptamine type 3 receptor antagonism with ondansetron as treatment for diarrhea-predominant irritable bowel syndrome: a pilot study. Mayo Clin Proc. 1992;67:732–738. doi: 10.1016/s0025-6196(12)60797-6. [DOI] [PubMed] [Google Scholar]
- 7.Maxton DG, Morris J, Whorwell PJ. Selective 5hydroxytryptamine antagonism: a role in irritable bowel syndrome and functional dyspepsia? Aliment Pharmacol Ther. 1996;10:595–599. doi: 10.1046/j.1365-2036.1996.30172000.x. [DOI] [PubMed] [Google Scholar]
- 8.Mangel AW, Northcutt AR. Review article: the safety and efficacy of alosetron, a 5-HT3 receptor antagonist, in female irritable bowel syndrome patients. Aliment Pharmacol Ther. 1999;13(Suppl 2):77–82. doi: 10.1046/j.1365-2036.1999.00010.x. [DOI] [PubMed] [Google Scholar]
- 9.Buhl C, Fibbe C, Frenzel H. Ondansetron bei Karzinoid-Syndrom. Dtsch Med Wochenschr. 1992;117:1821. [PubMed] [Google Scholar]
- 10.Platt AJ, Heddle RM, Rake MO, Smedley H. Ondansetron in carcinoid syndrome. Lancet. 1992;339:1416. doi: 10.1016/0140-6736(92)91235-z. (Letter). [DOI] [PubMed] [Google Scholar]
- 11.Schwörer H, Münke H, Stöckmann F, Ramadori G. Treatment of diarrhea in carcinoid syndrome with ondansetron, tropisetron, and clonidine. Am J Gastroenterol. 1995;90:645–648. [PubMed] [Google Scholar]
- 12.Anderson JV, Coupe MO, Morris JA, Hodgson HJ, Bloom SR. Remission of symptoms in carcinoid syndrome with a new 5-hydroxytryptamine M receptor antagonist. Br Med J. 1987;294:1129. doi: 10.1136/bmj.294.6580.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coupe MO, Anderson JV, Morris JA, Alstead EM, Bloom SR, Hodgson HJF. The effects of the 5-hydroxytryptamine (5HT3) receptor antagonist ICS 205–930 in the carcinoid syndrome. Aliment Pharmacol Ther. 1988;2:167–172. doi: 10.1111/j.1365-2036.1988.tb00684.x. [DOI] [PubMed] [Google Scholar]
- 14.Talley NJ, Phillips SF, Hadda A, et al. GR 38032F (ondansetron), a selective 5HT3 receptor antagonist, slows colonic transit in healthy man. Dig Dis Sci. 1990;35:477–480. doi: 10.1007/BF01536922. [DOI] [PubMed] [Google Scholar]
- 15.Gore S, Gilmore IT, Haigh CG, Brownless SM, Stockdale H, Morris AI. Colonic transit in man is slowed by ondansetron (GR38032F), a selective 5-hydroxytryptamine receptor (type 3) antagonist. Aliment Pharmacol Ther. 1990;4:139–144. doi: 10.1111/j.1365-2036.1990.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 16.Houghton LA, Foster J, Whorwell PJ, McDonald JN. Effect of alosetron, a new 5-HT3 antagonist, on gastrointestinal (GI) transit in normal healthy volunteers. Gastroenterology. 1995;108:A618. (Abstract). [Google Scholar]
- 17.Foster JM, Houghton LA, Whorwell PJ. Alosetron slows colonic transit in patients with irritable bowel syndrome (IBS) Gut. 1997;40(Suppl 1):A44. doi: 10.1046/j.1365-2036.2000.00762.x. (Abstract). [DOI] [PubMed] [Google Scholar]
- 18.Drewe J, Beglinger C, Meier R, Krause G, Steinborn C. Oral cilansetron produced increases in colonic transit time and gastric emptying in young and old healthy volunteers. Digestion. 1998;59(Suppl 3):665. (Abstract). [Google Scholar]
- 19.Stacher G, Gaupmann G, Schneider C, et al. Effects of a 5-hydroxytryptamine3 receptor antagonist (ICS 205–930) on colonic motor activity in healthy men. Br J Clin Pharmacol. 1989;28:315–322. doi: 10.1111/j.1365-2125.1989.tb05432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von der Ohe MR, Camilleri M, Kvols LK. A 5HT3 antagonist corrects the postprandial colonic hypertonic response in carcinoid diarrhea. Gastroenterology. 1994;106:1184–1189. doi: 10.1016/0016-5085(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 21.von der Ohe MR, Hanson RB, Camilleri M. Serotonergic mediation of post-prandial colonic tonic and phasic responses in humans. Gut. 1994;35:536–541. doi: 10.1136/gut.35.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Wijngaarden I, Hamminga D, van Hes R, et al. Development of high-affinity 5HT3 receptor antagonists. Structure-affinity relationships of novel 1,7-annelated indole derivatives. J Med Chem. 1993;36:3693–3699. doi: 10.1021/jm00075a026. [DOI] [PubMed] [Google Scholar]
- 23.Investigational Drug Brochure. 6th. Solvay Pharma Deutschland: Hannover; 1999. Cilansetron. [Google Scholar]
- 24.4th. Vol. 2. SAS Institute: Cary, NC; SAS/Stat User’s Guide; pp. 1351–1456. Version 6. [Google Scholar]
- 25.Moreno-osset E, Bazzocchi G, Lo S, et al. Association between postprandial changes in colonic intraluminal pressure and transit. Gastroenterology. 1989;96:1265–1273. doi: 10.1016/s0016-5085(89)80013-7. [DOI] [PubMed] [Google Scholar]
- 26.Bell AM, Pemberton JH, Hanson RB, Zinsmeister AR. Variations in muscle tone of the human rectum: recordings with an electromechanical barostat. Am J Physiol. 1991;260:G17–G25. doi: 10.1152/ajpgi.1991.260.1.G17. [DOI] [PubMed] [Google Scholar]
- 27.Morteau O, Julia V, Eeckhout C, Buéno L. Influence of 5-HT3 receptor antagonists in visceromotor and nociceptive responses to rectal distension before and during experimental colitis in rats. Fundam Clin Pharmacol. 1994;8:553–562. doi: 10.1111/j.1472-8206.1994.tb00837.x. [DOI] [PubMed] [Google Scholar]
- 28.Buéno L, Fioramonti J. Patterns of colonic motility. Clin Res Rev. 1981;1:91–100. [Google Scholar]
- 29.Stacher G, Steinringer H, Schmierer G. Effects of the synthetic enkephalin analogue FK 33–824 on colonic motor activity in healthy man. In: Christensen J, editor. Gastrointestinal motility. New York: Raven Press; 1980. pp. 443–450. [Google Scholar]
- 30.Ooms LAA, Degryse A-D, Janssen PAJ. Mechanism of action of loperamide. Scand J Gastroenterol. 1984;19(Suppl 96):145–155. [PubMed] [Google Scholar]
- 31.Buéno L, Fioramonti J. Action of opiates on gastrointestinal function. Clin Gastroenterol. 1988;2:123–139. doi: 10.1016/0950-3528(88)90024-3. [DOI] [PubMed] [Google Scholar]
- 32.Ferraz AAB, Cowles VE, Condon RE, Schulte WJ. Opioid and nonopioid analgesic drug effects on colon contractions in monkeys. Dig Dis Sci. 1995;40:1417–1419. doi: 10.1007/BF02285185. [DOI] [PubMed] [Google Scholar]
- 33.Degen LP, Phillips SF. How well does stool form reflect colonic transit? Gut. 1996;39:109–113. doi: 10.1136/gut.39.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saslow SB, Scolapio JS, Camilleri M, et al. Medium term effects of a new 5HT3 antagonist, alosetron, in patients with carcinoid diarrhoea. Gut. 1998;42:628–634. doi: 10.1136/gut.42.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steadman CJ, Phillips SF, Camilleri M, Haddad AC, Hanson RB. Variation of muscle tone in the human colon. Gastroenterology. 1991;101:373–381. doi: 10.1016/0016-5085(91)90014-c. [DOI] [PubMed] [Google Scholar]
- 36.Vassallo MJ, Camilleri M, Phillips SF, et al. Colonic tone and motility in patients with irritable bowel syndrome. Mayo Clin Proc. 1992;67:725–731. doi: 10.1016/s0025-6196(12)60796-4. [DOI] [PubMed] [Google Scholar]
- 37.von der Ohe MR, Hanson RB, Camilleri M. Comparison of simultaneous recordings of human colonic contractions by manometry and a barostat. Neurogastroenterol Mot. 1994;6:213–222. [Google Scholar]
- 38.Ford MJ, Camilleri M, Wiste JA, Hanson RB. Differences in colonic tone and phasic response to a meal in the transverse and sigmoid human colon. Gut. 1995;37:264–269. doi: 10.1136/gut.37.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Narducci F, Bassotti G, Gaburri M, Morelli A. Twenty four hour manometric recording of colonic motor activity in healthy man. Gut. 1987;28:17–25. doi: 10.1136/gut.28.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bassotti G, Gaburri M. Manometric investigation of high-amplitude propagated contractile activity of the human colon. Am J Physiol. 1988;255:G660–G664. doi: 10.1152/ajpgi.1988.255.5.G660. [DOI] [PubMed] [Google Scholar]
- 41.Bruley des Varannes S, Zerbib F, et al. Oral cilansetron increased threshold of gastric visceral perception in a placebo-controlled, double-blind study in healthy volunteers. Digestion. 1998;59(Suppl 3):226–227. (Abstract). [Google Scholar]
- 42.Delvaux M, Louvel D, Mamet J-P, Campos-oriola R, Frexinos J. Effect of alosetron on responses to colonic distension in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 1998;12:849–855. doi: 10.1046/j.1365-2036.1998.00375.x. [DOI] [PubMed] [Google Scholar]
- 43.Zerbib F, Bruley des Varannes S, Oriola RC, McDonald J, Isal JP, Galmiche JP. Alosetron does not affect the visceral perception of gastric distension in healthy subjects. Aliment Pharmacol Ther. 1994;8:403–407. doi: 10.1111/j.1365-2036.1994.tb00307.x. [DOI] [PubMed] [Google Scholar]
- 44.Klatt S, Bock W, Rentschler J, Beckh K, Adler G. Effects of tropisetron, a 5–HT3 receptor antagonist, on proximal gastric motor and sensory function in nonulcer dyspepsia. Digestion. 1999;60:147–152. doi: 10.1159/000007640. [DOI] [PubMed] [Google Scholar]
- 45.Zighelboim J, Talley NJ, Phillips SF, Harmsen WS, Zinsmeister AR. Visceral perception in irritable bowel syndrome. Rectal and gastric responses to distension and serotonin type 3 antagonism. Dig Dis Sci. 1995;40:819–827. doi: 10.1007/BF02064986. [DOI] [PubMed] [Google Scholar]
- 46.Goldberg PA, Kamm MA, Setti-carraro P, van der Sijp JRM, Roth C. Modification of visceral sensitivity and pain in irritable bowel sndrome by 5-HT3 antagonism (ondansetron) Digestion. 1996;57:478–483. doi: 10.1159/000201377. [DOI] [PubMed] [Google Scholar]
- 47.Hammer J, Phillips SF, Talley NJ, Camilleri M. Effect of a 5HT3-antagonist (ondansetron) on rectal sensitivity and compliance in health and the irritable bowel syndrome. Aliment Pharmacol Ther. 1993;7:543–551. doi: 10.1111/j.1365-2036.1993.tb00131.x. [DOI] [PubMed] [Google Scholar]