Abstract
Aims
The primary objective of this study was to describe the single dose pharmacokinetics of ribavirin in subjects with normal liver function and those with various degrees of stable chronic liver disease. Additionally this study assessed the safety and tolerability of ribavirin in this population.
Methods
Single oral 600 mg doses of ribavirin were administered to healthy male and female volunteers (n = 6) and patients with stable chronic liver disease (n = 17), in a parallel group study. Pharmacokinetic sampling and tolerability assessments were performed up to 168 h post dose.
Results
Single oral doses of 600 mg ribavirin were well tolerated by healthy volunteers and patients with varying degrees of hepatic dysfunction. Although mean Cmax increased with the severity of hepatic dysfunction, there was no change in extent of absorption or renal clearance of ribavirin.
Conclusions
There are no pharmacokinetic reasons for initial dose adjustment of ribavirin in patients with hepatic dysfunction.
Keywords: liver disease, pharmacokinetics, ribavirin
Introduction
Over the last three decades, in vitro and clinical studies with the nucleoside analogue ribavirin have shown it to have activity against a number of RNA and DNA viruses [1–3]. Initial studies of ribavirin monotherapy in patients with chronic hepatitis C revealed that serum ALT concentrations were reduced or normalized in a majority of patients, but serum levels of hepatitis C virus (HCV) RNA were unchanged [4]. Subsequent studies using the combination of ribavirin and interferon α-2b showed increased antiviral activity compared with interferon [5–8].
The single dose pharmacokinetics of ribavirin have been previously described in healthy volunteers [9] and patients infected with HIV [10] or compensated chronic hepatitis C [11]. Ribavirin is eliminated by both renal and metabolic routes [9, 10]. As some or all of its metabolism may be hepatic, it was important to assess the effects of hepatic dysfunction on ribavirin pharmacokinetics. Therefore, the primary objective of this single dose study was to describe the pharmacokinetics of ribavirin in subjects with normal liver function and those with various degrees of stable chronic liver disease. Additionally this study assessed the tolerability of ribavirin in subjects with chronic liver disease based on laboratory tests and reported adverse events.
Methods
This open label study assessed the single dose pharmacokinetics of ribavirin in a total of 23 male and female subjects (mean age 46.3 years, range 31–57). In addition to six healthy control subjects with normal liver function, 17 patients had stable chronic liver disease, confirmed by biopsy or by clinical, biochemical and radiological features. These patients were divided in two groups of five and one group of seven subjects according to Pugh’s modification [12] of Child’s classification of severity of liver disease [13] (Category A: n = 5, mean score 4.2; Category B: n = 7, mean score 6.5; Category C: n = 5, mean score 10.4). All subjects provided written informed consent prior to participating in this study, which was approved by the University of Texas Health Science Center Institutional Review Board. Patients with evidence of encephalopathy were assessed as being capable of providing informed consent. Subjects were determined to be eligible to participate in the study on the basis of a physical examination, medical history, electrocardiogram, vital signs, laboratory safety tests and urine screen for drugs of abuse. Subjects who had significant medical disorders, who were positive for HIV or HCV antibodies or hepatitis B surface antigen, or who had taken any investigational drugs within 3 months prior to the study were not permitted to participate in the study. In addition, healthy control subjects who had histories of drug or alcohol abuse, or any female subjects who were pregnant or breast feeding were also excluded.
All subjects were confined to the study centre from 12 h prior to dosing until 168 h post dosing. A light snack was consumed approximately 10 h prior to dosing, after which the subjects fasted until 4 h postdose. During this fasting period, no food or fluid except water was permitted. Following the overnight fast, subjects received a single oral dose of ribavirin 600 mg (3 × 200 mg capsules). Blood samples for determination of plasma ribavirin concentrations were drawn immediately prior to drug administration and then at 1, 2, 3, 4, 6, 8, 10, 12, 14, 16, 24, 36, 48, 72, 96, 120, 144 and 168 h after drug administration. These samples were collected directly into heparinized Vacutainer® tubes which had their tops removed, via a wide-bore cannula. The sample was gently mixed, and was centrifuged for 15 min at 3000 rev min−1 and 4 ° C. Plasma was separated and frozen to −80 ° C until assayed. Block urine samples were collected before dosing and for 168 h post dose to measure renal clearance of ribavirin. Tolerability assessments performed during the study included measurement of vital signs, drawing of safety laboratory tests and recording of adverse events.
Plasma concentrations of ribavirin were determined using a high performance liquid chromatography/mass spectrometric method validated with respect to linearity, precision, accuracy, limit of quantification (50 ng ml−1, LOQ) and selectivity. Urine concentrations of ribavirin were determined using a high performance liquid chromatographic-mass spectrometric method, validated with respect to linearity, precision, accuracy, limit of quantification (250 ng ml−1, LOQ) and selectivity. In brief, solid phase extraction was performed on samples using phenylboronic acid columns, after addition of the internal standard (13C3-ribavirin) and di-ammonium hydrogen orthophosphate buffer. Columns were eluted with a solution of formic acid in 50/50 methanol/water (v/v). The resulting eluate was injected into a Hewlett-Packard 1090 Series II h.p.l.c. with a SCIEX API 300 MS/MS detector, fitted with a hypersil 3.0 × 4.6 mm analytical column (3 µm particle size). The mobile phase consisted of acetonitrile (82%) and ammonium acetate (18%). Assay precision and bias were less than 11%, respectively, for all samples.
Individual plasma concentration-time data above the LOQ were used to determine the derived pharmacokinetic parameters using model-independent methods [14]. The maximum concentration (Cmax), time of maximum concentration (tmax) and the final quantifiable sampling time (0,t) were the observed values. The area under the plasma concentration-time curve from time zero to the final quantifiable sampling time [AUC(0,t)] was calculated using the linear trapezoidal method. The truncated AUC value [AUC(0,72 h)] was also calculated. Renal clearance (CLr) was calculated by dividing the amount excreted in the urine over 72 h [Ae(0,72 h)] by the AUC(0,72 h). Assuming a variability of 15% [10] for log-transformed AUC and Cmax, it was calculated that this study with six subjects per group should have been able to detect a 30% difference between groups with 80% power and α= 0.05. Analyses of variance were used to extract the effect due to the level of hepatic dysfunction for original scale and log-transformed AUC, Cmax and CLr, and for tmax in the original scale. The 95% confidence intervals for the differences in AUC, Cmax and CLr between control and patient groups were calculated. The power to detect a 20% difference in means between any two groups for an α level of 0.05 was calculated using the pooled residual error and the associated degrees of freedom from the analysis of variance.
Results
Pharmacokinetics
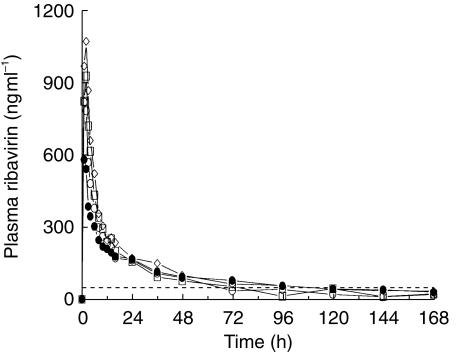
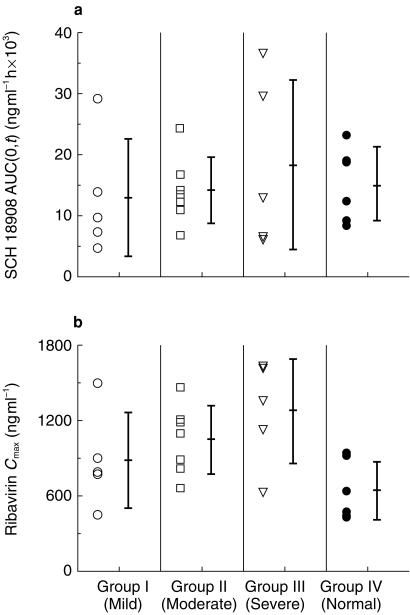
Twenty-three subjects (20 males and 3 females) between the ages of 31 and 57 years (mean 46.3 years) and weighing between 55 and 139 kg (mean 85.3 kg) were enrolled in and completed the study. Mean plasma concentration-time profiles for the four dose groups are shown in Figure 1 and mean plasma and urinary pharmacokinetic parameters are shown in Table 1. The mean plasma concentration-time curves for the four groups show similar characteristics: rapid absorption and distribution phases and long-terminal elimination phases. Mean tmax values were similar and occurred between 1.3 h and 1.6 h, indicating rapid absorption of ribavirin in all treatment groups. Individual AUC(0,t) and Cmax values for the four groups are presented in Figure 2a, b. The Cmax increased with the severity of hepatic dysfunction and the differences compared with control were significant (P = 0.029; 95% CI: mild: 86–213; moderate: 110–253; severe: 126–311; Table 1). However, there was a considerable overlap between the individual Cmax values among four treatment groups (Figure 2b). The intergroup differences in AUC(0,t) or CLr were not significant (AUC(0,t): P = 0.79; 95% CI: mild: 37–158; moderate: 48–185; severe: 49–210; Cmax: P = 0.029; 95% CI: mild: 41–156; moderate: 35–141; severe: 40–155).
Figure 1.
Mean plasma ribavirin concentration-time profiles following a single oral dose of 600 mg to subjects with normal liver function (◊) and with various degrees of chronic liver disease (○ mild, □ moderate, • severe). - - - LOQ 50 ng ml−1.
Table 1.
Mean (%CV) pharmacokinetic information for the four groups.
| Controls | Child-Pugh A | Child-Pugh B | Child-Pugh C | |
|---|---|---|---|---|
| n | 6 | 5 | 7 | 5 |
| Cmax (ng ml−1) | 643 (37) | 886 (43) | 1048 (26) | 1273 (33) |
| Ratio; CIb,c | 135%; 86–213 | 167%; 110–253 | 198%; 126–311 | |
| tmax (h) | 1.33 (39) | 1.60 (56) | 1.29 (38) | 1.60 (34) |
| AUC(0,72 h) (ng ml−1 h) | 10927 (29) | 11284 (54) | 12274 (25) | 14454 (57) |
| AUC(tf) (ng ml−1 h) | 15162 (40) | 13046 (74) | 14184 (38) | 18392 (75) |
| Ratio; CIb,c | 76%; 37–158 | 94%; 48–185 | 101%; 49–210 | |
| Ae(0,72 h) (mg) | 53.6 a (15) | 56.0 (44) | 57.3 (41) | 53.3 (33) |
| % Dose | 9 a (15) | 9 (44) | 10 (41) | 9 (33) |
| CLr (l h−1) | 5.35a (29) | 5.27 (34) | 4.73 (42) | 5.21 (68) |
| Ratio; CIb | 98%; 41–156 | 88%; 35–141 | 97%; 50–155 |
n = 5;
ratio = mean differences as percentage of control; 95% confidence intervals for ratio;
ratio and confidence interval based on log-transformed data.
Figure 2.
Individual ribavirin pharmacokinetic parameters in subjects with normal liver function and with various degrees of chronic liver disease.
Tolerability
Compared with baseline, there were no changes of clinical relevance in any laboratory parameters during or at completion of the study. Adverse events were reported by 2/23 subjects (9%). The two adverse events were erythema and abdominal pain. Both of these adverse events were of mild severity and were assessed as being unrelated to the study drug.
Discussion
The elimination of ribavirin occurs by both metabolism and renal elimination. Renal elimination of parent ribavirin is relatively minor compared with metabolic elimination, as only 5–15% of a single dose is eliminated via this route [9, 15], which implies that metabolism accounts for the majority of ribavirin’s elimination. A substantial part of this is due to first pass metabolism (absolute bioavailability estimates range from 33 to 65% [9, 10, 16, 17];. The site(s) of metabolism are unknown, however, it is possible that some metabolism occurs in the liver. As drug metabolism may be affected in patients with hepatic dysfunction [18, 19], it was possible that ribavirin clearance might be altered in patients with hepatic dysfunction compared with healthy control subjects.
This single dose study showed that the overall pharmacokinetic profile of ribavirin was not different in controls and patients with hepatic dysfunction. AUC, tmax and the terminal elimination phase profile (Figure 1) were similar between treatment groups, indicating that hepatic dysfunction did not have substantial influence on the apparent clearance (CL/F) of ribavirin following a single oral dose. As ribavirin does not bind at all to plasma proteins (data on file, Schering-Plough), between group differences in albumin or other protein concentrations would not affects these pharmacokinetic results. These findings are also consistent with mean single dose pharmacokinetic parameters reported in patients with compensated chronic hepatitis C (ribavirin monotherapy: Cmax: 782 ng ml−1; AUC (0,t): 13394 ng ml −1 h; ribavirin plus interferon-α2b: Cmax: 1030 ng ml−1; AUC (0,t): 16942 ng ml−1 h [11]).
These findings suggest that the liver is not the major organ associated with presystemic elimination of ribavirin. Two 14C -labelled mass balance studies have shown that the majority of orally administered doses of ribavirin (approximately 85%) is absorbed[15, Schering-Plough, data on file]. However absolute bioavailability is approximately 50% [9, 10, 16, 17]. Therefore, since liver dysfunction did not change the bioavailability of ribavirin, it is possible that the major site of first-pass elimination may be in the gastrointestinal tract. Renal clearance was similar between the four groups and is consistent with values previously reported in healthy volunteers (5.26 l h−1 [20]);. The fraction of parent drug eliminated in urine (Ae; 9–10% for all groups) was consistent with previous published reports (5–15% [9, 15]);. The similar renal clearance values in all groups is consistent with preserved renal function in the cirrhotic patients participating in this study.
The only pharmacokinetic parameter that was different between hepatic dysfunction patients and controls was Cmax. Mean Cmax and concentration-time profiles between 0 and 6 h were increased relative to the severity of hepatic dysfunction. However there was considerable overlap in individual Cmax values between the four groups, and in light of the high pharmacokinetic variability of this drug, this may be a chance finding.
In conclusion, this study did not show alteration of ribavirin pharmacokinetics in patients with hepatic dysfunction compared with control subjects. The main clinical implication of this study is that there are no pharmacokinetic reasons for initial dose adjustment of ribavirin in patients with hepatic dysfunction.
Acknowledgments
The assistance of Josephine Lim, Anita Durso and Brigid Siegel (SPRI) and Joan Finch, RN (Texas) is gratefully acknowledged.
References
- 1.Fernandez H, Banks G, Smith R. Ribavirin: a clinical overview. Eur J Epidemiol. 1986;2:1–14. doi: 10.1007/BF00152711. [DOI] [PubMed] [Google Scholar]
- 2.Sidwell R, Hoffman J, Kharp L, et al. Broad spectrum antiviral activity of virazole: 1-beta-d-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science. 1972;117:705–706. doi: 10.1126/science.177.4050.705. [DOI] [PubMed] [Google Scholar]
- 3.Sidwell RW, Robins RK, Hillyard IW. Ribavirin: an antiviral agent. Pharmacol Ther. 1979;6:123–146. doi: 10.1016/0163-7258(79)90058-5. [DOI] [PubMed] [Google Scholar]
- 4.Di Bisceglie AM, Conjeevaram HS, Fried MW, et al. Ribavirin as therapy for chronic hepatitis C. Ann Int Med. 1995;123:897–903. doi: 10.7326/0003-4819-123-12-199512150-00001. [DOI] [PubMed] [Google Scholar]
- 5.Davis GL, Estaban-mur R, Rustgi V, et al. Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. N Engl J Med. 1998;339:1493–1499. doi: 10.1056/NEJM199811193392102. [DOI] [PubMed] [Google Scholar]
- 6.McHutchison JG, Gordon SC, Schiff ER, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N Engl J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 7.Poynard T, Marcellin P, Lee SS, et al. Randomized trial of interferon α-2b plus ribavirin for 48 weeks or 24 weeks versus interferon α-2b plus placebo for 48 weeks the treatment of chronic infection with hepatitis C virus. Lancet. 1998;352:1426–1432. doi: 10.1016/s0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- 8.Reichard O, Norkrans G, Fryden A, et al. Randomized double-blind placebo-controlled study of interferon α-2b with and without ribavirin for chronic hepatitis C. Lancet. 1998;351:83–87. doi: 10.1016/s0140-6736(97)06088-1. [DOI] [PubMed] [Google Scholar]
- 9.Paroni R, Del Puppo M, Borghi C, et al. Pharmacokinetics of ribavirin and urinary excretion of the major metabolite 1,2,4-triazole-3-carboxamide in normal volunteers. Int J Clin Pharmacol Ther Toxicol. 1989;27:302–307. [PubMed] [Google Scholar]
- 10.Laskin OL, Longstreth JA, Hart CA, et al. Ribavirin disposition in high risk patients for acquired immunodeficiency syndrome. Clin Pharmacol Ther. 1987;41:546–555. doi: 10.1038/clpt.1987.70. [DOI] [PubMed] [Google Scholar]
- 11.Khakoo S, Glue P, Grellier L, et al. Ribavirin and interferon alpha-2b in chronic hepatitis C. assessment of possible pharmacokinetic and pharmacodynamic interactions. Br J Clin Pharmacol. 1998;46:563–570. doi: 10.1046/j.1365-2125.1998.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pugh R, Murray-lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 13.Child CG. Philadelphia: Saunders; The liver and portal hypertension. [Google Scholar]
- 14.Gibaldi M, Perrier D. 2. New York: Marcel Dekker; Pharmacokinetics; pp. 409–417. [Google Scholar]
- 15.Catlin DH, Smith RA, Samuels AI. 14C-ribavirin distribution and pharmacokinetic studies in rats, baboons and man. In: Smith RA, Kirkpatrick W, editors. Ribavirin: a Broad Spectrum Antiviral Agent. New York: Academic Press; 1980. pp. 83–98. [Google Scholar]
- 16.Preston SL, Drusano GL, Glue P, Nash J, Gupta SK, McNamara P. Pharmacokinetics and absolute bioavailability of ribavirin using stable-label isotope methodology in normal volunteers. Antimicrob Agents Chemother. 1999;43:2451–2456. doi: 10.1128/aac.43.10.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lertora JJL, Rege AB, Lacour JT, et al. Pharmacokinetics and long-term tolerance to ribavirin in asymptomatic patients infected with human immunodeficiency virus. Clin Pharmacol Ther. 1991;50:442–449. doi: 10.1038/clpt.1991.162. [DOI] [PubMed] [Google Scholar]
- 18.Branch RA. Drugs in liver disease. Clin Pharmacol Ther. 1998;64:462–465. doi: 10.1016/S0009-9236(98)90077-7. [DOI] [PubMed] [Google Scholar]
- 19.McLean AJ, Morgan DJ. Clinical pharmacokinetics in patients with liver disease. Clin Pharmacokinet. 1991;21:42–69. doi: 10.2165/00003088-199121010-00004. [DOI] [PubMed] [Google Scholar]
- 20.Glue P. The clinical pharmacology of ribavirin. Sem Liv Dis. 1999;19(Suppl 1):17–24. [PubMed] [Google Scholar]