Abstract
Aims
Fibrinogen receptor antagonists show a close relationship between plasma concentrations and inhibitory effect. Optimal efficacy at an acceptable bleeding risk requires low inter- and intrasubject variability on low peak trough fluctuation in receptor occupancy and therefore also of plasma concentrations. Therefore, the enteral absorption of lefradafiban, an orally available fibrinogen receptor antagonist prodrug, was investigated after local administrations to different sites of the gastrointestinal tract in order to investigate the feasibility of an oral extended release formulation.
Methods
Twelve healthy male subjects received in a randomised, open-labelled, four-period crossover trial four consecutive administrations of lefradafiban: 1. orally; 2. administration into the jejunum, 3. administration into the lower jejunum/ileum (300 cm distally to the teeth), and 4. administration into the lumen of the sigmoid region (30 cm proximally to the anus). Local intestinal administrations were performed through a gastrointestinal tube.
Results
Compared with oral administration, ratios [mean (two-sided 90% confidence intervals)] of maximum drug plasma concentrations and AUC(0,24 h) of fradafiban were 1.05 (0.80,1.39) and 1.06 (0.85,1.31) after jejunal, 0.98 (0.75,1.30) and 0.98 (0.79,1.21) after ileal, 0.52 (0.39,0.69) and 0.68 (0.55,0.85) after colonic administration. Urinary excretion of fradafiban was about 16% of the dose after oral, jejunal and ileal applications whereas after rectal administration about 11% were excreted.
Conclusions
Lefradafiban is absorbed throughout the entire gastrointestinal tract. Therefore, an extended release formulation seems to be feasible with regard to bioavailability.
Keywords: enteral absorption, fibrinogen receptor antagonist, fradafiban, lefradafiban
Introduction
Platelets play an important role in the pathogenesis of thrombembolic and atherosclerotic diseases. Activation of the platelet glycoprotein IIb/IIIa receptor has emerged as the key step in the induction of platelet aggregation, which may lead to haemostasis [1]. Recently, several orally active nonpeptide inhibitors of the glycoprotein IIb/IIIa receptor became available [2]. One of these new synthetic inhibitors is fradafiban, which is concerted from its prodrug lefradafiban following enteral absorption. Optimal efficacy at an acceptable bleeding risk requires low inter- and intrasubject variability on low peak trough fluctuation in receptor occupancy and therefore also of plasma concentrations, since a close relationship between the plasma concentrations and inhibitory effect has been established [3]. This might be achieved with oral administration of an extended release formulation. In order to test feasibility of a conventional extended release formulation, the enteral absorption of lefradafiban was investigated after local administration at different sites of the small and large intestine in the present human study.
Methods
Selection of subjects
The study protocol and informed consent forms were approved by the Human Ethics Committee of the University Clinic Basel/Kantonsspital prior to the start of the study. All subjects gave their written informed consent.
The study was performed in 12 healthy male volunteers aged between 22 and 48 years, having body weights between 60 and 88 kg and heights between 168 and 193 cm. Subjects were healthy as assessed by medical history, clinical examinations including ECG and laboratory investigations of blood and urine. Special exclusion criteria were: surgery of the gastrointestinal tract (except appendectomy), history of any disorder or event associated with an increased risk for bleeding, abnormal platelet aggregation in platelet rich plasma, thrombocyte counts < 150000 µl−1, or a positive test for occult blood in stools (Haemoccult®) at pre-examination.
Medication
Lefradafiban (10 mg) was dissolved in 15 ml of acidic aqueous solvent (containing tartaric acid) immediately before administrations so that absorption was unbiased by dissolution rates. Tartaric acid was used because of pH-dependent solubility of lefradafiban. The low dose was selected to mimic the low release rate occurring with extended release formulations and to avoid large amounts of acids, which might interfere with physiology of the intestinal tract. Drug and solvent were obtained from Boehringer Ingelheim Pharma KG, Biberach, Germany.
Administration
Lefradafiban (solution containing 10 mg in 15 ml) was administered as single doses as bolus injections directly into the jejunum, ileum and colon to the subjects by gastrointestinal tubes. The tubes were flushed with 10 ml of water after administration of drug. The volume was expected to have no relevant influence on peristalsis.
The subjects received four consecutive administrations in an open-label, randomised, four period cross-over design. These administrations are as follows: 1. orally; 2. administration into the jejunum, 3. administration into the lower jejunum/ileum (300 cm distally to the teeth), and 4. administration into the lumen of the sigmoid region (30 cm proximally to the anus). Local intestinal administrations were performed through a gastrointestinal tube (Cartmill feeding tube, Hollander Medizintechnik, Cham, Switzerland).
Before the intestinal drug administrations, subjects were orally intubated in supine position with a one-lumen gastrointestinal tube (Cartmill feeding tube, Hollander Medizintechnik, Cham, Switzerland). The tube has an inner diameter of 1.5 mm, outer diameter of approximately 2.5 mm, and a length of 3.5 m. Tubes were placed under fluoroscopic control with the tip placed in the upper jejunum or in the lower jejunum/ileum. The tubes were swallowed in the evening before the experiments and allowed to be transported distally by the peristaltic movements of the gut. The proximal part of the tube was then rerouted to the nose and firmly attached to the skin behind the ear. This procedure resulted in the minimum amount of discomfort. On the administration day, the correct position of the tube was checked by fluoroscopy and corrected, if necessary.
The retrograde tube administration into the colon was done 15 min before drug administration; the tube was placed with a rigid introduction instrument without prior enema or cathartics administration by a trained gastroenterologist. The tube was placed 30 cm proximally to the anus and firmly fixed externally to the skin to prevent movement. At the time of each administration, subjects were in supine position. After administration, the subjects remained intubated in supine position for 4 h. Then the tubes were carefully pulled out. Two subsequent administrations were separated by a washout period of at least 1 week.
Pharmacokinetic sampling
Blood samples (4 ml) were collected in EDTA-coated tubes from each volunteer at baseline (predose) and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 24 and 48 h after administration. Plasma was stored at −20 °C until analysed by high-pressure liquid chromatography (h.p.l.c). An urine sample was collected predose, and all urine voided during the time intervals 0–12 h, 12–24 h and 24–48 h postdose was collected.
Drug determinations
Fradafiban plasma and urine concentrations were analysed by an automated column switching h.p.l.c. assay using an internal standard. Chromatographic separation was achieved with ODS-Hypersil. Fluorescence detection (excitation at 310 nm, emission at 430 nm) was used for quantification. The validated ranges covered a range of 1–500 ng ml−1. Minimum assay precision and assay accuracy values in quality control samples (n = 31) were < 5%.
Pharmacokinetic parameters
Maximum plasma concentrations (Cmax) of fradafiban and times to reach peak plasma concentration (tmax) were obtained directly from the measured data. The individual profiles of fradafiban plasma concentrations and urinary excretion were simultaneously evaluated by compartmental methods employing TOPFIT software [4]. The model comprised a first-order formation process of fradafiban from lefradafiban, first-order absorption of fradafiban from the gut lumen, a two-compartment disposition and a first-order renal elimination. Fitting was performed for different treatments using the same disposition and elimination constants as for the oral administration. In addition, noncompartmental methods were used for calculation of area under the plasma concentration/time curve (AUC(0,∞), the total mean residence time (MRT) and MRT of absorption as well as apparent total clearance (CL/F), as defined in [4].
Adverse events
At each visit, the occurrence of adverse events was assessed and evaluated. In particular, signs of nose and gum bleeding, petechiae and haematuria were assessed.
Dietary and general instructions
The study started in the morning after an overnight fast of 10 h with only water allowed. Only caffeine-free beverages were allowed for the first 12 h after drug administration. Subjects had to refrain from smoking during the study until 12 h after drug administration. No food was permitted for the first hours after each drug administration. The subjects were restricted to not more than 50 ml water per hour. A standardized liquid lunch (500 ml Ensure®, Abbott Laboratories, Cham, Switzerland) was given 4 h after drug administration. This lunch contained a caloric content of 500 kcal. It was to be swallowed by the subjects within 5 min.
Results
Administrations of lefradafiban were well tolerated. There were no bleeding events. Headache of mild to moderate intensity was observed in many subjects. One subject reported mild diarrhoea. No event was considered to be drug related. All resolved spontaneously without therapy or sequelae.
Pharmacokinetics of fradafiban
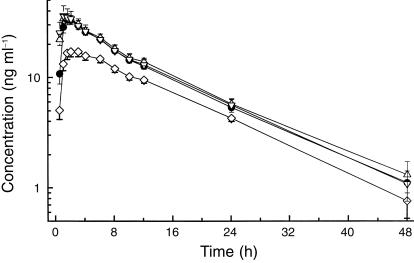
Plasma levels and urinary excretion of fradafiban could be quantified, with the exception of subject 8, after administration of drug into the colon. In this subject, no fradafiban at all could be detected, whereas all other subjects showed plasma concentrations being at least tenfold above the lower limit of quantification. Therefore, colonic administration of subject 8 was excluded from statistical evaluation. Plasma drug concentrations of fradafiban increased rapidly after drug intake (Figure 1; Table 1). After oral administration, Cmax occurred at 1.72 h and amounted to 33.72 ng ml−1. Plasma profiles could be well described with a linear two-compartment model with half-lives of disposition and elimination of 0.12 and 10.16 h, respectively. Apparent total and renal clearances were 410.3 and 67.58 ml min−1. Renal elimination was 17% of the applied dose. No statistically significant differences were observed in pairwise comparisons between oral, jejunal and ileal administrations except for the parameter tmax, which showed statistically significant (but biologically irrelevant) differences between some treatments. In comparison with oral and small intestinal administrations, colonic administration showed a reduced bioavailability, a decreased Cmax, and an increased MRT of absorption. Whereas, after oral and small intestinal administration between 16.2 and 17.1% of the applied dose was excreted into urine, this amount decreased to 10.9% after rectal administration.
Figure 1.
Mean ± s.e. mean plasma concentrations of fradafiban after local administrations to different parts of the intestinal tract (n = 12). •oral, ▵ jejunum, ▿ ileum, ⋄ colon.
Table 1.
Pharmacokinetic parameters of fradafiban after local administration to different parts of the intestine in 12 healthy male volunteers.
| Parameter | p.o. | Jejunum | Ileum | Colon | P value |
|---|---|---|---|---|---|
| MRTtotal (h) | 14.25 ± 0.67 | 14.10 ± 0.65 | 14.18 ± 0.71 | 15.79 ± 0.80 | NS |
| MRTabs (h) | 4.21 ± 0.17 | 4.05 ± 0.17 | 4.14 ± 0.26 | 5.56 ± 0.20 | < 0.001(a) |
| AUC(0, ∞) (ng ml−1 h) | 427.5 ± 29.1 | 460.4 ± 43.7 | 439.2 ± 55.7 | 290.0 ± 19.1 | 0.00715(a) |
| tmax, model (h) | 1.72 ± 0.11 | 1.37 ± 0.08 | 1.38 ± 0.12 | 2.33 ± 0.13 | < 0.0001(b) |
| Cmax, model (ng ml−1)] | 33.72 ± 2.98 | 35.58 ± 3.12 | 38.20 ± 8.07 | 17.57 ± 1.79 | < 0.0003(a) |
| AE (%) | 17.0 ± 1.4 | 16.2 ± 1.4 | 17.1 ± 2.5 | 10.9 ± 0.9 | < 0.0137(a) |
Data represent mean ± s.e mean.
colonic administration was significantly different (P < 0.05) from each of the others in pairwise comparisons
all pairwise comparisons were statistically significantly different except for comparison of jejunal and ileal administration; NS = not significantly different. Cmax: maximum plasma concentrations; tmax: times of Cmax: AUC: areas under the plasma concentration/time curve; Ae (%): amount of drug excreted renally (% of dose). To account for different molecular masses of lefradafiban and fradafiban, a correction factor of 1.196 was applied.
Discussion
The results show that lefradifiban is absorbed along the entire gastrointestinal tract. The time course of fradafiban plasma concentrations and all pharmacokinetic parameters derived either from plasma concentrations or urinary excretion, show that rate and extent of absorption were not significantly different for oral administration and into the small intestine (e.g. jejunum and ileum). However, rate and extent of absorption were significantly decreased after colonic administration.
In this study, no tubes with blocking balloons were used to prevent luminal drug transport along the gastrointestinal tract. In addition, no marker technique was applied to quantify the net transport of drug across the gut wall. Therefore, no inferences could be drawn with regard to the capacity of the investigated gut segments to absorb lefradafiban. On the other hand, our design allowed the drug to be transported freely to more distal parts of the gastrointestinal tract. This was assumed to mimic more appropriately the fate of drug released from an extended release dosage form.
The reduced surface area available for absorption may explain the diminished bioavailability after colonic administration. On the basis of the strong relationship between pharmacokinetic and pharmacodynamic effects of fradafiban, our results suggest that changes in gastric emptying or GI transit may be without any significant effects on the pharmacokinetics of fradafiban and hence bleeding potential. Development of an extended release formulation is feasible with regard to bioavailability.
References
- 1.Tcheng JE. Glycoprotein IIb/IIIa receptor inhibitors: putting the EPIC, IMPACT II, RESTORE, and EPILOG trials into perspective. Am J Cardiol. 1996;78:35–40. doi: 10.1016/s0002-9149(96)00490-0. [DOI] [PubMed] [Google Scholar]
- 2.Simpendorfer C, Kottke-Marchant K, Lowrie M, et al. First chronic platelet glycoprotein IIb/IIIa integrin blockade. A randomized, placebo-controlled pilot study of xemilofiban in unstable angina with percutaneous coronary interventions. Circulation. 1997;96:76–81. doi: 10.1161/01.cir.96.1.76. [DOI] [PubMed] [Google Scholar]
- 3.Müller TH, Weisenberger H, Brickl R, Narjes H, Himmelsbach F, Krause J. Profound and sustained inhibition of platelet aggregation by Fradafiban, a nonpeptide platelet glycoprotein IIb/IIIa antagonist, and its orally active prodrug, Lefradafiban, in men. Circulation. 1997;96:1130–1138. doi: 10.1161/01.cir.96.4.1130. [DOI] [PubMed] [Google Scholar]
- 4.Heinzel G, Woloszczak R, Thomann P. Topfit 2.0. Pharmacokinetic and pharmacodynamic data analysis system for the PC. Stuttgart-Jena-New York: Gustav Fischer; 1993. [Google Scholar]