Abstract
Aims
To characterize milk/plasma (M/P) ratio and infant dose, for citalopram and demethylcitalopram, in breast-feeding women taking citalopram for the treatment of depression, and to determine the plasma concentration and effects of these drugs in their infants.
Methods
Seven women (mean age 30.6 years) taking citalopram (median dose 0.36 mg kg−1 day−1) and their infants (mean age 4.1 months) were studied. Citalopram and demethylcitalopram in plasma and milk were measured by high-performance liquid chromatography over a 24 h dose interval. Infant exposure was estimated (two separate methods) as the product of milk production rate and drug concentration in milk, normalized to body weight and expressed as a percentage of the weight-adjusted maternal dose.
Results
Mean M/PAUC values of 1.8 (range 1.2–3) and 1.8 (range 1.0–2.5) were calculated for citalopram and demethylcitalopram, respectively. The mean maximum concentrations of citalopram and demethylcitalopram in milk were 154 (95% CI, 102–207) µg l−1 and 50 (23–77) µg l−1. Depending on the method of calculation, mean infant exposure was 3.2 or 3.7% for citalopram and 1.2 or 1.4% for demethylcitalopram. Citalopram (2.0, 2.3 and 2.3 µg l−1) was detected in three of the seven infants. Demethylcitalopram (2.2 and 2.2 µg l−1) was detected in plasma from two of the same infants. No adverse effects were seen in the infants, all were within appropriate percentile limits for weight and all had normal Denver developmental quotients.
Conclusions
The mean combined dose of citalopram and demethylcitalopram (4.4–5.1% as citalopram equivalents) transmitted to infants via breast milk is below the 10% notional level of concern. Plasma concentrations of these drugs in the infants were very low or absent and there were no adverse effects. These data support the safety of the use of citalopram in breast feeding women. Nevertheless, each decision to breast feed should always be made as an individual risk:benefit analysis.
Keywords: citalopram, demethylcitalopram, human milk, infant dose, M/P ratio
Introduction
Citalopram, a bicyclic isobenzofuran derivative [1] is a selective serotonin reuptake inhibitor. It is a racemic mixture of the (–)-R-and (+)-S-enantiomers, with the latter having the most potent and selective effect on 5-hydroxytryptamine uptake. Bioavailability after oral administration is approximately 80%, with peak plasma levels occurring 2–4 h after a dose [2, 3]. The volume of distribution is 12–16 l kg−1[2] and the elimination half-life of citalopram is approximately 37 h [3]. Citalopram is lipid soluble and is metabolized in the liver mainly to a primary metabolite N-demethylcitalopram, which is then converted to N-didemethylcitalopram [4]. Three different cytochrome P450 isoforms are thought to be involved in its disposition (CYP2C19, CYP3A4 and CYP2D6 [5]);. The demethylated metabolites are less potent in vitro as serotonin reuptake inhibitors and are less able to penetrate to the brain [6]. The plasma protein binding of citalopram and its metabolites is approximately 50–80% [2]. Citalopram is also a weak inhibitor of cytochrome P4502D6 and has a minimal propensity to cause drug interactions with other CYP2D6 drug substrates [2].
A major depressive disorder will occur in around 13% of women in the postnatal period, and a substantive number of these women will benefit from the use of antidepressant medication [7]. In recent years the drive to breast feed babies has undergone a resurgence for biological, psychological and social reasons. Lactating women who are recommended to commence an antidepressant have understandable concerns about the possible transfer of psychotropic medication into the breast milk and thence to their neonate. To assist in this area of patient management, we have studied the transfer of citalopram and demethylcitalopram into milk in seven lactating women and related this data to plasma concentrations and effects in their breast-fed infants.
Methods
Patients
Seven breast-feeding women (mean age 31 years, range 24–36 years; mean body weight 62.6 kg, range 52–70 kg) and their infants (3 M and 4 F; mean age = 4.1 months, range 1.9–6.6 months) were enrolled in the study. The median dose of citalopram ingested by the women was 0.36 (range 0.29–0.58) mg kg−1 day−1. Therapy with citalopram had commenced a mean of 97 (range 49–183) days prior to the study day, and all participants were considered to be at steady-state at the time of study.
Study protocol
The study was approved by the Ethics Committee of King Edward Memorial and Princess Margaret Hospitals, and written informed consent was obtained from all participants.
Data collection
The mothers were admitted to the research ward at 07.30 h, and had a venous catheter inserted into a forearm vein immediately prior to the morning dose of citalopram at 08.00 h. Venous blood samples (8 ml, heparinized) were collected via the catheter at 0, 2, 4, 6 and 8 h postdose, and also by venepuncture at 12 and 24 h. At the same time intervals, both breasts were emptied via an electric or manual breast pump. Milk volumes were recorded and 15 ml aliquots were retained for drug assay. A sample of milk was also collected directly into a 1 ml blood-gas syringe (Bard-Parker, Becton-Dickinson, NJ, USA) and pH was measured using a NOVA StatProfile® blood gas analyser (NOVA Biomedical, Waltham MA, USA). Preliminary experiments established that the pH and pCO2 of samples collected using this procedure did not change significantly over a 9 h period (data not shown). Creamatocrit (% fat in milk) for each milk sample was measured as previously described [8]. The remainder of the milk was bottle-fed to the infants as required. The women were discharged from hospital after 8 h and milk and plasma samples at 12 and 24 h postdose were collected at the patient's home. All women gave consent for a venous blood sample (0.5–1 ml, heparinized) to be taken from their infants. Infant blood samples were collected at a mean of 6.5 (6.2, 6.8) h after the maternal dose.
For all studies, infant health and well being were evaluated by enquiry of the mother together with a full clinical examination by a specialist neonatologist (RK). Infant body weight for age was checked against gender-specific population percentile graphs and a Denver developmental screening test was performed [9, 10]. Results for the latter were expressed as the quotient of age as assessed by the Denver test as a percent of chronological age.
Materials
Citalopram and demethylcitalopram standards were donated by Lundbeck Australia Pty Ltd, and desipramine hydrochloride by Novartis Pharmaceuticals Australia Pty Ltd. All solvents and other chemicals were of analytical or h.p.l.c. grade.
High performance liquid chromatography (h.p.l.c.)
Following the addition of desipramine (100 ng) as an internal standard, 1 ml aliquots of plasma were made alkaline with 0.1 ml of 1 m NaOH and the analytes were extracted into 10 ml 1% isoamylalcohol in hexane by shaking vigorously for 5 min. After centrifugation (1500 g for 5 min), the organic phase (9 ml) was back-extracted into 0.2 ml 0.05 m HCl by vortexing for 1 min, and 0.05–0.1 ml aliquots of the acid phase were injected into the h.p.l.c. Milk samples were extracted in a similar manner except that the ‘method of addition’ was used. Rac-citalopram and rac-demethylcitalopram concentrations in milk were determined by taking four equal aliquots of each milk sample (1 ml) and spiking three of these with increasing concentrations of authentic citalopram and demethylcitalopram. The samples were then extracted and analysed as for plasma. The h.p.l.c. system consisted of a Merck RP Select B C18 column (250 × 4.6 mm), a mobile phase of 40% acetonitrile containing 0.01% sodium chloride and 0.01% H3PO4 (1.6 ml min−1), with u.v. detection at 210 nm. Plasma drug concentrations were interpolated from a standard curve (peak height ratio analyte: desipramine vs analyte concentration (r2 > 0.995). For milk, a standard curve (peak height ratio analyte: desipramine vs added analyte concentration (r2 > 0.995) was constructed and drug concentrations were determined from the negative x-axis intercept. Coefficients of variation (CV) were measured at 25 and 250 µg l−1 for both citalopram and demethylcitalopram. Intra-day CV (both analytes) ranged from 1.7 to 5.5% for plasma and 4.7–6.4% for milk, while interday CV ranged from 2.9 to 8.2% for plasma and from 5.8 to 13.2% for milk. The limit of detection for both analytes in milk and plasma was 1 µg l−1.
Measurement of plasma protein binding
Protein binding for citalopram and demethylcitalopram (one sample from each of five volunteers) was measured by ultrafiltration using Amicon Centrifree YM-30 centrifugal filter devices (Millipore Corporation, MA, USA) and the h.p.l.c. method described above.
Measurement of octanol:buffer partition coefficients
Citalopram and demethylcitalopram (each 10 mg l−1) were dissolved in 0.02 m phosphate buffer (pH 7.2) and aliquots were equilibrated with an equal volume of n-octanol by shaking vigorously for 10 min. The concentration of each analyte in the buffer was determined before and after the extraction process using the above h.p.l.c. method and log10P values calculated from these data.
Statistical evaluation of data
Data have been summarized as mean (95% CI, or range), or median (range) as appropriate. Difference between mean infant dose calculated by two different methods was assessed using a paired Student's t-test. Differences between milk pH or creamatocrit between patients and collection times were assessed by 2-way anova. Repeated measures 2-way anova was used to investigate the relationship between M/P and milk pH, creamatocrit and subjects for both citalopram and demethylcitalopram. 2-way anova was also used to investigate inter and intravolunteer variability in milk pH and creamatocrit.
Calculation of milk/plasma ratios and infant dose
Area under the concentration-time profiles (AUC(0, 24 h)) was calculated using the log trapezoidal rule [11] for plasma, and rectangular areas for milk (Σ concentration × collection time). Milk/plasma ratios (M/PAUC) were calculated from these AUC data, and also from milk and plasma concentrations measured at each collection time (M/P). The absolute infant citalopram or demethylcitalopram (as citalopram equivalents) dose was calculated by two separate methods, both assuming an oral bioavailability of 100%. In method A, the measured cumulative drug excretion over 24 h (Σ milk concentration for each collection period × volume) was divided by the infant's body weight to give a dose in mg kg−1. In method B, an average infant intake of 0.15 l milk kg−1 day−1 was assumed [12], and this value was multiplied by the average milk concentration (AUCmilk/dose interval time) to give a dose in mg kg−1. For both methods, the infant dose was then expressed as a percentage of the maternal weight-normalized dose. M/P for citalopram was also calculated directly from a knowledge of its pKa (9.5; data on file, Lundbeck Australia Pty Ltd), plasma protein binding and log10P (octanol:buffer, pH 7.2) according to the method of Begg et al.[13].
Results
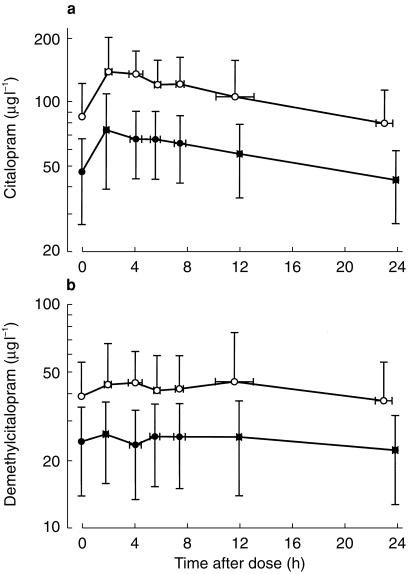
Mean plasma and milk concentration-time profiles for citalopram and demethylcitalopram (n = 7) are shown in Figure 1. Peak drug concentrations occurred at a mean of 3.9 h and 5.7 h after dose for citalopram and demethylcitalopram, respectively (Table 1). The mean Cmax for citalopram was 154 µg l−1 (102, 157 µg l−1) and 50 µg l−1 (23,77 µg l−1) for demethylcitalopram (Table 1). However, average concentrations across the dose interval were somewhat lower with mean values of 97 µg l−1 (56, 138 µg l−1) and 36 µg l−1 (15,58 µg l−1) for citalopram and demethylcitalopram, respectively. The mean M/PAUC ratio was 1.8 (1.2, 2.3) for citalopram and 1.8 (1.0, 2.5) for demethylcitalopram (Table 1).M/P-values calculated at each collection time were subjected to repeated measures 2-way anova using milk pH, creamatocrit and volunteers as independent factors, but no significant relationships were present (P > 0.05) for either citalopram or demethylcitalopram.
Figure 1.
Plasma (•) and milk (○) concentration-time profiles for citalopram (a) and demethylcitalopram (b) in seven mothers over a 24 h dose interval (commencing 08.00 h) at steady-state. Data are presented as mean ± 95% CI. The negative error bars on selected plots have been omitted for clarity.
Table 1.
Milk concentrations and M/P ratios for citalopram and demethylcitalopram.
| Citalopram | Demethylcitalopram | |||||||
|---|---|---|---|---|---|---|---|---|
| Volunteer | tmax (h) | Maximum1 milk concentration (µg l−1) | Average2 milk concentration (µg l−1) | M/PAUC | tmax (h) | Maximum milk concentration (µg l−1) | Average milk concentration (µg l−1) | M/PAUC |
| 1 | 2.6 | 210 | 107 | 1.4 | 11.1 | 67 | 48 | 1.4 |
| 2 | 3.8 | 103 | 56 | 0.9 | 3.8 | 32 | 23 | 1.0 |
| 3 | 3.8 | 82 | 53 | 1.4 | 2.9 | 27 | 23 | 1.1 |
| 4 | 7.8 | 186 | 141 | 2.0 | 3.8 | 48 | 34 | 1.5 |
| 5 | 3.5 | 232 | 168 | 1.9 | 10.8 | 109 | 83 | 1.9 |
| 6 | 3.8 | 119 | 83 | 2.6 | 1.8 | 35 | 29 | 2.1 |
| 7 | 1.9 | 147 | 68 | 2.3 | 5.7 | 34 | 15 | 3.3 |
| Mean | 3.9 | 154 | 97 | 1.8 | 5.7 | 50 | 36 | 1.8 |
| (95% CI) | (2.2, 5.6) | (102, 207) | (56, 138) | (1.2,2.3) | (2.2, 9.2) | (23, 77) | (15, 58) | (1.1, 2.5) |
Maximum value recorded during the dose interval.
Calculated as AUC(0,24h)/dose interval.
Table 2 summarizes the estimated infant dose of citalopram and demethylcitalopram as citalopram equivalents. Infant dose was calculated by two different methods. The mean (95% CI) absolute infant doses calculated by method A (actual cumulative drug excretion) were 3.2% (1.9, 4.6) and 1.2% (0.7, 1.7) for citalopram and demethylcitalopram, respectively. Doses calculated by method B (assuming an estimated infant milk intake of 0.15 l kg−1 day−1) were 3.7% (2.6, 4.8) for citalopram and 1.4% (0.9, 1.9) for demethylcitalopram. There were no significant differences between the mean infant doses calculated by either method. The mean milk pH for the whole group was 7.23 (7.09, 7.37). Two-way anova showed that milk pH varied significantly between volunteers (F = 14.8, P < 0.001) but not between collection times. Mean creamatocrit for the whole group was 4.8% (4.1, 5.5). There were significant differences in creamatocrit between volunteers (F = 2.8, P = 0.024) and between collection times (F = 4.6, P = 0.002).
Table 2.
Estimated infant dose for citalopram and demethylcitalopram (as citalopram equivalents), and infant plasma concentrations of citalopram and demethylcitalopram.
| Citalopram | Demethylcitalopram | |||||
|---|---|---|---|---|---|---|
| Volunteer | Infant dose as % of maternal dose Method A1 | Method B2 | Infant plasma concentration (µg l−1) | Method A1 | Infant dose as % of maternal dose Method B2 | Infant plasma concentration (µg l−1) |
| 1 | 3.9 | 3.6 | 2 | 1.8 | 1.7 | ND3 |
| 2 | 1.6 | 3.0 | ND | 0.6 | 1.2 | ND |
| 3 | 1.3 | 2.0 | ND | 0.6 | 0.9 | ND |
| 4 | 5.6 | 5.9 | 2.3 | 1.6 | 1.5 | 2.2 |
| 5 | 3.9 | 4.4 | 2.3 | 1.9 | 2.3 | 2.2 |
| 6 | 2.7 | 4.0 | ND | 1.0 | 1.5 | ND |
| 7 | 3.6 | 3.0 | ND | 1.0 | 0.7 | ND |
| Mean (95% CI) | 3.2 (1.9, 4.6) | 3.7 (2.6, 4.8) | 1.2 (0.7, 1.7) | 1.4 (0.9, 1.9) | ||
Infant dose (cumulative dose over 24 h in µg/(infant body wt in kg × 1000)/mother's dose in mg kg−1) as a percentage.
Infant dose (average milk concentration in µg l−1 × 0.15 l kg−1 day−1 × 1000)/mother's dose in mg kg−1) as a percentage.
ND = less than detection limit (1 µg l−1).
Low concentrations (around 2–2.3 µg l−1) of citalopram and demethylcitalopram were detected in plasma from three and two of the seven infants, respectively (Table 2). None of the mothers reported any adverse effects in their infants and a full clinical examination revealed no motor or tone abnormalities. Infant weights at the time of study (mean = 6.2 kg, range 3.7–9.1 kg) were within the same (or greater) percentiles as they were at birth (mean = 3.2, range 2.5–3.8 kg). Values for the Denver development quotient were normal for age (median = 100%, range 100–119%).
Log P octanol:buffer pH 7.2-values for citalopram and demethylcitalopram were 1.452 and 0.242, respectively. Mean plasma protein binding (five patients) was 70.9% (65.2–76.6) for citalopram and 67.5% (60.7–74.3) for demethylcitalopram. Calculation of the theoretical M/P for citalopram [14], using the above log P value, a mean milk pH of 7.2 and assuming 71% protein binding gave a value of 1.3. Varying the percent protein binding in this calculation (from 65 to 77% = the 95% CI) gave a predicted M/P varying from 1.5 to 1.1, respectively.
Discussion
Although both citalopram and its major metabolites have enantiomers with modest differences in their pharmacokinetics [15, 16], in the present study we were primarily interested in maximum infant exposure and have therefore confined our attention to the racemates of citalopram and its major metabolite demethycitalopram.
Milk pH averaged 7.23 with significant intervolunteer variation, but no significant variation across the various sampling times. Creamatocrit showed significant variation both between volunteers and across collection times. However, neither milk pH nor creamatocrit were significant predictors of M/P after intervolunteer variability was isolated. We were not able to include plasma protein binding in this analysis as we had made only one measurement from five of the seven volunteers. The citalopram concentration-time profile in milk showed a similar mean peak:trough concentration ratio (1.6) to that for its demethyl metabolite (1.4). M/PAUC also was similar (1.8) for both citalopram and demethylcitalopram. The similar extent of transfer of these analytes into milk occurred despite the higher log P value for citalopram. Overall, our data suggest that lipid solubility, milk pH and creamatocrit are not the major determinant variables for transfer of these drugs into milk. In this regard, it has previously been noted that plasma protein binding can often be highly influential in drug transfer to milk [13]. Theoretical prediction of the M/P for citalopram by the method of Begg et al.[13] gave a value of 1.3, which was at the lower end of the 95% CI for the mean measured value (1.0–2.5). This confirmed that this method is useful in the absence of actual patient data. M/P changed inversely with percent binding of citalopram in plasma. Increasing percent bound from 71 to 77% decreased predicted M/P from 1.3 to 1.1 (15%).
There are two published papers describing the excretion of citalopram into breast milk. Jensen [17] studied a single mother and baby pair and quoted an M/P ≈ 3 for both citalopram and N-demethylcitalopram. Peak milk concentrations occurred 3–9 h after dose. Both citalopram and N-demethylcitalopram were detected in trace amounts in infant's plasma after 3 weeks exposure to citalopram via breast milk. The plasma concentration of citalopram in the infant was 2.3 µg l−1 (7 nm) and the dose received by the infant was ≈ 5% of weight adjusted maternal dose. In the second study by Spigset et al.[18], data from two breast feeding mothers with depression, and one healthy volunteer were reported. In the depressed patients, M/P for citalopram ranged from 1.66 to 1.88 based on single paired measurements in plasma and milk. Data for N-demethylcitalopram were not provided. The relative infant dose of citalopram was calculated to be 0.7–5.9% of the maternal dose. No adverse effects were observed in the infants. Based on AUC data for citalopram in the healthy volunteer subject, M/P and relative infant dose were estimated to be 1 and 1.8% (of the maternal dose), respectively. Values for M/P and estimated infant dose from the above studies were similar to the definitive data from our present investigation. We found an M/PAUC of 1.8 for both citalopram and demethylcitalopram with a similar degree of interpatient (Table 1) and intrapatient (data not shown) variability. Thus, in our view, AUC measurements remain the preferred data source for calculating M/P.
Two different methods for calculating infant dose were tested. Both gave similar values for infant dose, confirming that milk production in this cohort of mothers (0.13 l kg−1 day−1) was similar to the population average (0.15 l kg−1 day−1) [12]. This is different from the situation with sertraline [19] where an apparently lower milk production resulted in lower estimates of infant dose with method A.
In summary, no adverse effects were reported by the mothers, or noted on clinical examination, in any of the breast-fed infants in the study. All had body weights that were within appropriate percentile limits for age and they showed normal development for age as assessed by the Denver developmental screening test. Moreover, drug transfer was lower (mean of around 5%) than the notional 10% level of concern [12], and only very low plasma concentrations of drug were found in less than half of the infants. Taken together, these data suggest that citalopram is likely to be safe for use during breast feeding. Nevertheless, each decision to breast feed should always be made as an individual risk:benefit analysis.
Acknowledgments
We acknowledge funding from the Women and Infants Research Foundation, Lundbeck Australia Pty Ltd and Medela Inc USA. We thank Ruth Barrett-Lennard, Neville Butcher and Shane Powell for assistance with data collection and analysis.
References
- 1.Lader M. Citalopram – a new antidepressant. Prim Care Psychiat. 1996;2:49–57. [Google Scholar]
- 2.Noble S, Benfield P. Citalopram. A review of its pharmacology, clinical efficacy and tolerability in the treatment of depression. CNS Drugs. 1997;8:410–431. [Google Scholar]
- 3.Joffe P, Larsen FS, Pedersen V, Ring-Larsen H, Aaes-Jorgensen T, Sidhu J. Single-dose pharmacokinetics of citalopram in patients with moderate renal insufficiency or hepatic cirrhosis compared with healthy subjects. Eur J Clin Pharmacol. 1998;54:237–242. doi: 10.1007/s002280050452. [DOI] [PubMed] [Google Scholar]
- 4.Oyehaug E, Ostensen ET, Salvesen B. High-performance liquid chromatographic determination of citalopram and four of its metabolites in plasma and urine samples from psychiatric patients. J Chromatogr Biomed Appl. 1984;308:199–208. [PubMed] [Google Scholar]
- 5.Rochat B, Amey M, Gillet M, Meyer UA, Baumann P. Identification of three cytochrome P450 isozymes involved in N-demethylation of citalopram enantiomers in human liver microsomes. Pharmacogenetics. 1997;7:1–10. doi: 10.1097/00008571-199702000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Baumann P, Larsen F. The pharmacokinetics of citalopram. Rev Contemp Pharmacother. 1995;6:287–295. [Google Scholar]
- 7.O'hara M, Swain M. Pares and risk of posnatal depression: a meta-analysis. Int Rev Psychiat. 1996;8:37–54. [Google Scholar]
- 8.Silprasert A, Dejsarai W, Keawvichit R, Amatayakul K. Effect of storage on the creamatocrit and total energy content of human milk. Human Nutrit Clin Nutrit. 1987;41:31–36. [PubMed] [Google Scholar]
- 9.Frankenburg WK, Dodds JB. The Denver development screening test. J Pediat. 1967;71:181–191. doi: 10.1016/s0022-3476(67)80070-2. [DOI] [PubMed] [Google Scholar]
- 10.Rossiter EJ. The use of developmental screening and assessment instruments by paediatricians in Australia. J Paediat Child Health. 1993;29:357–359. doi: 10.1111/j.1440-1754.1993.tb00534.x. [DOI] [PubMed] [Google Scholar]
- 11.Thomann P. Non-compartmental analysis methods manual. In: Heinzel G, Woloszcak R, Thomann P, Gustav Fischer Stuttgart, editors. Topfit, Version 2 0 Pharmacokinetic and Pharmacodynamic Data Analysis System for the PC. 1993. pp. 2-5–2-66. [Google Scholar]
- 12.Bennett PN. Use of the monographs on drugs. In: Bennett PN, editor. Drugs and Human Lacataion. 2. Elsevier; 1996. pp. 67–74. [Google Scholar]
- 13.Begg EJ, Atkinson HC, Duffull SB. Prospective evaluation of a model for the prediction of milk: plasma drug concentrations from physicochemical characteristics. Br J Clin Pharmacol. 1992;33:501–505. doi: 10.1111/j.1365-2125.1992.tb04077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Begg EJ, Atkinson HC. Modelling of the passage of drugs into milk. [Review][29 refs] Pharmacol Ther. 1993;59:301–310. doi: 10.1016/0163-7258(93)90072-l. [DOI] [PubMed] [Google Scholar]
- 15.Sidhu J, Priskorn M, Poulsen M, Segonzac A, Grollier G, Larsen F. Steady-state pharmacokinetics of the enantiomers of citalopram and its metabolites in humans. Chirality. 1997;9:686–692. doi: 10.1002/(SICI)1520-636X(1997)9:7<686::AID-CHIR9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Rochat B, Amey M, Baumann P. Analysis of the enantiomers of citalopram and its demethylated metabolites in plasma of depressive patients using chiral reverse-phase liquid chromatography. Ther Drug Monit. 1995;17:273–279. doi: 10.1097/00007691-199506000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Jensen PN, Olesen OV, Bertelsen A, Linnet K. Citalopram and desmethylcitalopram concentrations in breast milk and in serum of mother and infant. Ther Drug Monit. 1997;19:236–239. doi: 10.1097/00007691-199704000-00021. [DOI] [PubMed] [Google Scholar]
- 18.Spigset O, Carieborg L, Ohman R, Norstrom A. Excretion of citalopram in breast milk. Br J Clin Pharmacol. 1997;44:295–298. doi: 10.1046/j.1365-2125.1997.t01-1-00576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kristensen JH, Ilett KF, Dusci LJ, et al. Distribution and excretion of sertraline and N-desmethylsertraline in human milk. Br J Clin Pharmacol. 1998;45:453–457. doi: 10.1046/j.1365-2125.1998.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]