Abstract
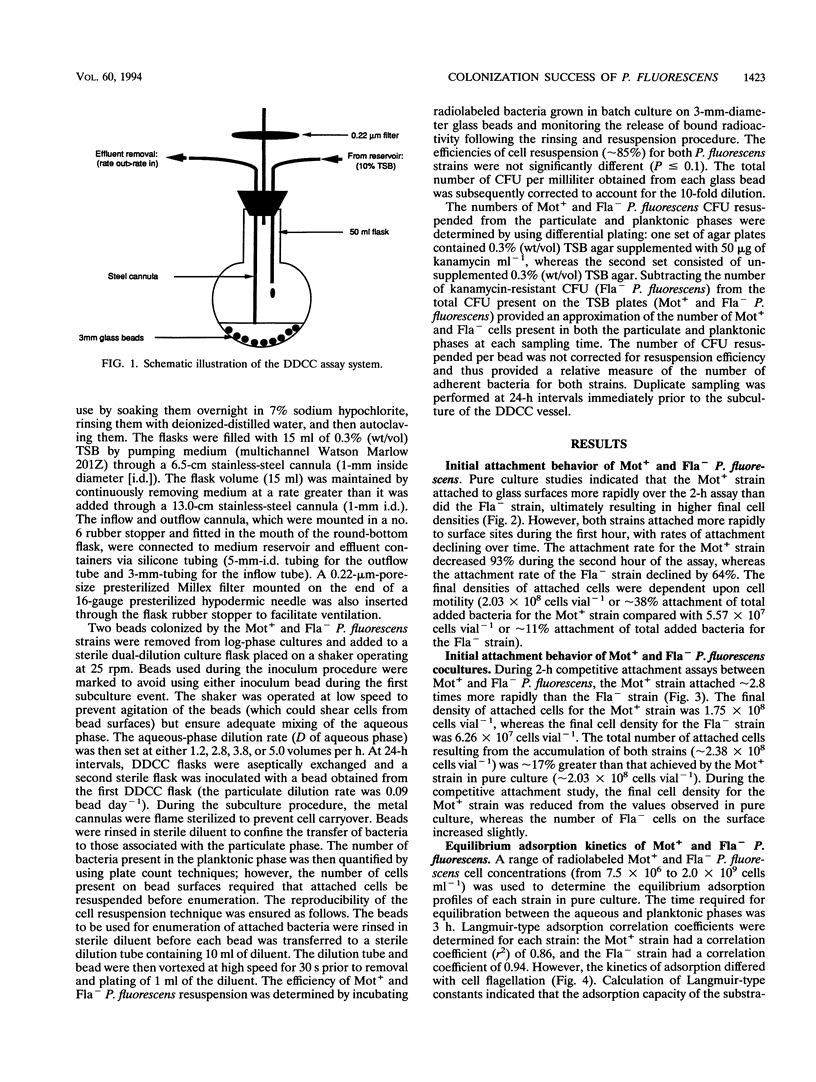
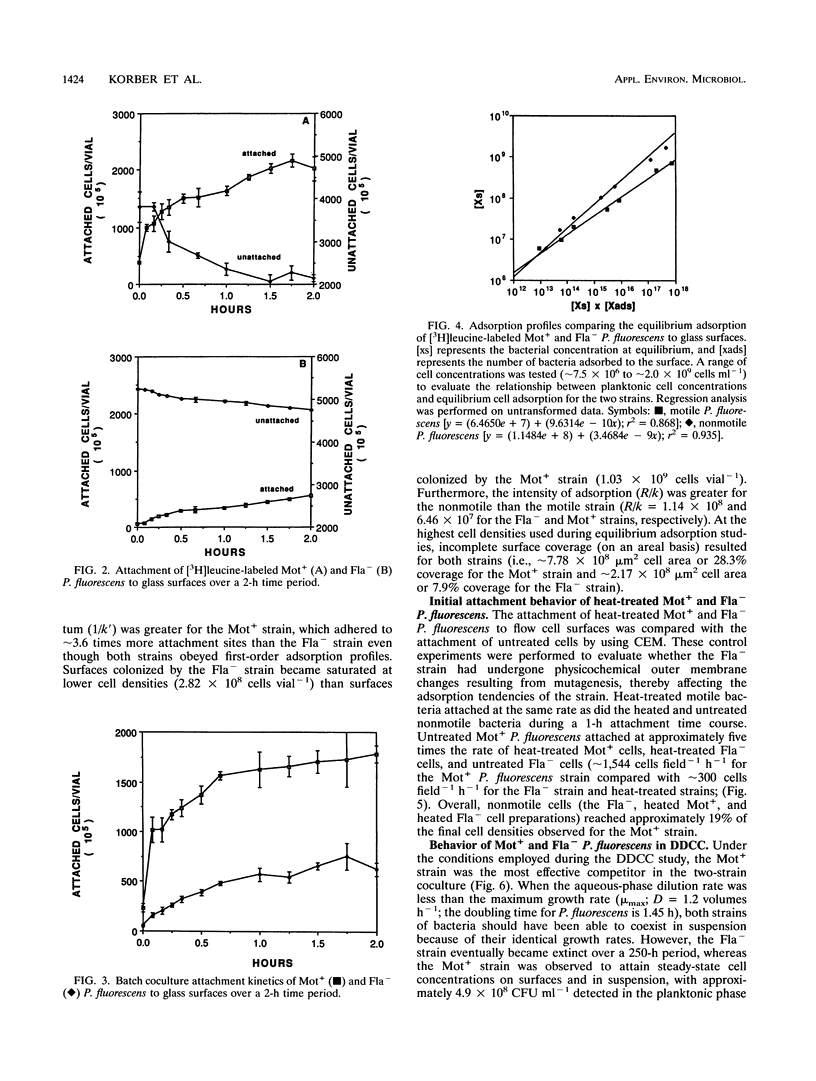
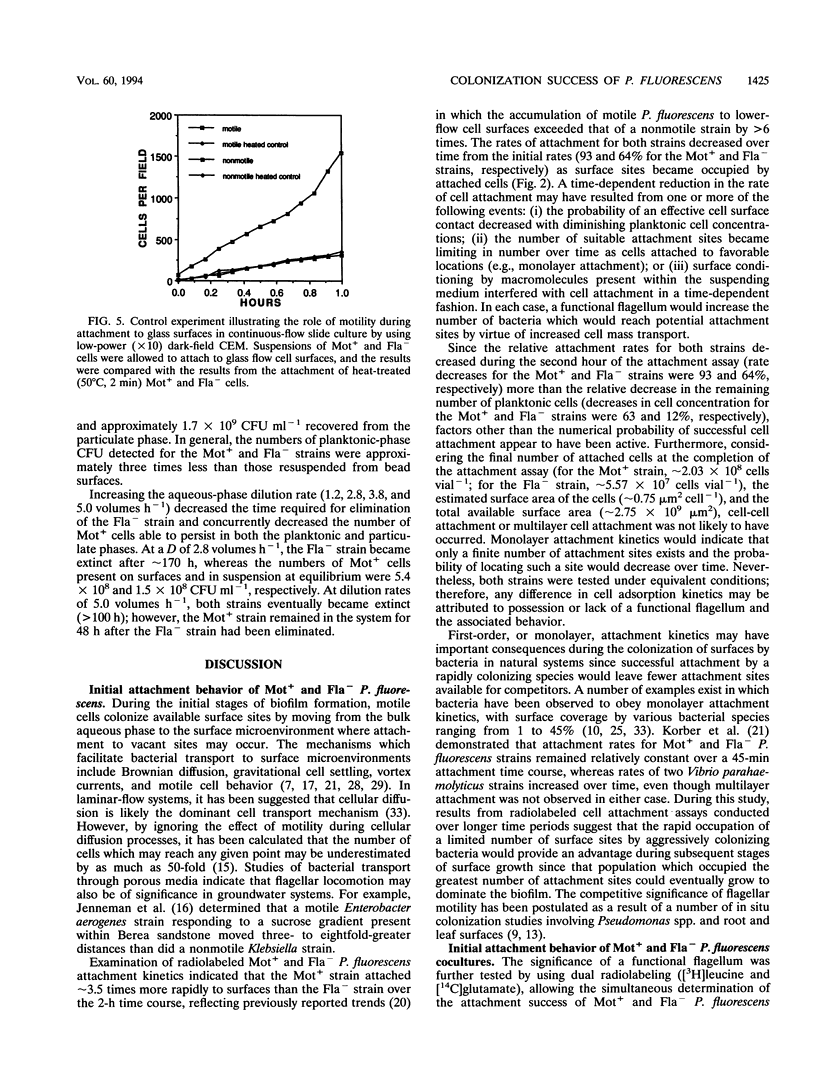
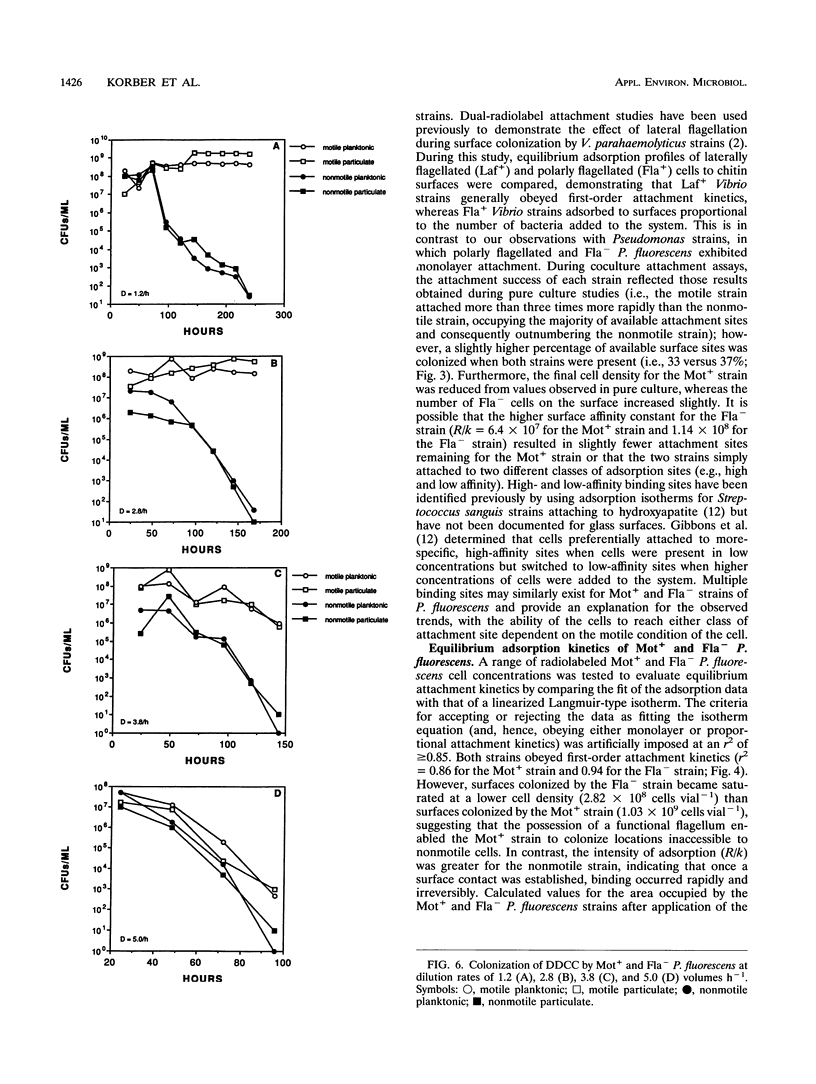
The colonization of glass surfaces by motile and nonmotile strains of Pseudomonas fluorescens was evaluated by using dual-dilution continuous culture (DDCC), competitive and noncompetitive attachment assays, and continuous-flow slide culture. Both strains possessed identical growth rates whether in the attached or planktonic state. Results of attachment assays using radiolabeled bacteria indicated that both strains obeyed first-order (monolayer) adsorption kinetics in pure culture. However, the motile strain attached about four times more rapidly and achieved higher final cell densities on surfaces than did the nonmotile strain (2.03 × 108 versus 5.57 × 107 cells vial-1) whether evaluated alone or in cocultures containing motile and nonmotile P. fluorescens. These kinetics were attributed to the increased transport of motile cells from the bulk aqueous phase to the hydrodynamic boundary layer where bacterial attachment, growth, and recolonization could occur. First-order attachment kinetics were also observed for both strains by using continuous-flow slide culture assays analyzed by image analysis. The DDCC system contained both aqueous and particulate phases which could be diluted independently. DDCC results indicated that when cocultures containing motile and nonmotile P. fluorescens colonized solid particles, the motile strain replaced the nonmotile strain in the system over time. Increasing the aqueous-phase rates of dilution decreased the time required for extinction of the nonmotile strain while concurrently decreasing the overall carrying capacity of the DDCC system for both strains. These results confirmed that bacterial motility conveyed a selective advantage during surface colonization even in aqueous-phase systems not dominated by laminar flow.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks M. K., Bryers J. D. Bacterial species dominance within a binary culture biofilm. Appl Environ Microbiol. 1991 Jul;57(7):1974–1979. doi: 10.1128/aem.57.7.1974-1979.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belas M. R., Colwell R. R. Adsorption kinetics of laterally and polarly flagellated Vibrio. J Bacteriol. 1982 Sep;151(3):1568–1580. doi: 10.1128/jb.151.3.1568-1580.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao L., Ramsdell G. The effects of wall populations on coexistence of bacteria in the liquid phase of chemostat cultures. J Gen Microbiol. 1985 May;131(5):1229–1236. doi: 10.1099/00221287-131-5-1229. [DOI] [PubMed] [Google Scholar]
- De Weger L. A., van der Vlugt C. I., Wijfjes A. H., Bakker P. A., Schippers B., Lugtenberg B. Flagella of a plant-growth-stimulating Pseudomonas fluorescens strain are required for colonization of potato roots. J Bacteriol. 1987 Jun;169(6):2769–2773. doi: 10.1128/jb.169.6.2769-2773.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson A. G. Behavior of mixed cultures of microorganisms. Annu Rev Microbiol. 1977;31:63–87. doi: 10.1146/annurev.mi.31.100177.000431. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Moreno E. C., Etherden I. Concentration-dependent multiple binding sites on saliva-treated hydroxyapatite for Streptococcus sanguis. Infect Immun. 1983 Jan;39(1):280–289. doi: 10.1128/iai.39.1.280-289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefele D. M., Lindow S. E. Flagellar Motility Confers Epiphytic Fitness Advantages upon Pseudomonas syringae. Appl Environ Microbiol. 1987 Oct;53(10):2528–2533. doi: 10.1128/aem.53.10.2528-2533.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch P. Budding bacteria. Annu Rev Microbiol. 1974;28(0):391–444. doi: 10.1146/annurev.mi.28.100174.002135. [DOI] [PubMed] [Google Scholar]
- Jenneman G. E., McInerney M. J., Knapp R. M. Microbial Penetration through Nutrient-Saturated Berea Sandstone. Appl Environ Microbiol. 1985 Aug;50(2):383–391. doi: 10.1128/aem.50.2.383-391.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J. R., Korber D. R., Caldwell D. E. Behavioral analysis of Vibrio parahaemolyticus variants in high- and low-viscosity microenvironments by use of digital image processing. J Bacteriol. 1992 Sep;174(17):5732–5739. doi: 10.1128/jb.174.17.5732-5739.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J. R., Korber D. R., Hoyle B. D., Costerton J. W., Caldwell D. E. Optical sectioning of microbial biofilms. J Bacteriol. 1991 Oct;173(20):6558–6567. doi: 10.1128/jb.173.20.6558-6567.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maigetter R. Z., Pfister R. M. A mixed bacterial population in a continuous culture with and without kaolinite. Can J Microbiol. 1975 Feb;21(2):173–180. doi: 10.1139/m75-025. [DOI] [PubMed] [Google Scholar]
- Mayfield C. I., Inniss W. E. A rapid, simple method for staining bacterial flagella. Can J Microbiol. 1977 Sep;23(9):1311–1313. doi: 10.1139/m77-198. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Cotta M. A., Dombrowski D. B. Rumen Bacterial Competition in Continuous Culture: Streptococcus bovis Versus Megasphaera elsdenii. Appl Environ Microbiol. 1981 Jun;41(6):1394–1399. doi: 10.1128/aem.41.6.1394-1399.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. L., Doetsch R. N. Studies on negative chemotaxis and the survival value of motility in Pseudomonas fluorescens. J Gen Microbiol. 1969 Mar;55(3):379–391. doi: 10.1099/00221287-55-3-379. [DOI] [PubMed] [Google Scholar]
- Stanley P. M. Factors affecting the irreversible attachment of Pseudomonas aeruginosa to stainless steel. Can J Microbiol. 1983 Nov;29(11):1493–1499. doi: 10.1139/m83-230. [DOI] [PubMed] [Google Scholar]



