Abstract
Aims
Urinary salbutamol post‐inhalation has been shown to be an index of lung deposition. The possibility of using the urinary method for prolonged periods of inhalation (such as nebulized therapy) has been evaluated.
Methods
On separate study days volunteers received salbutamol 5 × 100 µg via either oral administration (ORAL), oral with 5 g oral charcoal (ORAL + C), inhaled from a metered dose inhaler (MDI) or MDI plus 5 g oral charcoal (MDI + C). Each dose was separated by 2 min, i.e. administration time of 8 min. Urine samples were provided at 0, 30, 40, 60 and 120 min postdose. Also seven subjects inhaled 5×100 µg doses from the MDI on five separate occasions and provided urine 0–30 min post dose.
Results
No salbutamol was detected in urine samples following ORAL + C. The mean (s.d.) amounts of salbutamol excreted in the urine in the first 30 min post ORAL, MDI and MDI + C were 0.42 (0.55), 11.01 (3.77) and 11.60 (3.68) µg, respectively. The ratio of urinary salbutamol following MDI and MDI + C to ORAL in the 0–30 min collection period was 26.2 and 27.8, and between 30 and 40 min postdose was 5.1 and 4.7, respectively. There was no difference between urinary salbutamol over the first 30 min following MDI and MDI + C with a mean ratio (90% confidence interval) of 95.6 (84.0, 107.2). The mean (s.d.) coefficient of variation for the 30 min urinary salbutamol elimination following inhalation of 5 × 100 µg doses from the MDI by seven subjects (on 5 separate study days) was 9.4 (2.3) %.
Conclusions
The 30 min urinary salbutamol method can be used for an inhalation period of up to 8 min to identify the relative bioavailability to the lung. Samples taken after this time period are affected by excretion of the oral absorbed fraction. Most nebulisers deliver their dose within this administration time.
Keywords: 30 min, prolonged inhalation, relative lung bioavailability, salbutamol, urinary excretion
Introduction
We have previously shown that the amount of salbutamol excreted in the urine over the first 30 min after an inhaled dose is an index of lung deposition which can be used to compare different techniques, methods or products [1]. This method, the relative bioavailability of salbutamol to the lungs following inhalation, was validated using a total administration time of < 3 min. To extend our studies to nebulized therapy, validation of this index for a longer inhalation period is necessary. It has been recommended that during nebulization the administration time should be no longer than 10 min [2] and most nebulisers are inhaled over approximately 5 min [3]. When the fill volume in the nebuliser chamber is increased the dose available for nebulization does not change but the nebulization time is increased [4]. For some nebuliser systems a fill volume of 4 ml has been recommended [4]. In house assessment of standard hospital nebuliser systems with a 4-ml fill showed that nebuliser time can be up to 8 min
Nebulisers are inefficient systems with only about 20% of the original dose available for inhalation [5] and of this 2–19% [5, 6] is deposited into the lungs. These amounts are dependent on the system and also on the breathing pattern of the patient [7]. To evaluate the potential of the urinary salbutamol method [1] for nebuliser therapy, especially the lack of any interference from the swallowed fraction, we have determined if administration over an 8 min period is suitable. A longer administration period could prolong the lag phase for significant oral absorption thus samples provided later than 30 min post inhalation could be used in these circumstances. However the slower rate of dosage delivery to the lungs may not allow sufficient time for the renal elimination of significant amounts of salbutamol. In this assessment we have mimicked delivery from a nebuliser by using a MDI spaced out over this time period. 5 × 100 µg from a MDI has been used because this would be equal to the amount available for inhalation when 2.5 µg salbutamol is nebulized [5]. Sampling times after the 30 min post start of dosing have also been used to determine if the greater variability of the salbutamol excretion method reported by Lipworth et al.[8] could be caused by interference due to oral absorption of this swallowed fraction of the inhaled dose.
Methods
Local Ethics Research Committee approval was obtained and all patients gave signed informed consent. On separate study days, each subject received either an oral dose (ORAL) or an oral dose immediately followed by 5 g activated charcoal (Carbomix, Penn Pharmaceuticals) in 50 ml of water (ORAL + C), inhaled from a Ventolin (Glaxo Wellcome plc, UK) metered dose inhaler (MDI) or inhaled from the MDI followed by 5 g activated charcoal (MDI + C). The study dose was 5 × 100 µg with each 100 µg administration separated by 2 min, thereby producing an administration period of 8 min. The 100 µg oral doses were dissolved in 20 ml of water with rinsing using another 20 ml of water.
All subjects were highly trained in the inhalation techniques used and were very experienced with this type of study. Each subject refrained from drinking caffeine-containing beverages for at least 4 h before each study and until after the last urine sample had been given. They were allowed a light breakfast and fluid intake was not controlled. All doses were given 4 h post breakfast. The order of each study day administration was randomised with a 7 day break between each. Urine samples were provided at 0, 30, 40, 60 and 120 min post (start of first) dose. All samples were stored at −20 °C prior to the determination of their salbutamol content using a high performance liquid chromatography assay [1]. On a separate occasion seven volunteers inhaled 5×100 µg salbutamol from a Ventolin MDI and provided a urine sample 30 min post dose. This procedure was repeated 5 times on separate study dates.
Results
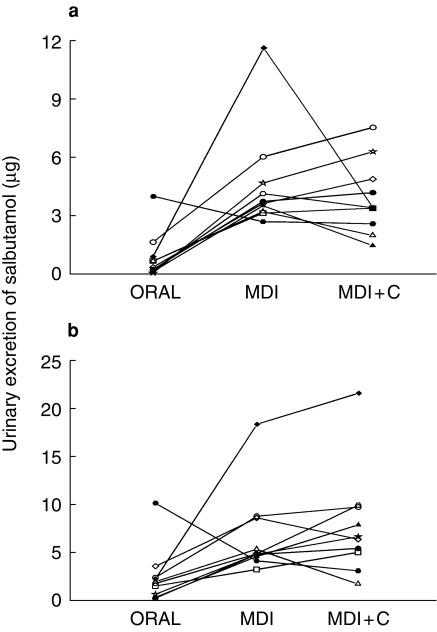
Ten (five female) healthy volunteers completed the study. Their mean (s.d.) age was 28.1 (7.0) years and weight 69.6 (11.2) kg. No salbutamol was detected in any of the samples following oral administration with charcoal (ORAL + C). The individual amounts of Salbutamol excreted in the urine during the first 30 min, post start of dosing, and between 30 and 40 min are shown in Figure 1. These highlight the individual variability. The data in Figure 1a show that three volunteers did not excrete any salbutamol in the urine in the first 30 min with a range up to 1.54 µg. After MDI dosing the range of salbutamol excreted during this time period was 4.57–15.31 µg and following MDI + C it was 4.31–15.67 µg. The mean (s.d.) amounts of salbutamol excreted are shown in Table 1. The ratio of ORAL:MDI and MDI + C was 1 : 26.2 and 1 : 27.8, respectively, for salbutamol urinary excretion in the first 30 min and 1 : 5.5 and 1 : 4.7 between 30 and 40 min postdose. Statistical comparison (t-test) between MDI and MDI + C for each collection period showed no difference and for the first 30 min the mean ratio (90% confidence interval) was 95.6 (84.0–107.0). Comparison of MDI and MDI + C to ORAL were significant (t-test, P < 0.01 for 0–30 and 30–40, P < 0.05 for 40–60) except for the 60–120 min collection period. Seven (four females) volunteers, mean (s.d.) age 29.1 (4.5) years weighing 66.1 (14.4 kg), repeated the inhalation of 5×100 µg salbutamol from the MDI on 5 occasions. Their coefficient of variation for the amount of salbutamol excreted in their urine during the first 30 min was 13.4, 9.5, 7.4, 7.4, 10.3, 7.2 and 11.0%. The mean (s.d.) coefficient of variation was, therefore, 9.4 (2.3)%.
Figure 1.
a) Individual urinary salbutamol excretion, in the first 30 min, after oral (ORAL) metered dose inhaler (MDI) and metered dose inhaler with oral administration of charcoal (MDI + C) and b) individual urinary salbutamol excretion, in the first 30–40 min, after oral (ORAL) metered dose inhaler (MDI) and metered dose inhaler with oral administration of charcoal (MDI + C).
Table 1.
Mean (s.d.) urinary salbutamol (µg) following 5 × 100 µg salbutamol doses.
| Collection period (min) | ORAL | MDI | MDI + C |
|---|---|---|---|
| 0–30 | 0.42 (0.55) | 11.01 (3.77) | 11.66 (3.68) |
| 30–40 | 0.84 (1.20) | 4.63 (2.63) | 3.92 (1.89) |
| 40–60 | 2.48 (2.89) | 6.81 (4.47) | 7.81 (5.54) |
| 60–120 | 11.53 (10.23) | 14.22 (5.34) | 10.35 (6.55) |
| Cumulative amount | 15.27 (14.05) | 36.66 (12.23) | 33.75 (14.28) |
Discussion
The amount of salbutamol excreted in the urine over 30 min post inhalation from a MDI and oral dosing when the inhalation period was < 3 min was reported as 0.72 (0.56) and 8.24 (3.2) µg, respectively [1]. Thus, this variability of salbutamol excreted was 71 and 35%, respectively, with a mean ORAL:MDI ratio of 11.4. This compares with respective values of 0.42 (0.55) µg and 11.01 (3.77) µg in this study and variabilities of 134.0% and 34%, respectively, with a mean ratio of 26.2. The difference in the oral data could be due to the slower rate of dosing thereby extending the lag phase for significant oral absorption. This would account for the higher variability for the oral dosing (with three subjects excreting no salbutamol) and the larger ratio for the ORAL:MDI ratio. Only one subject repeated this and the original study.
Clark & Lipworth [8] have compared their plasma salbutamol concentration technique with the 30 min urinary salbutamol method [1]. They measured these following 12 doses inhaled over 6 min and the urine samples they used were provided 30 min after the last dose. This corresponds to a 36 min post start of dose according to our method. Clark & Lipworth [8] showed that due to the larger variability with their urinary excretion data then the plasma salbutamol method was more sensitive. Although this could be anticipated because the urinary method involves renal clearance, the results from our study indicate that between 30 and 40 min post start of dosing the urinary salbutamol data from samples taken 36 min postdose [8] would contain more drug delivered via the oral route. Between 30 and 40 min the ratio of ORAL:MDI and: MDI + C were much lower than the 0–30 minute values thereby highlighting the end of the lag phase for absorption. The elimination portion of MDI without charcoal would represent the absorption rate due to a flip flop model [1]. The Dundee group [9–11] focus on plasma salbutamol concentrations within the first half hour as their index of relative lung bioavailability, after an inhalation period over 6 min. The results of this study highlight that the majority of drug delivered to the systemic concentration when the administration is 6 min will have been delivered via the pulmonary route and thus validates their plasma method as an index of lung deposition.
The lack of a difference between the urinary data of MDI and MDI + C in the first 30 min indicates that oral charcoal is not necessary to compare the relative bioavailability of salbutamol to the lungs. The 90% confidence intervals of this ratio were within the 80–120% required for bio-equivalence. However due to the low number of subjects a type II error could have occurred. By omitting charcoal 0–24 h urinary collections can give an indication of the total dose delivered to the body [1]. Also if charcoal was used in patient studies then any other oral medication may not be absorbed.
It has been shown that salbutamol is detectable in urine samples post nebulization [11]. Thus, the urinary pharmacokinetic method based on 30 min urinary excretion, can be extended to evaluate the in vivo performance of nebulisers provided that the nebulization times are less than 8 min. This simple method has been shown to have a linear relationship with dose [12] which is essential because nebulized therapy involves the administration of large doses (5 mg compared with 100 µg from an MDI). In vitro studies have highlighted a large variability in the emitted dose and the particle size characteristics of nebulized steroids [13] and terbutaline [14]. However, clinical studies use bronchodilatory response which may not be able to demonstrate differences because of the large doses commonly used [15–19]. In contrast we have shown a relationship between urinary salbutamol following inhalation and the methacholine dose to reduce the FEV1 by 20% PD20[20]. The intra-subject variability for the seven volunteers repeating the 30 min urinary excretion following inhalation of 5×100 µg doses was 9.4 (2.3)%. This is consistent to values previously reported by Hindle et al. when four doses were inhaled [1].
Although the inhaled doses were much lower than the standard doses from a nebuliser, in terms of the amount available for inhalation they were equal to a 2.5-mg nebulized dose. Hardy et al.[6] have suggested that 50% of a nebulized dose is swallowed. This is consistent with what we have reported after inhalation with an MDI in that 57.4% of the inhaled dose was excreted in the urine during the first 24 h post dosing [1]. Furthermore, since nebulisers only provide 20% of the dose available for inhalation to the subject then the total systemic delivery from a 2.5-mg nebulized dose should be the same as that when five doses of salbutamol are inhaled from an MDI, as in this study.
Studies are now in progress to evaluate the in vivo performance of different nebulized systems using this urinary pharmacokinetic method. In these studies we are comparing 2.5 mg nebulized dose with five doses of salbutamol inhaled through a metered dose inhaler spaced out over a time interval equivalent to that of the nebulized system.
References
- 1.Hindle M, Chrystyn H. Determination of the relative bioavailability of salbutamol to the lung following inhalation. Br J Clin Pharmacol. 1992;34:311–315. doi: 10.1111/j.1365-2125.1992.tb05921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.British Thoracic Society. Current best practices for nebuliser treatment. Journal ?? 1977;52(Suppl 2):s4–s24. [Google Scholar]
- 3.O'callaghan C, Clark AR, Milner AD. Why nebulise for more than five minutes? Arch Dis Child. 1989;64:1270–1273. doi: 10.1136/adc.64.9.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kendrick AH, Smith EC, Denyer J. Nebulizers-fill volume, residual volume, and matching of nebulizer to compressor. Resp Med. 1995;89:157–159. doi: 10.1016/0954-6111(95)90241-4. [DOI] [PubMed] [Google Scholar]
- 5.Clay MM, Clark SW. Wastage of drug from nebulisers: a review. J R Soc Med. 1987;80:38–39. doi: 10.1177/014107688708000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardy JG, Newman SP, Knoch M. Long deposition for four nebulisers. Respir Med. 1993;87:461–465. doi: 10.1016/0954-6111(93)90074-a. [DOI] [PubMed] [Google Scholar]
- 7.Nikander K. Some technical, physicochemical and physiological aspects of nebulistation of drugs. Eur Resp Rev. 1997;44:168–172. [Google Scholar]
- 8.Lipworth BJ, Clark DJ. Lung bioavailability of chlorofluorocarbon free, dry powder and chlorofluorocarbon containing formulations of salbutamol. Br J Clin Pharmacol. 1996;41:247–249. doi: 10.1111/j.1365-2125.1996.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 9.Lipworth BJ, Clark DJ. Effects of airway calibre on lung delivery of nebulised salbutamol. Thorax. 1997;52:1036–1039. doi: 10.1136/thx.52.12.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipworth BJ, Clark DJ. Lung delivery of non-CFC salbutamol via small Volume metal spacer and large Volume plastic spacer devices compared with an open vent jet nebuliser. Br J Clin Pharmacol. 1998;45:160–163. doi: 10.1046/j.1365-2125.1998.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark DJ, Tan KS, Lipworth BJ. Evaluation of plasma and urinary salbutamol levels in COPD. Eur J Clin Pharmacol. 1996;51:91–93. doi: 10.1007/s002280050166. [DOI] [PubMed] [Google Scholar]
- 12.Tomlinson HS, Corlett SA, Chrystyn H. Effect of dose on the relative lung bioavailability of salbutamol. J Aerosol Med. 1995;8:P193. [Google Scholar]
- 13.Smalldone GC, Cruz-Rivera M, Nikander K. In-vitro determination of inhaled mass and particle distribution of budesonide nebulising suspensions. J Aerosol Med. 1998;11:113–125. [Google Scholar]
- 14.Johnson MA, Newman SP, Bloom R, Talaee N, Clarke SW. Delivery of albuterol and ipratropium bromide from two nebuliser systems in chronic stable asthma. Efficacy and pulmonary deposition. Chest. 1989;96:1–10. doi: 10.1378/chest.96.1.6. [DOI] [PubMed] [Google Scholar]
- 15.Chou KJ, Cunningham SJ, Crain EF. Metered dose inhalers with spacers vs nebulizers for pediatric asthma. Arch Pediatr Adolesc Med. 1995;149:201–205. doi: 10.1001/archpedi.1995.02170140083015. [DOI] [PubMed] [Google Scholar]
- 16.Idris AH, McDermott MF, Raucci JC, et al. Emergency department treatment of severe asthma: metered dose inhaler plus holding chamber is equivalent in effectiveness to nebulizer. Chest. 1993;103:665–672. doi: 10.1378/chest.103.3.665. [DOI] [PubMed] [Google Scholar]
- 17.Kerem E, Levison H, Schuh S, et al. Efficacy of albuterol administered by nebuliser vs spacer in children with acute asthma. J Pediatr. 1993;123:313–317. doi: 10.1016/s0022-3476(05)81710-x. [DOI] [PubMed] [Google Scholar]
- 18.Parkin PC, Saunders NR, Diamond SA, Winders PM, MacArthur C. Randomised trial spacer v. nebuliser for acute asthma. Arch Dis Child. 1995;72:239–240. doi: 10.1136/adc.72.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin YZ, Hsieh KH. Metered dose inhaler and nebuliser in acute asthma. Arch Dis Child. 1995;72:214–218. doi: 10.1136/adc.72.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chrystyn H, Allen MB, Corlett SA, Tomlinson HS. Simultaneous measurement of pharmacodynamic and pharmacokinetic parameters which can be used to evaluate the equivalence of inhaled salbutamol. Am J Respir Crit Care Med. 1998;157:A636. [Google Scholar]