Abstract
Aims
To investigate the effect of acute P-glycoprotein inhibition by the multidrug-resistance (MDR) modulator valspodar (SDZ PSC 833; PSC) on the pharmacokinetics, and potentially adverse pharmacodynamic effects of morphine, and its principal pharmacologically active metabolites, morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G).
Methods
In a double-blind, three-way crossover study, the pharmacokinetic and potentially adverse pharmacodynamic effects (reaction time, transcutaneous PCO2, blood pressure) of morphine were compared with and without acute inhibition ofP-glycoprotein by PSC. The effects of PSC alone were also evaluated. The study was performed in 18 healthy male volunteers and pharmacodynamic effects analysed by measuring the area under the effect (AUE) curve. 150 mg PSC (or its placebo) was given as an i.v. infusion over 2 h. With the expected inhibition of Pgp 1 h after starting PSC infusion, 7.5 morphine HCl (or its placebo) was infused over 2 h.
Results
The infusion of PSC resulted in blood concentrations expected to inhibit Pgp mediated transport. While the pharmacokinetics of plasma morphine and M6G. were unaffected there was a small but statistically significant increase in the AUC and Cmax of M3G (11.8 and 8.3%, respectively). The t½ and tmax were unaffected. The pharmacokinetic parameters of PSC were not affected by coadministration with morphine. PSC did not significantly affect the adverse events of morphine, as assessed by spontaneous reporting. Compared with PSC alone, morphine elicited an increase in reaction time (Emax 48 ms, compared with the predose absolute reaction time of 644 ms), which was not detected by the alertness-drowsiness score, indicating only slight sedation. There was a significant decrease in systolic blood pressure (Emin−9 mmHg), and a trend for a fall in diastolic blood pressure (Emin−14.5 mmHg) and respiratory rate (Emin−1.8 breath·min−1). For all these parameters, the effects of PSC/morphine were similar to that of PSC alone, suggesting some attenuation of morphine's effect. In contrast, morphine caused a significant increase in PCO2 (Emax 0.69 kPa) compared to PSC alone, indicating slight respiratory depression. This increase was similar to that of the PSC/morphine combination.
Conclusions
Acute inhibition of P-glycoprotein by PSC in this setting does not affect the pharmacokinetic or safety-related pharmacodynamic profile of morphine in a clinically significant manner.
Keywords: healthy volunteers, morphine-3-glucuronide, morphine-6-glucuronide, morphine, multidrug resistance, P-glycoprotein, SDZ PSC 833
Introduction
Multidrug resistance (MDR) is a major problem in chemotherapy of malignancies. One important explanation is the reduced accumulation of anticancer drugs in tumour cells caused by increased active drug efflux [1, 2]. This MDR phenotype has been shown to result from increased expression of a gene designated as human mdr-1 which produces a membrane glycoprotein termed P-glycoprotein 170 (Pgp) [3].
Valspodar (3′-keto-Bmt1]-[Val2]-cyclosporin; SDZ PSC833; PSC), an analogue of cyclosporin D, is being developed for its high potency to reverse the resistance to chemotherapy of cancer cells with the MDR phenotype by inhibiting the action of Pgp [4]. It is devoid of nephrotoxic and immunosuppressive adverse effects.
PSC reverses Pgp-mediated multidrug resistance in chemotherapy resistant cells in vitro as well as in cancer patients by inhibiting the binding of anticancer drugs to Pgp and reducing their extracellular efflux [5–7]. This results in an enhanced tissue availability of Pgp substrates [8–10].
While PSC is highly effective in selectively reversing Pgp-mediated MDR, it is also expected to inhibit the physiological function of Pgp in various tissues, including the brain [11, 12], liver [13] and kidney [14]. Therefore, the net brain uptake of digoxin and quinidine could be significantly enhanced in mice after oral intake of PSC [15, 16]. Since PSC will be used primarily to treat cancer patients who may receive morphine concomitantly for pain control, it is of direct interest that also morphine and its active metabolite morphine-6-glucuronide (M6G) are substrates of Pgp [17, 18]. This raises the possibility that there may be a clinically significant interaction between PSC and morphine, leading to increased brain concentrations of morphine and M6G by inhibition of their Pgp-mediated back-extrusion through the blood–brain barrier. Such an interaction could lead to potentially hazardous respiratory depression in these patients, justifying study under controlled clinical conditions. While the effects of PSC on morphine disposition have not been studied in animals, the finding that there is a two-fold increased net brain uptake [11, 12] in mdr1a knock-out mice lends additional support to the notion that Pgp may affect of brain morphine disposition.
Recently, an interaction of a Pgp-inhibitor with pharmacokinetic and pharmacodynamic effects of morphine was demonstrated in rats [19]. However, no data on such an interaction are yet available in humans. Therefore, the pharmacokinetics of PSC and morphine after intravenous administration was investigated in the present human study in healthy volunteers. Because of the target population of PSC, pharmacodynamic variables focused on PCO2 and drowsiness (reaction time and visual analogue scale) rather than the potential analgesic effects of morphine.
Methods
Subjects
Eighteen healthy male subjects participated in this study. Subjects were between 20 and 43 years of age (mean 27 years) with heights between 161 and 190 cm (mean 179 cm) and weights between 56 and 84 kg (mean 71 kg). They were all within 10% of their ideal body weight and had normal physical and laboratory findings in their prestudy evaluations. Concomitant medication was not allowed. Special exclusion criteria were positive findings in urinary drug screen (alcohol, benzodiazepines, amphetamines, cannabinoids, cocaine, and opiates) and for cotinine to reduce the likelihood of inclusion of subjects at risk for drug abuse. Prior to the start of the study, the Ethics Committee of the University Hospital in Basel approved the study protocol (June 1997) and written informed consent of the subjects was obtained.
Drugs
PSC and its matching placebo were supplied by Novartis Ltd, Basel, CH. Vials contained 500 mg PSC in 10 ml of vehicle. Placebo contained 10 ml of vehicle. Morphine hydrochloride (MO) was purchased from Sintetica, Medrisino, CH. Infusion pump syringes were filled as appropriate with the four different infusion solutions (150 mg PSC in 75 ml of vehicle, 75 ml of vehicle, 7.5 mg morphine hydrochloride in 40 ml of saline, and 40 ml of saline) under aseptic conditions by the University of Basel Hospital Pharmacy. This was done by personnel not directly involved in the study, making it possible to deliver infusions in a double-blind fashion.
Study design and drug administrations
The study was performed according to a double-blind, placebo-controlled, three-way cross-over design. Subjects were randomised to receive each of the three treatments (PSC + MO; PSC + placebo; vehicle + MO) once with a 14–21 day washout period between doses. PSC (150 mg) or vehicle was administered as a 2 h intravenous infusion; morphine hydrochloride (7.5 mg) or saline were infused intravenously over 2 h, starting 1 h after the initiation of the PSC infusion. Infusions were performed using syringe perfusion pumps (Perfusor compact, B. Braun Medical AG, Sempach, Switzerland). All post-dose times refer to the beginning of the infusion of PSC or its vehicle.
Drug administrations were in the morning after an overnight fast of at least 12 h. A light meal was given 4.5 h post dose and consumption of fruit tea and decaffeinated coffee was permitted together with the meal. Eight hours after dosing, a sandwich was given, followed by supper 10 h post dose. Subjects had to consume the entire contents of each meal within 30 min. Mineral water was allowed at all times prior to and following drug administration.
Subjects were asked to lie supine throughout the course of the day's measurements in a quiet room. They were allowed to read/write, but no movies were available as this was considered to potentially affect pharmacodynamic measurements.
Blood pressure, pulse, and respiratory rate were assessed after the subject had rested in the supine position for at least 3 min. As an additional measure, arterial oxygen saturation was measured by means of a pulse oxymeter (Nellcor® N-1000E, Nellcor GmbH, Idstein, BRD) with the sensor being placed on the finger.
Transcutaneous PCO2
In order to quantify the pharmacodynamic effect of the drug treatments, potential respiratory depression was determined by measuring transcutaneous partial pressure of CO2 (tcPCO2). For this purpose, a TCM-3 Combi monitor (Radiometer, Copenhagen, Denmark) equipped with a tcPO2/PCO2 electrode, was used. For measurement, a drop of distilled water was placed on the skin and the sensor was attached by means of an adhesive ring. The tcPCO2 was reported to the nearest 0.1 kPa.
Reaction time
Potential sedatory drug effects were evaluated by measuring the BonnDet device [20]. This microcomputer controlled device recorded interstimulus intervals as working speed and latency between stimulus (coloured lights) and appropriate reactions (coloured buttons) as reaction time. The computer controlled a fast on-line feedback loop between performance and working speed as to yield a constant failure rate of 50% over 540 stimuli. The duration of the test was between 5 and 10 min. Subjects were made familiar with the procedures prior to starting the protocol so as to avoid habituation effects. The mean reaction time and measure of variability were reported to the nearest millisecond.
Alertness-drowsiness score
The visual-analogue scale (VAS) for drowsiness was a horizontal line of 15 cm length. The left-most end stated ‘wide awake’, the other end ‘very tired’. The subjects were asked to mark the line according to his/her own subjective assessment of alertness-drowsiness. Subjects were not allowed to refer to their assessments at previous time points in order to avoid bias.
Pharmacokinetics
Blood samples were drawn through an indwelling cannula inserted in a forearm vein (contra-lateral arm to that with infusion line) before administration (baseline) and at different time points up to 11 h (morphine) and 96 h (PSC) afterwards. For determination of PSC, 2 ml of whole blood were collected into an EDTA coated (polypropylene) tube. For morphine and morphine metabolites, 5 ml whole blood was collected into heparinized tubes, centrifuged at 4 °C for 15 min at approximately 800 × gravity and plasma was separated. The tubes were kept frozen at ≤−20 °C pending analysis.
Determination of whole blood PSC concentrations was performed by radioimmunoassay analysis using the PSC radioimmunoassay kit (ANAWA Trading SA, Wangen-Zürich, Switzerland). Determinations were done in duplicate. The between-run accuracy ranged from −3.8% to + 5.2%, while between-run coefficients of variation ranged from 4.4% to 7.8%. The assay limit of quantification (LOQ, accuracy within ± 20% and coefficient of variation ≤ 20%) was set to the lowest calibration sample concentration, i.e. to 28.1 ng ml−1.
Plasma concentrations of morphine and its metabolites were determined by a specific LC-MS-MS method as described by Tyrefors et al.[21]. Plasma samples were spiked with the deuterated internal standard N-CD3 morphine and each sample analysed by h.p.l.c. The within-study assay validation for morphine showed high accuracy (93.2–102.2%) and precision (4.5–6.2%). Similarly values were found for both M3G (95.3–102.5% and 10.9–13.1%) and M6G (97.7–103.9% and 8.7–13.2%). The limit of quantification (LOQ, mean recovery within 80 and 120% and coefficient of variation ≤ 20%) was set to the lowest concentration of morphine in the QC samples, i.e. to 1.00 ng ml−1. For morphine 3-β-D glucuronide, the LOQ was set up to 5.00 ng ml−1 and for morphine 6-β-d glucuronide to 2.00 ng ml−1.
The concentration time curves of PSC in blood and morphine in plasma were evaluated by noncompartmental analysis using WinNonlin Pro (version 2.0) and the following parameters determined. Area under the concentration-time curve (AUC(0,last)) from time zero to the last measurable sampling point was calculated by the linear trapezoidal method. Terminal elimination rate constant λz was determined from the slope of the regression line of ln(Ct) vs time, where Ct is the concentration at time t. The AUC measured from time zero and extrapolated to infinity (AUC(0,∞) was calculated as AUC(0,last) + Ctλz. The maximum concentration observed post-dose and the time of occurrence were defined as Cmax and tmax, respectively. Elimination half-life (t½) was defined as 0.693 λz. Apparent clearance (CL) was defined as Dose·AUC(0,∞)−1 and the apparent volume of distribution at steady-state (Vss) was calculated as MRTinf·CL, where MRTinf is the mean residence time extrapolated to infinity calculated as (AUMC(0,∞)/AUC(0,∞)-t/2, t is the infusion duration, and AUMC(0,∞) (area under the first moment curve) determined as AUMC(0,last) + (tlast·Clast) λz+ Clast λz2. Apparent volume of distribution (Vz) was defined as Dose·AUC(0,∞)−1·λz1 (this term is also known as Varea).
The pharmacodynamic measurements, which were repeatedly recorded were summarized by means of: the area under the effect-time curve (AUE) from time 0 to last available observation by the linear-trapezoidal rule, maximum effect (Emax), and time to first occurrence of maximal effect (tmax). AUE, Emax and tmax were calculated for the change from baseline (predose). For Emax, the value at predose, i.e. at time = 0, was not included in determining the maximum effect.
Pharmacokinetic and pharmacodynamic variables were analysed by an analysis of variance (anova)-model accounting for sequence, period, and treatment effect, as well as possible interaction. All statistical calculations were performed using SAS software (version 6.12). The alpha-level was set to 0.05 and no alpha adjustment was made for multiple testing.
Results
Data are given as means ± standard deviation (s.d.). All administrations of morphine were well tolerated (see Table 1). Several mild to moderate adverse reactions (such as fatigue, sedation, postural hypotension, nausea, headache, and paraesthesia) were observed, however, the frequency was similar between the treatments.
Table 1.
Adverse events.
| Subject | PSC | PSC + Morphine | Morphine |
|---|---|---|---|
| 1 | hypoaesthesia * | ||
| 2 | headache * | ||
| 3 | hypoaesthesia *, fatigue * | ||
| 4 | hypoaesthesia *, fatigue * | ||
| 5 | flushing *, GI-discomfort * | ||
| 6 | fatigue * | fatigue * | |
| 7 | flushing *, somnolence * fatigue † | ||
| 8 | conjunctivitis ‡, fatigue * | flushing *, somnolence * | flushing *, fatigue * |
| 9 | metallic taste * | ||
| 10 | flushing †, GI-discomfort † | flushing †, GI-discomfort † paraesthesia* | flushing †, GI-discomfort † |
| 11 | drop-out | flushing *, GI-discomfort * | drop-out |
| 12 | headache †, fatigue † | ||
| 13 | flushing *, dyspnea * | flushing †, nausea † dyspnea †, hypoaesthesia * paraesthesia * | flushing *, fatigue † |
| 14 | orthostatic reaction * | ||
| 15 | fatigue † | ||
| 16 | paraesthesia * | hypoaesthesia * | |
| 18 | flushing *, dyspnaea * nausea *, tachycardia * | ||
| 111 | nausea * | fatigue *, headache † |
intensity:
mild
moderate
severe.
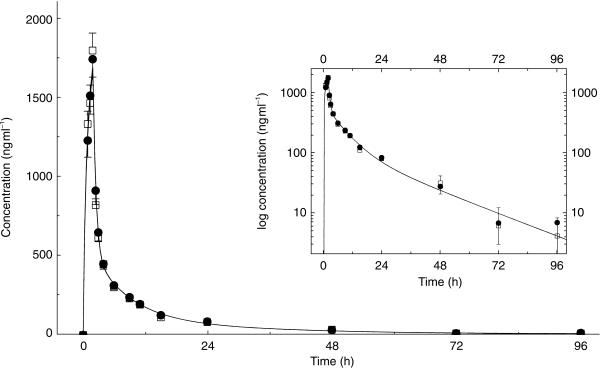
The main pharmacokinetic variables of PSC for the 18 healthy subjects are given in Figure 1 and Table 2. All the pharmacokinetic parameters of PSC were unaffected by the coadministration of morphine hydrochloride (Figure 1 and Table 2).
Figure 1.
Mean (± s.e. mean) blood concentrations of PSC after intravenous administration of 150 mg PSC over 2 h with (•) and without (□) coadministration of an intravenous infusion of 7.5 mg morphine hydrochloride over 2 h starting 1 h later (n = 18). Insert: log-linear representation of data.
Table 2.
Pharmacokinetic parameters of PSC.
| PSC | PSC + morphine | Ratio (%) | 95% confidence interval | P value | |
|---|---|---|---|---|---|
| Cmax (ng·ml−1) | 1810 ± 461 | 1759 ± 481 | 97.2 | 86.8–108.9 | NS |
| AUC(0, last) (ng ml−1h) | 8490 ± 3479 | 8517 ± 3316 | 100.7 | 90.3–112.3 | NS |
| t1/2, (h) | 16.2 ± 9.8 | 16.9 ± 8.3 | 0.71 * | −3.03–4.45 | NS |
| AUC(0,∞) (ng ml−1 h) | 9695 ± 4314 | 9660 ± 3804 | 100.7 | 91.0–111.5 | NS |
| Vz (l) | 355 ± 141 | 373 ± 109 | 108.1 | 89.5–130.5 | NS |
| Vs s (l) | 227 ± 66.9 | 233 ± 58.3 | 103.5 | 90.2–118.9 | NS |
| CL (l h−1) | 17.5 ± 5.4 | 17.3 ± 5.0 | 99.3 | 89.7–109.9 | NS |
n = 18; data are given as mean ± s.d.
difference in h; P > 0.05.
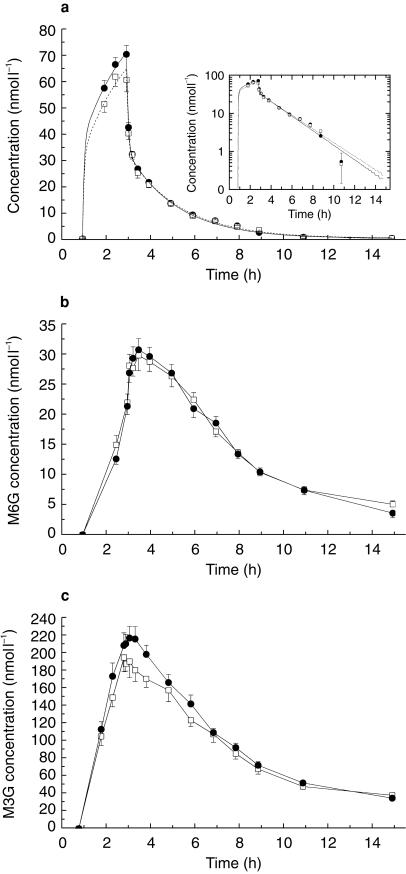
The plasma concentration-time profiles of morphine are given in Figure 2a. There was no significant difference in the pharmacokinetic parameters (Table 3) measured in the presence of PSC. The plasma concentrations of M6G were lower-and those of M3G significantly higher-than the concentrations of morphine (Figure 2a,b, Table 4), given alone or in the presence of PSC. No differences in the pharmacokinetic parameters of M6G were observed after coadministration of morphine hydrochloride and PSC compared to morphine hydrochloride alone. However, Cmax, AUC(0,last) and AUC(0,∞) of M3G were significantly increased 8.3%, 11.8 and 7.2% after PSC coadministration.
Figure 2.
Mean (± s.e. mean) plasma concentrations of (a) morphine, (b) morphine-6-glucuronide and (c) morphine-3-glucuronide after intravenous administration of 7.5 mg morphine hydrochloride over 2 h with (•) and without (□) coadministration of an intravenous infusion of 150 mg PSC over 2 h starting 1 h earlier (n = 18). Insert: log-linear representation of data.
Table 3.
Pharmacokinetic parameters of morphine.
| Morphine | PSC + Morphine | Ratio | 95% confidence interval | P value | |
|---|---|---|---|---|---|
| tmax (h) | − † | − † | |||
| Cmax (ng ml−1) | 18.6 ± 4.7 | 20.4 ± 3.6 | 109.9 | 93.2–127.6 | NS |
| AUC(0,last) (ng l−1 h) | 45.5 ± 10.6 | 48.6 ± 8.6 | 107.0 | 94.0–121.9 | NS |
| t½ (h) | 2.4 ± 1.7 | 2.3 ± 0.9 | −0.13 * | −1.08–0.82 | NS |
| AUC(0,∞) (ng ml−1 h) | 49.7 ± 9.2 | 52.6 ± 9.8 | 106.0 | 98.1–114.4 | NS |
| Vz (l) | 571 ± 540 | 470 ± 124 | 98.9 | 73.2–133.8 | NS |
| Vss (l) | 406 ± 372 | 305 ± 50 | 91.1 | 69.8–118.9 | NS |
| CL (l h−1) | 156 ± 28.3 | 147 ± 22.7 | 94.8 | 87.4–102.8 | NS |
n = 18; data are given as mean ± s.d.
difference in hours; P > 0.05
assumed to be time of termination of infusion.
Table 4.
Pharmacokinetic parameters of morphine glucuronides.
| Morphine | Morphine + PSC | Ratio | 95% confidence interval | P value * | |
|---|---|---|---|---|---|
| Morphine-6-glucuronide | |||||
| tmax (h) | 2.3 ± 0.5 | 2.3 ± 0.2 | n.a. | n.a. | NS |
| Cmax (ng ml−1) | 16.1 ± 3.4 | 16.1 ± 3.4 | 98.1 | 89.6–107.5 | NS |
| AUC(0,last) (ng ml−1 h) | 75.1 ± 17.9 | 72.5 ± 18.8 | 94.9 | 86.7–103.9 | NS |
| t1/2 (h) | 3.5 ± 1.0 | 3.7 ± 1.5 | 0.19(1) | −0.79–1.16 | NS |
| AUC(0,∞) (ng ml−1h) | 89.5 ± 21.3 | 87.0 ± 20.8 | 97.4 | 88.4–107.4 | NS |
| Morphine-3-glucuronide | |||||
| tmax (h) | 2.2 ± 0.3 | 2.3 ± 0.4 | n.a. | n.a. | NS |
| Cmax (ng ml−1) | 99.8 ± 32.3 | 108.0 ± 25.1 | 108.3 | 100.5–116.7 | 0.039 |
| AUC(0,last) (ng ml−1h) | 569 ± 154 | 624 ± 126 | 111.8 | 104.6–119.5 | 0.0026 |
| t1/2 (h) | 5.0 ± 1.4 | 4.8 ± 2.1 | −0.14 (1) | −0.89–0.60 | NS |
| AUC(0,∞) (ng ml−1 h) | 700 ± 194 | 743 ± 162 | 107.2 | 100.7–114.1 | 0.045 |
n = 18; data are given as mean ± s.d.
difference in hours; n.a. = not applicable.
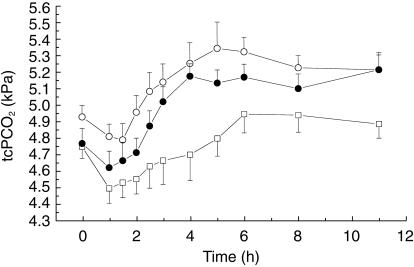
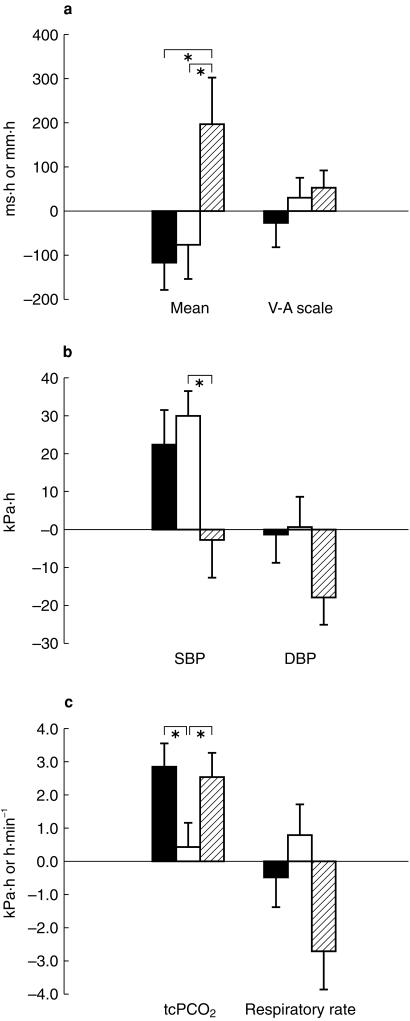
Morphine caused a slight and prolonged respiratory depression (Figures 3 and 5), significantly elevating the AUE for PCO2 (2.57 kPa·h) compared with PSC alone (0.44 kPa·h). This effect of morphine was not influenced by coadministration with PSC (2.9 kPah). The average absolute increase (Emax) was 0.73 kPa for the PSC/morphine group vs 0.69 kPa for morphine (Figure 5). Pulse oxymetry did not show any significant differences between the three treatments. Following morphine there was a trend for an early fall in respiratory rate (Figure 5), as captured by AUE (−20.4 breath min−1 h), compared with PSC alone (5.9 breath min−1 h). For the combination of PSC/morphine there was only a slight fall (−3.8 breath min−1 h). None of these changes was significant.
Figure 3.
Mean (± s.e. mean) transcutaneously measured partial pressure of CO2 (tcPCO2) after intravenous administration of morphine (○), PSC (□), and their combination (•) (n = 18).
Figure 5.
Effect of PSC/morphine (filled bar), PSC (open bar) or morphine (hatched bar) on AUE (calculated as change from baseline) of (a) reaction time and visual-analogue scale (b) systolic and diastolic blood pressure and (c) tcPCO2 and respiratory rate. *P < 0.05. Bars are mean (s.e. mean n = 19).
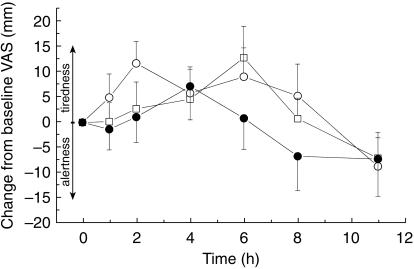
Morphine resulted in a statistically significant prolongation (Figure 5) of the mean reaction time (198 ms h), as assessed by AUE curve, compared with PSC alone (−76.8 ms h). The absolute change (Emax) was however, slight, the prolongation being 48 ms compared to an absolute predose reaction time of 644 ms. When given in combination with PSC the prolongation was completely abrogated (−118 ms h). The indications for sedation seen with reaction time were not detected by the alertness-drowsiness score (Figures 4 and 5). The mean AUE ranged from −27.8–52.0 mm h and the maximal effect from 21.3 to 26.0 mm occurring between 4.2 and 5.2 h after start of treatment. None of the differences was statistically significant.
Figure 4.
Mean (± s.e. mean) change in visual analogue drowsiness scale from baseline (mm) (morphine (○), morphine + PSC (•), and PSC (□); n = 18).
Compared with PSC alone (Figure 5), morphine elicited a fall in AUE of systolic and diastolic blood pressure, the effect being statistically significant only for the former. The maximal fall of systolic blood pressure from baseline was −8.9 mmHg for morphine, −3.1 mmHg for PSC and −4.0 mmHg for the combination.
Discussion
The main purpose of this study was to investigate the potential pharmacokinetic and pharmacodynamic interaction between the Pgp inhibitor PSC and morphine or its metabolites in humans. These interactions could be of considerable clinical importance for several reasons. PSC is being developed to reverse the resistance of cancer cells to chemotherapy. This effect is achieved by inhibition of the membrane efflux pump Pgp, the gene product of the MDR1 gene. The inhibition of Pgp results in substantial alteration of drug tissue availability of concomitantly administered drugs with a potential to cause adverse effects of those with a narrow safety margin. Of particular importance is the possibility of enhanced brain disposition caused by a reduction in drug back-extrusion across the blood brain barrier [11, 12]. Cancer patients will often require treatment with morphine. Because morphine and its active metabolite M6G are substrates of Pgp, a clinically significant interaction could be dangerous.
In the present study we have shown that the pharmacokinetics of morphine was virtually unaffected by coadministration of PSC. The apparent terminal half-lives of morphine were 2.3 ± 0.9 h and 2.4 ± 1.7 h, after administrations of morphine hydrochloride with and without coadministration of PSC, respectively. These values were comparable. However, they were slightly higher than those values reported in the literature of 1.78 ± 0.4 h [22] and 1.8 ± 0.4 h [23]. Based on these results, we conclude that PSC administered in clinically relevant doses (as in this study) did not significantly change the pharmacokinetics of morphine to a clinically relevant degree.
In this study, direct measurements of changes in brain disposition of morphine and its metabolites were not possible; we therefore relied on peripheral blood measurements to facilitate interpretation of the drug interaction and the central pharmacodynamic effects. Three safety related pharmacodynamic variables were measured: visual analogue scale of sedation as a subjective measurement, reaction time as a ‘neurological’ assessment, and transcutaneous partial pressure of CO2 as a physiological and safety assessment. VAS was subject to a large intersubject variation, which is presumably the reason why significant differences between the treatments could not be detected. This may also be related to the moderately low dose of morphine. The dose of morphine was chosen with respect to safety, since data in mdr1a knock-out mice suggested at least a two-fold increased net brain uptake [11, 12]. However, it is not clear, whether reaction time measurements are appropriate parameters for the assessment of cerebral opioid effects since chronic administration of methadone or morphine in cancer patients did not change reaction times when compared with controls [24, 25]. Since the focus of this study was on variables related to adverse effects, analgesia was not measured. Additionally, there was concern that stimuli used to generate analgesic measurements might affect respiration, confounding the interpretation of the PCO2 values [26].
There are several possible explanations as to why augmentation of the central effects of morphine wasn't seen in the present study. The simplest is that in man, as opposed to mice, the relative contribution of BBB Pgp back-extrusion to the disposition of morphine is of minor importance in relation to its passive diffusion into the brain and its CSF clearance. A second explanation is that PSC has caused an elevation in brain morphine. But, since coadministration of PSC increased plasma concentrations of M3G, one possible explanation is an inhibitory effect of M3G on the effects of morphine and M6G at the µ-receptor. This inhibitory effect of M3G was demonstrated in rats and mice [27, 28], where M3G was able to completely antagonize the analgesic action of morphine and M6G. In addition, M3G also abolished the respiratory depression induced by morphine and M6G [29, 30] in rats. In human volunteers, M3G is inactive or has an opposite effect on miosis or saliva production induced by morphine and M6G [31]. A third possible explanation is that the chosen methods of pharmacodynamic measurement were not sufficiently sensitive. Methods such as measurement of pupil diameter (known to be sensitive to the central effects of morphine), or assessment of the sensitivity to CO2 rebreathing were considered and might have had a higher chance to detect a possible PSC/morphine interaction. However, our objective was to determine whether there was a clinically–relevant interaction resulting in a safety problem which was addressed by measurement of resting PCO2, vital signs and assessment of the subjects' adverse events reports. In addition, measurement of pupil diameters possibly would have jeopardised assessment of adverse events in a double-blind manner.
In rats, chronic inhibition of Pgp does not affect volume of distribution and clearance of morphine [19]. M3G plasma levels were increased, which is similar to our findings albeit to a smaller degree. In addition to tissue distribution, inhibition of Pgp will also decrease biliary and renal excretion of its substrates such as morphine and the more hydrophilic metabolites M6G and M3G. Furthermore, Pgp inhibition decreases tubular secretion of drugs [14]. There is evidence that the affinity of morphine metabolites to renal Pgp is different. In patients with renal dysfunction, changes in disposition and elimination were significantly pronounced for morphine than for M3G and M6G [32]. This may indicate that M3G and M6G are substrates with higher affinity to Pgp than morphine itself. After i.v. administration to humans, a 5-times greater fraction of the administered dose of M3G was recovered in the urine than of M6G [23]. Pgp inhibition may therefore have a greater effect on tubular secretion of M3G compared with M6G. As a consequence, Pgp inhibition will more likely increase M3G than M6G plasma concentrations.
This increase in M3G may prevent the respiratory depression induced by morphine and M6G. Therefore, acute Pgp inhibition will most likely not enhance respiratory depression in cancer patients receiving morphine for pain control.
On the other hand, we have shown that PSC pharmacokinetics are not influenced significantly by coadministration of the Pgp substrate morphine. This is consistent with the finding that PSC, although being a potent inhibitor of Pgp, is not itself a substrate of this transporter [4]. Therefore, a change in PSC disposition or elimination by inhibition of Pgp was not expected.
Therefore, on the basis of clinically relevant measures, it can be concluded that concomitant treatment with morphine and PSC does not increase morphine toxicity.
Acknowledgments
The study was financially supported in part by a research grant of Novartis Ltd, Basel to Jürgen Drewe, MD.
The authors gratefully acknowledge the expert contribution of Michele Pongowski, Novartis to the writing of the clinical trial protocol. Also we gratefully acknowledge Drs C. Gerbeau and M. Guerret for performing the drug analytics.
References
- 1.Fojo A, Akiyama S, Gottesman MM, Pastan I. Reduced drug accumulation in multiply drug-resistant human KB carcinoma cell lines. Cancer Res. 1985;45:3002–3007. [PubMed] [Google Scholar]
- 2.Inaba M, Kobayashi H, Sakurai Y, Johnson RK. Active efflux of daunorubicin and adriamycin in sensitive and resistant sublines of P388 leukemia. Cancer Res. 1979;39:2200–2203. [PubMed] [Google Scholar]
- 3.Gottesman MM, Hrycyna CA, Schoenlein PV, Germann UA, Pastan I. Genetic analysis of the multidrug transporter. Ann Rev Genet. 1995;29:607–649. doi: 10.1146/annurev.ge.29.120195.003135. [DOI] [PubMed] [Google Scholar]
- 4.Archinal-Mattheis A, Rzepka RW, Watanabe T, et al. Analysis of the interactions of SDZ PSC 833 ([3′-keto-Bmt1]- [Val2]-Cyclosporine), a multidrug resistance modulator, with P- glycoprotein. Oncol Res. 1995;7:603–610. [PubMed] [Google Scholar]
- 5.Ehrlich PH, Moustafa ZA, Archinal-Mattheis AE, Newman MJ, Bair KW, Cohen D. The reversal of multidrug resistance in multicellular tumor spheroids by SDZ PSC 833. Anticancer Res. 1997;17:129–133. [PubMed] [Google Scholar]
- 6.Sonneveld P, Marie JP, Huisman C, et al. Reversal of multidrug resistance by SDZ PSC 833, combined with VAD (vincristine, doxorubicin, dexamethasone) in refractory multiple myeloma. A phase I study. Leukemia. 1996;10:1741–1750. [PubMed] [Google Scholar]
- 7.Watanabe T, Tsuge H, Oh-Hara T, Naito M, Tsuruo T. Comparative study on reversal efficacy of SDZ PSC 833, cyclosporin A and verapamil on multidrug resistance in vitro and in vivo. Acta Oncol. 1995;34:235–241. doi: 10.3109/02841869509093961. [DOI] [PubMed] [Google Scholar]
- 8.Floren LC, Bekersky I, Benet LZ, et al. Tacrolimus oral bioavailability doubles with coadministration of ketoconazole. Clin Pharmacol Ther. 1997;62:41–49. doi: 10.1016/S0009-9236(97)90150-8. [DOI] [PubMed] [Google Scholar]
- 9.Kim RB, Fromm MF, Wandel C, et al. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest. 1998;101:289–294. doi: 10.1172/JCI1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Asperen J, van Tellingen O, Sparreboom A, et al. Enhanced oral bioavailability of paclitaxel in mice treated with the P-glycoprotein blocker SDZ PSC 833. Br J Cancer. 1997;76:1181–1183. doi: 10.1038/bjc.1997.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schinkel AH. Pharmacological insights from P-glycoprotein knockout mice. Int J Clin Pharmacol Ther. 1998;36:9–13. [PubMed] [Google Scholar]
- 12.Schinkel AH, Wagenaar E, Mol CA, van Deemter L. P-glycoprotein in the blood–brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest. 1996;97:2517–2524. doi: 10.1172/JCI118699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Müller M, Jansen PL. Molecular aspects of hepatobiliary transport. Am J Physiol. 1997;272:G1285–1303. doi: 10.1152/ajpgi.1997.272.6.G1285. [DOI] [PubMed] [Google Scholar]
- 14.Miller DS, Fricker G, Drewe J. P-Glycoprotein-mediated transport of a fluorescent rapamycin derivative in renal proximal tubule. J Pharmacol Exp Ther. 1997;282:440–444. [PubMed] [Google Scholar]
- 15.Kusuhara H, Suzuki H, Terasaki T, Kakee A, Lemaire M, Sugiyama Y. P-Glycoprotein mediates the efflux of quinidine across the blood–brain barrier. J Pharmacol Exp Ther. 1997;283:574–580. [PubMed] [Google Scholar]
- 16.Mayer U, Wagenaar E, Dorobek B, Beijnen JH, Borst P, Schinkel AH. Full blockade of intestinal P-glycoprotein and extensive inhibition of blood–brain barrier P × glycoprotein by oral treatment of mice with PSC833. J Clin Invest. 1997;100:2430–2436. doi: 10.1172/JCI119784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Callaghan R, Riordan JR. Synthetic and natural opiates interact with P-glycoprotein in multidrug-resistant cells. J Biol Chem. 1993;268:16059–16064. [PubMed] [Google Scholar]
- 18.Huwyler J, Drewe J, Klusemann C, Fricker G. Evidence for P-glycoprotein-modulated penetration of morphine-6-glucuronide into brain capillary endothelium. Br J Pharmacol. 1996;118:1879–1885. doi: 10.1111/j.1476-5381.1996.tb15619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Letrent SP, Pollack GM, Brouwer KR, Brouwer KL. Effect of GF120918, a potent P-glycoprotein inhibitor, on morphine pharmacokinetics and pharmacodynamics in the rat. Pharm Res. 1998;15:599–605. doi: 10.1023/a:1011938112599. [DOI] [PubMed] [Google Scholar]
- 20.Langewitz W, Stephan JA, Otten H. A new self-adjusting reaction time device (BonnDet) with high test-re-test reliability. J Psychophysiol. 1987;1:67–77. [Google Scholar]
- 21.Tyrefors N, Hyllbrant B, Ekman L, Johansson M, Langstrom B. Determination of morphine, morphine-3-glucuronide and morphine-6-glucuronide in human serum by solid-phase extraction and liquid chromatography-mass spectrometry with electrospray ionisation. J Chromatogr A. 1996;729:279–285. doi: 10.1016/0021-9673(95)01090-4. [DOI] [PubMed] [Google Scholar]
- 22.Lötsch J, Stockmann A, Kobal G, et al. Pharmacokinetics of morphine and its glucuronides after intravenous infusion of morphine and morphine-6-glucuronide in healthy volunteers. Clin Pharmacol Ther. 1996;60:316–325. doi: 10.1016/S0009-9236(96)90058-2. [DOI] [PubMed] [Google Scholar]
- 23.Hasselström J, Säwe J. Morphine pharmacokinetics and metabolism in humans. Enterohepatic cycling and relative contribution of metabolites to active opioid concentrations. Clin Pharmacokin. 1993;24:344–354. doi: 10.2165/00003088-199324040-00007. [DOI] [PubMed] [Google Scholar]
- 24.Vainio A, Ollila J, Matikainen E, Rosenberg P, Kalso E. Driving ability in cancer patients receiving long-term morphine analgesia. Lancet. 1995;346:667–670. doi: 10.1016/s0140-6736(95)92281-4. [DOI] [PubMed] [Google Scholar]
- 25.Hauri-Bionda R, Bar W, Friedrich-Koch A. [Driving fitness/driving capacity of patients treated with methadone] Schweiz Med Wochenschr. 1998;128:1538–1547. [PubMed] [Google Scholar]
- 26.Borgbjerg FM, Nielsen K, Franks J. Experimental pain stimulates respiration and attenuates morphine-induced respiratory depression: a controlled study in human volunteers. Pain. 1996;64:123–128. doi: 10.1016/0304-3959(95)00088-7. [DOI] [PubMed] [Google Scholar]
- 27.Faura CC, Olaso MJ, Garcia Cabanes C, Horga JF. Lack of morphine-6-glucuronide antinociception after morphine treatment. Is morphine-3-glucuronide involved? Pain. 1996;65:25–30. doi: 10.1016/0304-3959(95)00198-0. [DOI] [PubMed] [Google Scholar]
- 28.Smith MT, Watt JA, Cramond T. Morphine-3-glucuronide – a potent antagonist of morphine analgesia. Life Sci. 1990;47:579–585. doi: 10.1016/0024-3205(90)90619-3. [DOI] [PubMed] [Google Scholar]
- 29.Gong QL, Hedner J, Bjorkman R, Hedner T. Morphine-3-glucuronide may functionally antagonize morphine-6-glucuronide induced antinociception and ventilatory depression in the rat. Pain. 1992;48:249–255. doi: 10.1016/0304-3959(92)90065-J. [DOI] [PubMed] [Google Scholar]
- 30.Gong QL, Hedner T, Hedner J, Bjorkman R, Nordberg G. Antinociceptive and ventilatory effects of the morphine metabolites: morphine-6-glucuronide and morphine-3-glucuronide. Eur J Pharmacol. 1991;193:47–56. doi: 10.1016/0014-2999(91)90199-z. [DOI] [PubMed] [Google Scholar]
- 31.Westerling D, Persson C, Hoglund P. Plasma concentrations of morphine, morphine-3-glucuronide, and morphine-6-glucuronide after intravenous and oral administration to healthy volunteers: relationship to nonanalgesic actions. Ther Drug Monit. 1995;17:287–301. doi: 10.1097/00007691-199506000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Sear JW, Hand CW, Moore RA, McQuay HJ. Studies on morphine disposition: influence of renal failure on the kinetics of morphine and its metabolite. Br J Anaesth. 1989;62:28–32. doi: 10.1093/bja/62.1.28. [DOI] [PubMed] [Google Scholar]