Abstract
Aims
Pseudoephedrine (PSE) is a readily available over-the-counter nasal decongestant which is structurally similar to amphetamine and is included on the International Olympic Committee's list of banned substances. However to date, little research has supported its putative ergogenic effect. This study investigated whether a 180 mg dose of PSE ingested 45 min prior to exercise enhanced short-term maximal exercise performance and/or altered related physiological variables.
Methods
A randomised, double-blind, crossover study in 22 healthy male athletes.
Results
Maximum torque (mean ±s.d., n = 22) produced in an isometric knee extension exercise was 321.1 ± 62.0 Nm (PSE) and 295.7 ± 72.4 Nm (placebo), and peak power obtained on the ‘all-out’ 30 s cycle test was 1262.5 ± 48.5 W (PSE) and 1228.4 ± 47.1 W (placebo) (P < 0.01, P < 0.03, respectively). Subjects were estimated to be producing 96.9 ± 2.4% of their maximal possible isometric leg extension force after PSE ingestion, but only 95.3 ± 2.4% when PSE was not ingested. Bench press tasks and total work during the cycle test were not affected by the ingestion of PSE. Lung function was altered following ingestion of PSE (P < 0.05) with FEV1 and FVC significantly increased (P < 0.02, P < 0.01, respectively) although the FEV1/FVC ratio was not altered. Heart rate was significantly elevated by the ingestion of PSE immediately following the 30 s cycle sprint (P < 0.01) however, lactate concentration was not altered by the ingestion of PSE.
Conclusions
The administration of a 180 mg dose of PSE increased maximum torque, produced in an isometric knee extension and produced an improvement in peak power during maximal cycle performance, as well as improving lung function.
Keywords: amphetamines, bronchodilation, drugs in sport, ephedrine, ergogenics, exercise performance, muscle activation, noradrenaline, stimulants, sympathomimetics
Introduction
There is a wide range of nutrients, supplements and pharmacological agents available to athletes that purport to provide beneficial performance changes or improved recovery from training. Stimulants are one type of drug that have been extensively used throughout history with little scientific data substantiating claims of performance enhancement [1]. The International Olympic Committee (IOC) has banned drugs that affect or mimic the sympathetic nervous system (sympathomimetics), such as ephedrine (EPH), phenylpropanolamine and pseudoephedrine (PSE). This is mainly due to their chemical similarity to amphetamines and the assumption that these drugs may be ergogenic in nature.
The abuse of such substances in athletic competition is potentially harmful and therefore information regarding the effectiveness and side-effects of such drugs is required. The acquired information could be used to discourage athletes from the abuse of such substances and to encourage sport administrators to take positive action against this form of doping.
The sympathomimetic group of drugs (e.g. ephedrine, PSE and amphetamines) increases systolic and diastolic blood pressure, heart rate, peripheral vascular tone, respiratory stimulation, bronchial tube dilation, pupillary dilation, and relaxation of the smooth muscles of the gastrointenstinal tract. Further, the sympathomimetics promote vasoconstriction of cutaneous blood vessels, vasodilation in the skeletal muscles, and a redistribution of blood from the skin and portions of the splanchnic bed to the skeletal muscles and brain. This may result in increased core body temperature. An increased heart rate and myocardial contractility with a greater venous return due to increased vascular tone may increase cardiac output and therefore blood supply to working muscles [2, 3]. These effects are more pronounced with higher doses and may cause complications during maximal effort exercise.
The ephedrines and related phenylisopropylamines (amphetamines) act indirectly through the action of endogenous adrenaline and noradrenaline (NA) present in the organism. There are two separate mechanisms present. The first mechanism is the liberation of NA from storage sites in nerve tissue, whilst the second mechanism depends on sympathetic nerve activity and upon intensification and prolongation of the effect of amines [2].
Research has previously been carried out investigating ergogenic effects of amphetamines but in recent years, positive drug tests for amphetamines have been rare. PSE on the other hand is frequently detected in athlete's urine samples but at this time it is uncertain whether this drug has an ergogenic property.
PSE is available over-the-counter for therapeutic use and in Australia from 1996 to 1997, there were 11 positive urine tests for PSE [4]. However studies investigating the effectiveness of PSE in improving athletic performance have reported no ergogenic effect of a therapeutic dose of PSE [5–9]. Thus, it is unclear whether PSE ingestion significantly improves sporting performance.
Pseudoephedrine exhibits central nervous system stimulation, bronchodilation and vasoconstrictive properties when taken in doses of 60 mg 3–4 times per day [3]. It is claimed that PSE can be used to obtain an amphetamine-like euphoria that could suppress the central component of fatigue [10, 11]. Other ergogenic effects could be due to skeletal muscle stimulation, changed lung function due to bronchial dilation, or altered fuel utilization. These speculated ergogenic effects have yet to be scientifically confirmed. Currently it would appear that dangerous physiological consequences of PSE far exceed any performance enhancement effect.
There is concern about the inclusion of PSE on the banned list of substances by the IOC. Many athletes continue to test positive for PSE (concentration of PSE > 10 µg ml−1 of urine) so it is important for potential ergogenic effects to be researched and documented. Athletes competing in short duration maximal effort sports are more commonly found to be using PSE than their counterparts in longer aerobic type events.
Very little research has examined its effects on anaerobic or short duration exercise. Walton et al. [9] observed trends in improved anaerobic performance (isometric torque and 30 s maximal cycle performance) following a 120 mg dose of PSE. It seems that no ergogenic effect exists in aerobic exercise following ingestion of a therapeutic dose but if athletes were to use PSE for performance enhancement it is likely that a higher dose would be used. The purpose of the present study was to determine whether a higher than therapeutic dose of pseudoephedrine (180 mg) enhanced short-term maximal exercise and/or altered physiological variables related to the specific tasks.
Methods
Subjects
Twenty-two healthy male athletes volunteered from the University student population (Table 1). The subjects played basketball, rugby union, rugby league or Australian Rules Football at the recreational or representative level. Testing occurred at the end of their respective sporting seasons; so all subjects were assumed to be fit. No subjects reported injury prior to the commencement of the study and all subjects completed medical assessment and informed consent forms prior to testing to ensure there were no contraindications. Ethics approval for this study was obtained from Southern Cross University Human Research Ethics Committee.
Table 1.
Subject physical characteristics (n = 22).
| Variable | Minimum | Maximum | Mean ±s.d. |
|---|---|---|---|
| Age (years) | 18.0 | 25.0 | 21.0 ± 2.8 |
| Height (m) | 1.67 | 1.98 | 1.83 ± 0.09 |
| Mass (kg) | 64.4 | 105.0 | 83.4 ± 10.8 |
Experimental design and protocols
A randomised double-blind crossover experimental design was used. After a familiarization session subjects attended two experimental sessions separated by 7 days. The familiarization session consisted of all subjects performing all exercise tests. Prior to one session (PSE), 180 mg of PSE was ingested orally (three 60 mg tablets Sudafed, Warner Lambert, Australia), while placebo (two tablets of Blackmores Silica Compound containing sodium phosphate (200 mg) and silicon dioxide (25 mg) in a lactose base) was ingested orally prior to the other session (placebo). This placebo was used because the texture and colour were very similar to PSE. The tastes were different but instructions were given to subjects to swallow as quickly as possible and minimize contact with the tongue. The laboratory researcher involved in the collection of data was blind to the substance ingested since the substance preparation and condition allocation (randomised) was carried out by an associate not involved in the study. In all sessions water was allowed ad libitum.
Subjects were asked to avoid alcohol consumption, other medications, and intense exercise for the 24 h prior to each session. Testing sessions were scheduled for the same day each week and at the same time of day to avoid influences of circadian rhythms that operate for many variables [12, 13].
After PSE or placebo ingestion, subjects performed a standardized warm up which consisted of 5 min cycling on a stationary cycle ergometer (Monark 868) at a workload of 200 Watts. Subjects subsequently performed static stretches of the quadriceps, hamstrings, pectoralis and triceps muscle groups. Any other subject-preferred stretches were performed with each one being held for approximately 10 s. Testing started 45 min after ingestion of PSE or placebo. The drug administration and testing times were selected on the basis that a testing session took approximately 60 min to complete and PSE exerts its effects approximately 40 min after ingestion and reaches peak levels in the plasma at approximately 2 h [6, 14].
Effectiveness of blinding
The effectiveness of the blinding and placebo was assessed throughout the testing procedures by simple questions relating to subject's perception of what experimental condition they thought they were in. The blinding was deemed to be effective.
Procedures
Each subject performed all tests on 1 day. The order of testing was the same for each subject and the duration of each test and rest between tests were constant within and between subjects. The order of testing was such that carry over fatigue was minimal (upper body tasks were followed by lower body tasks).
Isometric knee extension and motor unit activation (Figure 1)
Figure 1.
Equipment and subject position for assessment of isometric knee extension and muscle activation. Straps around the chest and waist secure the subject to minimize movement.
During the 45 min rest period after dosing, electrodes for electrical stimulation were placed over the quadriceps of the subject's dominant leg (preferred leg to kick a ball). The skin was shaved on the anterior surface of the thigh and cleansed with alcohol swabs and two pairs of stimulating electrodes were attached prior to the warm-up (Tens electrode, Uni-match, USA). The cathodes were placed over the proximal quadriceps with the respective anodes placed over the distal portions of the superficial vastii. Precise electrode placement was determined during pilot testing to give the maximal possible twitch torque during an isometric contraction at the 90° knee angle. The dominant leg extensor strength was assessed on an isokinetic dynamometer (Kin-Com) at a 90° knee joint angle. Movement of the trunk and at the hip was prevented by straps securing the trunk, hips and thighs to the backrest and seat, respectively. During the test the subject's inactive leg hung freely from the bench. Subjects performed three maximal contractions after completing a standard sequence of warm-up contractions (3 × 30%, 2 × 50%, 1 × 70%, 1 × 90% of maximum torque produced during the familiarization session). Maximum voluntary contractions (MVCs) were interspaced with 2 min rest periods. Loud verbal encouragement and visual feedback of torque output were provided during all maximal efforts. Peak torque was recorded for data analysis.
Muscle activation was determined by a modified version of the method described by Newham et al. [15]. Brief trains (250 ms) of electrical stimulation were evoked in relaxed muscle and later superimposed over maximal voluntary contractions. Electrical stimulation (ES) was applied via an AMLAB stimulator (Associative Measurement Pty Ltd, Australia). The stimulation parameters are shown in Table 2.
Table 2.
Stimulation parameters used in the determination of motor unit activation.
| Parameters | Description |
|---|---|
| Electrode description | 7.62 × 12.7 cm Tens electrode, part no. 610, pigtail pin connection, Uni-match, USA |
| Pulse shape | Rectangular |
| Pulse width | 0.5 ms |
| Pulse frequency | 100Hz |
| Current | Adjusted to subject tolerance (Range 70–100mA) |
The ES was triggered by the onset of torque development and delayed for 1 s to allow maximum voluntary torque to reach a plateau. Trials in which torque was not held stable prior to stimulation were rejected. The peak torque during the ES period was recorded.
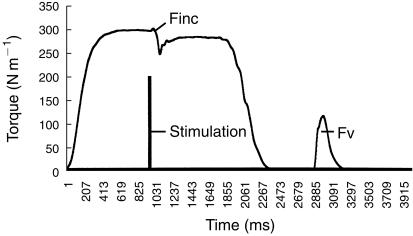
Full activation was assumed to occur when increased ES failed to induce any increment in torque output. When full activation was not achieved the extent of muscle activation failure (AF) was calculated by dividing the increment in torque (Finc) due to stimulation by the torque generated in the preceding evoked contraction (Fv) (AF = Finc/Fv). The extent of muscle activation (MA) was then calculated by subtracting the AF from 1. The extent of muscle activation in the strongest contraction was used for data analysis (Figure 2).
Figure 2.
The increment in force (Finc) due to electrical stimulation was divided by the force generated in the preceding evoked contraction (Fv) to establish activation failure (AF). Muscle activation (%MA) was calculated as follows:% MA = 1 − (Finc/Fv).
1 RM bench press (Figure 3)
Figure 3.
The bench press task involves the subject lowering the bar and weight to the chest (approximately 90° elbow angle) and then pushes the weight back to the starting position (illustrated). Hand position is standardised within subjects.
A one repetition maximum (1RM) bench press on a modified Smith machine (Plyopower Technologies, Lismore, Australia) was used as a test for muscular strength. The modified Smith machine was used in an attempt to standardize technique differences between subjects and eliminate the need for previous bench press experience. The weight on the bar was gradually increased until one repetition could just be completed with the bar being lowered to the chest and raised again.
70% 1RM bench press
Repetitions of 70% 1RM bench press (measured in kilograms, kg) to fatigue were carried out by using a counter set at 1 s intervals to set the pace of the repetitions, which was 1 s for the eccentric phase and 1 s for the concentric phase of the lift. The test ceased when the subject either failed to complete the lift or the cadence was not maintained.
30 second ‘all-out’ cycle test (Figure 4)
Figure 4.

The wind-braked cycle ergometer used in this study allows the measurement of maximal power. The subjects's feet were securely fastened with tape and toe straps to ensure feet were not pulled out of the pedals during the test.
This consisted of a 30 s ‘all-out’ cycle sprint on an air-braked front access cycle (Exertech Exercise Technology). A blood sample was taken immediately prior to the test from a small puncture to the finger, as well as at 1, 3, 6, and 10 min intervals after the test. Anaerobic work was defined as the total amount of work performed in the 30 s period (TW measured in kilojoules, kJ) while the peak anaerobic power was identified as the greatest power achieved during the work interval (PP measured in watts, W). TW and PP achieved over the 30 s period were recorded using a work monitor unit (Exertech Exercise Technology). The reliability of the 30 s ‘all-out’ cycle sprint was determined in this laboratory to be acceptable (Intra-class correlation [ICC] = 0.98, Technical error of measurement [TEM] = 1.7%). To ensure this high reliability the preparation, environmental conditions and warm up were all standardized across the experiment. Equal verbal encouragement was given from session to session across all subjects.
Lactate analysis
Blood was collected from the finger pricks in 75 µl Haematocrit-capillary tubes (Code-No 910 02 75) and a 25 µl sample was drawn and analysed in a YSI 1500 Sport L-Lactate Analyser.
Heart rate
Maximal heart rate was recorded immediately after the cycle test (n = 7) with the recovery heart rate being observed for 10 min after the test using a Polar Sport Tester heart rate monitor. Technical difficulties in recording heart rate were encountered resulting in only seven of the subjects having complete recovery heart rates measured for both experimental conditions.
Forced vital capacity
A single maximal breath test using a spirometer (Vitalograph, Ireland) was used to assess lung function changes due to effects of bronchodilation. Two tests were performed before and after drug administration. The best result of the two trials was used for analysis. The volume expired from maximum inspiration to rapid forced maximum exhalation, termed forced vital capacity (FVC measured in litres, l) was measured. The forced expired volume in one second (FEV1 measured in litres, l) was also measured and, using these two values, the forced expiratory ratio at the first second (FER1 measured as a percentage,%) was calculated as a measure of airway resistance.
Statistical analysis
A two-factor multivariate repeated measures analysis of variance with one within subject factor (drug) and one between subject factor (order of administration) was performed on all data. Wilk's Lambda uni-variate tests were performed to identify which of the dependent variables manifested significantly (P < 0.05) different responses for the two drugs (SPSS Statistical Software, Illinios, USA). Dependent variables analysed were torque and muscle activation (MA) in the leg extension, peak power (PP) and total work (TW) during the 30 s ‘all-out’ cycle sprint, weight lifted in the 1RM bench press (1RM WT), and repetitions (REPS) in the 70% 1RM lift to fatigue. The lactate response and lung function results were all analysed in the same manner. Logarithmic functions were fitted to each individual's heart rate response (r2 = 0.85–0.98) and a repeated measures anova performed to compare the coefficients and constants of the plotted lines. Alpha level was set at P < 0.05 for all tests.
Results
No significant interaction between drug (PSE or placebo) and order (first session or last session) indicates that order of administration did not significantly affect the differences between PSE and placebo in any of the variables analysed. Since effects of the drug were independent of order, the whole sample (both orders combined) was used to test the drug effect. A significant difference (P < 0.05) was detected between PSE and placebo on at least one linear combination of the performance variables analysed. The PSE and placebo were found to differ significantly in their effects on torque, the difference being 25.5 Nm or 8.6% (Table 3). The muscle activation (%) differed between the two conditions by 1.6% but this difference was not statistically significant (P = 0.07) (Table 3).
Table 3.
Performance tasks and changes in physiological variables (mean ±[s.d.], n = 22) in two experimental conditions; Pseudoephedrine (PSE) and placebo (PLA), *indicates significant difference between treatments, P < 0.05.
| Variable | PSE | Placebo | P value | 95% CI |
|---|---|---|---|---|
| Leg extension | ||||
| Torque (N m−1) | 321.1 [62.0] | 295.7 [72 4] | 0.01* | 15.3, 40.0 (r = 0.91) |
| M. activation (%) | 96.9 [2, 4] | 95.3 [2, 4] | 0.07 | −0.3, 2.7 (r = 0.20) |
| 30 s cycle sprint | ||||
| Peak power (W) | 1262.5 [48 5] | 1228.4 [47 1] | 0.03* | 4.4, 61.5 (r = 0.95) |
| Total work (kJ) | 26.1 [3, 8] | 25.6 [3, 8] | 0.14 | −0.2, 1.1 (r = 0.92) |
| Bench press | ||||
| 1RM Weight (kg) | 88.9 [2, 21] | 88.0 [1, 22] | 0.38 | −1.2, 2.7 (r = 0.98) |
| 70% repetitions | 10.9 [2, 8] | 11.1 [2, 8] | 0.75 | −1.3, 1.1 (r = 0.53) |
| Heart rate | ||||
| (n = 7, beats min−1) | ||||
| Constant | 175.5 [2, 20] | 166.3 [26 3] | 0.01* | −0.6, 5.8 (r = 0.88) |
| Coefficient | −37.6 [29 6] | −40.1 [9, 16] | 0.71 | |
| Lactate (mmol l−1) | ||||
| Peak value | 13.1 [1, 8] | 13.3 [2, 7] | 0.70 | −1.2, 1.3 (r = 0.43) |
Heart rate constant: The constant value was obtained by applying a line of best fit to the recovery heart rate data. This value represents the maximum heart rate or heart rate recorded immediately after the cycle sprint. Heart rate coefficient: The coefficient value was obtained by applying a line of best fit to the recovery heart rate data. This value represents the slope of the plotted line or decay of the heart rate during recovery. 95% CI: 95% confidence interval for the differences between PSE and PLA conditions. M. activation (%): Muscle activation.
The 1RM bench press and 70% 1RM bench press to fatigue tests were not significantly altered between drug conditions (Table 3). The total work performed during the 30 s ‘all-out’ cycle test was also unchanged between conditions (Table 3).
Peak power on the 30 s ‘all-out’ cycle test was also different between the groups with a 34-W difference or 2.8% change (Table 3). The logarithmic functions that were plotted for each individual's heart rate response were significantly different between PSE and placebo conditions (P = 0.01). Technical difficulties resulted in only seven subject's recovery heart rate being recorded. The constant of the equations (maximum heart rate) were significantly different between conditions (175 beats min−1[PSE] and 165 beats min−1[placebo]) whereas the coefficient (heart rate recovery) of the logarithmic functions were not (−37.5 [PSE] and −40.1 [placebo]). This suggests that the heart rate is elevated immediately following exercise and remains elevated but recovers at a similar rate regardless of substance ingested. Peak blood lactate concentrations following the 30 s ‘all-out’ cycle test are illustrated (Table 3). The lactate concentration rose from approximately 4 mmol l−1 immediately after the test to 13 mmol l−1 after 10 min recovery. The concentrations were not significantly different between PSE and placebo.
With respect to lung function (Table 4), FEV1 and FVC were significantly (P = 0.02 and P = 0.01, respectively) altered following ingestion of PSE but the FER1 values were not. The changes from prepost were not different following ingestion of placebo but following ingestion of PSE there were positive increases in the FEV1 and FVC values. FEV1 increased by 0.15 l following ingestion of PSE and was unchanged following ingestion of placebo, FVC values increased by 0.12 l in the PSE condition and decreased 0.04 l in the placebo condition. The FER1 values were not significantly changed due to greater variation within and between subjects.
Table 4.
Lung function assessment values (mean ±[s.d.], n = 22) in two experimental conditions; Pseudoephedrine (PSE) and placebo(PLA), *indicates significant difference in the prepost change between treatments, P < 0.05.
| Variable | Pre | Post | P value | Difference Pre-Post |
|---|---|---|---|---|
| Lung function (l) | ||||
| FEV1 (PSE) | ||||
| FEV1 (PLA) | 5.21 [0.7] | 5.36 [0.6] | 0.15 [0.7] | |
| 5.19 [0.6] | 5.17 [0.6] | −0.02 [0.6] | ||
| 95% CI for difference | 0.02* | 0.12, 0.40 | ||
| pre-post between conditions | (r = 0.30) | |||
| FVC (PSE) | 6.46 [0.5] | 6.58 [0.6] | 0.12 [0.6] | |
| FVC (PLA) | 6.51 [0.5] | 6.47 [0.6] | −0.04 [0.6] | |
| 95% CI for difference | 0.01* | 0.12, 0.36 | ||
| pre-post between conditions | (r = 0.39) | |||
| FER1 (%) (PSE) | 79.59 [8.1] | 80.61 [7.7] | 0.95 [7.9] | |
| FER1 (%) (PLA) | 78.90 [7.2] | 79.26 [7.2] | 0.36 [7.2] | |
| 95% CI for difference | 0.45 | −0.90, 2.23 | ||
| pre-post between conditions | (r = 0.20) | |||
Discussion
The results show that performance enhancement can be brought about in some performance tasks following ingestion of 180 mg PSE.
The greater torque produced in the isometric knee extension task following ingestion of PSE has been observed previously with a trend towards an increase in MVC (P < 0.11) being reported two hours after ingestion of 120 mg PSE [9]. However, the results of this present study are the first to show statistical significance. Gillies et al. [7] showed no change in torque produced in isometric knee extensions after subjects ingested a 120 mg dose 2 h prior to testing. A possible explanation for the different results across the studies may be due to variation in dosage (180 mg vs 120 mg) and time delay between dosing and testing (45 min vs 120 min).
Coinciding with the increase in force development was a slight, although insignificant, increase (P < 0.07) in muscle activation (%) as calculated by the method introduced by Newham et al. [15]. The reason for using this method of muscle stimulation was first to provide an insight into any changes that may occur with maximum voluntary contractions following ingestion of PSE and second to allow a possible explanation to be made regarding mechanisms of change. As the results presented here were approaching statistical significance, it is possible that with a larger sample size, or less intersubject variability, greater statistical power would be reached and a more clear result obtained. Muscle activation of 96.9% (PSE) and 95.3% (placebo) suggest that when the electrical stimulation was superimposed over the voluntary MVC in the PSE condition slightly more muscle activation was achieved. At this level of muscle activation there is limited scope for enhancement. The difference is only small but the reliability of this measurement has been established in our lab with an intraclass correlation coefficient of 0.95. It has been suggested that amphetamines exert a direct affect on muscle cells, producing hyper-irritability or hyper-excitability [2]. This could possibly lead to greater recruitment of motor units allowing muscle groups to contract more forcefully [16]. This same effect may also be produced with PSE.
In a study performed on strips of intercostal muscles from dogs, concentrations of ephedrine greater than 10−4 m increased quantal content of the endplate potential (21%) and increased the probability of quantal release (16%) [17]. This may explain the excitation effect observed in our study but the other results [17] contradict this by indicating an inhibitory effect at the endplate. PSE is a less active stereoisomer of ephedrine and the plasma concentration following a 25 mg dose of ephedrine is approximately 5 × 10−7 m in humans [18]. At this concentration ephedrine had no effect on neuromuscular transmission in vitro suggesting that either no or very little effect would be expected from the less potent PSE at therapeutic dosages. The mechanism of increased muscle activation therefore remains to be explained.
Whilst no studies have previously shown a change in isometric strength following ingestion of PSE, many have reported improvements following ingestion of amphetamines [19–21]. Because PSE and EPH are similar in mode of action to amphetamines, with the similarity being the main reason that the two substances are banned in athletic competition, the results obtained in the present study can be compared to amphetamines.
In the present study, no change in bench press performance was recorded although there were improvements in lower body tasks. This pattern has been observed previously with an increase in maximal isometric knee extension strength but no change in elbow flexion strength following ingestion of (+)-amphetamine [19]. This observation was attributed to the direct proportionality of the effect of the drug on strength to the number of motor units recruited, the greater number of motor units involved in a particular task the greater the effect of amphetamines. It is possible therefore that subjects performed no better in the upper body tasks after PSE ingestion because there are less motor units to be affected by the drug because of the smaller muscle mass. This may be due to muscle mass or the training status of the subjects used in this study.
In the 30 s ‘all-out’ cycle test, peak power was significantly different between conditions (1262.5 W (PSE) and 1228.4 W (placebo)). Walton et al. [9] observed similar performance changes and showed a greater absolute and relative mean power output in a 30 s Wingate test following a 120 mg dose of PSE. The mechanisms responsible for the particular performance change are unclear. Very high motivation is essential as a supra-maximal effort is required from the subject to perform as much work as possible in 30 s. It is possible given the reported effects of PSE on the CNS [22] that increased psychological drive (through increased sympathetic stimulation) is one mechanism by which performance could be improved; it is also possible the improvement is due to local muscular factors.
Lactate concentration and heart rate response were not significantly affected by PSE ingestion. The lactate concentration was very similar at each time point of recovery between the two experimental conditions and is likely to be related to the observation of no changes in total work in the 30 s cycle test. This result appears to be different to the trends observed by Walton et al. [9] where lactate production was reported to increase following PSE ingestion (P < 0.15).
Recovery heart rate however, showed a trend towards a higher heart rate following ingestion of PSE in the seven subjects reported. The statistical analysis revealed a significantly higher heart rate between conditions immediately following the 30 s cycle sprint but no difference in the rate of heart rate recovery. The technical difficulties that resulted in only seven subjects having complete data for recovery heart rate reduced sample size and therefore statistical power. It is thought that the differences observed would reach statistical significance with more subjects. While some authors have found increases in heart rate following PSE ingestion [5, 6, 19, 23] others have not [7, 9]. The results of the present study support the suggestion that heart rate maybe elevated by PSE ingestion. This is likely to be due to sympathetic nervous system stimulation resulting in increased rate of contraction in cardiac muscle [23].
There were significant differences in the lung function tests between the PSE and placebo conditions. The differences were small and likely to be clinically insignificant. Subjects were able to expire more air in the first second of the test (FEV1), and the total amount of air moved voluntarily in one maximal breath increased (FVC). As PSE stimulates the sympathetic nervous system, one of its effects is to dilate the airways (bronchodilation). The effect of PSE on the lung function values was expected and is supported by results obtained in clinical trials [23]. This is the main reason PSE is used as a decongestant and also explains the easy access to the drug over-the-counter. Indeed, the tasks that were improved were anaerobic in nature so a relationship between this physiological variable and performance is unlikely. The increase in torque produced during the isometric knee extension and the increased peak power involve forces that rely on immediate sources of high energy phosphate compounds not the use of oxygen [24].
A 180 mg dose of PSE improved peak power in the 30 s ‘all-out’ cycle sprint and increased the torque produced in isometric knee extension (MVC) in the present study. Lung function was also improved, but upper body bench press tasks (strength and strength endurance) were not changed between conditions (PSE vs placebo). The lactate response was identical between conditions and heart rate was elevated significantly (10 beats min−1) immediately after the cycle sprint. The results of this study suggest that PSE ingestion 45 min before exercise can improve lower body strength and power, and improve lung function. However in light of the cardiovascular effect reported and other side-effects reported in previous studies, the small increase in performance, especially that of isometric muscle contraction (which is not transferable to the field) is far out-weighed by the risks associated with this drug.
Acknowledgments
Did this study receive any financial support?
References
- 1.Verroken M. Drug use and abuse in sport. In: Mottram DR, editor. Drugs in Sport. London: E & F.N. Spon; 1996. pp. 18–55. [Google Scholar]
- 2.Ivy JL. Amphetamines. In: Williams MH, editor. Ergogenic Aids in Sport Champaign, Ill. Human Kinetic Publishers; 1983. pp. 101–127. [Google Scholar]
- 3.Gilman AG, Rall TW, Nies AS, Taylor P. The Pharmacological Basis of Therapeutics. New York: Pergamon Press; 1991. p. 214. [Google Scholar]
- 4.ASDA. Annual Report: Summary of Entries on Register of Notifiable Events ACT. Australia: Australian Sports Drug Agency; 1998. p. 51. [Google Scholar]
- 5.DeMeersman R, Getty D, Schaefer DC. Sympathomimetics and exercise enhancement: all in the mind? Pharmacol Biochem Behav. 1987;28:361–365. doi: 10.1016/0091-3057(87)90453-9. [DOI] [PubMed] [Google Scholar]
- 6.Clemons JM, Crosby SL. Cardiopulmonary and subjective effects of a 60 mg dose of pseudoephedrine on graded treadmill exercise. J Sports Med Phys Fitness. 1993;33:405–412. [PubMed] [Google Scholar]
- 7.Gillies H, Derman WE, Noakes TD, Smith P, Evans A, Gabriels G. Pseudoephedrine is without ergogenic effects during prolonged exercise. J Appl Physiol. 1996;81:2611–2617. doi: 10.1152/jappl.1996.81.6.2611. [DOI] [PubMed] [Google Scholar]
- 8.Swain RA, Harsha DM, Baenziger J, Saywell RM. Do pseudoephedrine or phenylpropanolamine improve maximum oxygen uptake and time to exhaustion? Clin J Sports Med. 1997;7:168–173. doi: 10.1097/00042752-199707000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Walton C, Parise J, Tarnopolsky MM. The effects of pseudoephedrine on exercise performance and neuromuscular function. Can J Appl Physiol. 1997;???:63P. [Google Scholar]
- 10.Smith DA, Perry PJ. The efficacy of ergogenic agents in athletic competition. Part II. other performance enhancing agents. Ann Pharmacother. 1992;26:653–659. doi: 10.1177/106002809202600510. [DOI] [PubMed] [Google Scholar]
- 11.Woolverton WL, Johanson CE, De La Garza R, Ellis S, Seiden LS, Schuster CR. Behavioural and neurochemical evaluation of phenylpropanolamine. J Pharmacol Exp Ther. 1986;237:926–930. [PubMed] [Google Scholar]
- 12.Rodahl A, O'brien M, Firth RGR. Diurnal variation in performance of competitive swimmers. J Sports Med. 1976;16:72–76. [PubMed] [Google Scholar]
- 13.Winget CM, DeRoshia CW, Holley DC. Circadian rhythms and athletic performance. Med Sci Sports Exerc. 1985;17:498–516. [PubMed] [Google Scholar]
- 14.George A. Central nervous system stimulants. In: Mottram DR, editor. Drugs in Sport. London: E & FN Spon; 1996. pp. 86–112. [Google Scholar]
- 15.Newham DJ, McCarthy T, Turner J. Voluntary activation of human quadriceps during and after isokinetic exercise. J Appl Physiol. 1991;71:2122–2126. doi: 10.1152/jappl.1991.71.6.2122. [DOI] [PubMed] [Google Scholar]
- 16.Fuller JM, Hines CW. D-Amphetamine levels in brain and other tissues of isolated and aggregated mice. Biochem Pharmacol. 1967;16:11–16. [Google Scholar]
- 17.Sieb JP, Engel AG. Ephedrine: effects on neuromuscular transmission. Brain Res. 1993;623:167–171. doi: 10.1016/0006-8993(93)90025-i. [DOI] [PubMed] [Google Scholar]
- 18.Basalt RC, Cravey RH. Disposition of Toxic Drugs and Chemicals in Man. 3. Chicago, Ill: Year Book Medical Publishers; 1989. pp. 320–322. [Google Scholar]
- 19.Chandler JV, Blair SN. The effect of amphetamines on selected physiological components related to athletic success. Med Sci Sports Exerc. 1980;12:65–69. [PubMed] [Google Scholar]
- 20.Lovingood BW, Blyth CS, Peacock WJ, Lindsay RB. Effects of d-amphetamine sulfate, caffeine, and high temperature on human performance. Res Q. 1967;38:65–71. [PubMed] [Google Scholar]
- 21.Graham G, Bos R. AAHPER National Convention. Texas: Houston; 1972. The effect of d-amphetamine sulfate on integrated action potentials and local muscular fatigue. [Google Scholar]
- 22.Badewitz-Dodd LH, Tuckwell KR. MIMS. Australia: Medimedia Australia Pty Ltd; 2000. p. 275. [Google Scholar]
- 23.Empey DW, Young GA, Letley E, et al. Dose–response study of the nasal decongestant and cardiovascular effects of pseudoephedrine. Br J Clin Pharmacol. 1980;9:351–358. doi: 10.1111/j.1365-2125.1980.tb01061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Margaria R, Oliva RD, Di Prampero PE, Cerretelli P. Energy utilization in intermittent exercise of supramaximal intensity. J Appl Physiol. 1969;26:752–756. doi: 10.1152/jappl.1969.26.6.752. [DOI] [PubMed] [Google Scholar]