Abstract
Aims
The pharmacokinetics and pharmacodynamics of oral dofetilide, a novel, class III antiarrhythmic drug, were assessed during administration either twice or three times daily.
Methods
Dofetilide was administered orally to three groups of healthy subjects in daily doses of 1000 µg (n = 8), 1500 µg (n = 8), or 2500 µg (n = 9) as twice daily and three times daily treatment regimens, with the two regimens assigned randomly as a two-way crossover for each subject and separated by at least a 6 day washout period.
Results
Pharmacokinetic analysis indicated a rise in plasma dofetilide concentrations until steady state was attained on day 3. Ctrough had a linear dependence on dose for both the twice daily and three times daily dosing regimens. The maximum concentration attained (Cmax) and the area under the concentration vs time curve (AUC(0,τ) increased linearly with dose for each dosing regimen on both days 1 and 5 of dosing. Cmax occurred at 2 h. Pharmacodynamic measurements showed that the QTc interval increased in a dose-dependent manner and that the time to maximum QTc was 2 h after dosing. A linear relationship was determined between plasma dofetilide concentration and the prolongation of the QTc interval. The slope of this line was significantly greater on day 1 (ranging from 12.9 to 14.2 ms/ng ml−1) than on day 5 (ranging from 9.9 to 12.8 ms/ng ml−1).
Conclusions
The pharmacokinetics and pharmacodynamics of dofetilide are predictable and based on a linear relationship for both twice daily and three times daily dosing regimens. The QT responsiveness to dofetilide is greater on day 1 than on day 5.
Keywords: antiarrhythmic, pharmacokinetics, pharmacodynamics, QTc interval
Introduction
Dofetilide is a novel, class III antiarrhythmic agent that selectively blocks the delayed rectifier, a repolarizing potassium current (IKr) [1, 2]. Dofetilide thereby prolongs the duration of both atrial and ventricular action potentials and effective refractory periods without affecting cardiac conduction [3, 4]. The results of ligand binding studies revealed that dofetilide, unlike other class III agents such as ±-sotalol and amiodarone, possesses little affinity for other ionic channels or receptors, including β-adrenergic receptors [2].
Clinical studies have demonstrated the efficacy of dofetilide in the treatment of supraventricular reentrant tachydysrhythmias. Dofetilide has been shown to restore and maintain sinus rhythm in patients with atrial fibrillation and flutter [5–9], a disease especially prevalent in the elderly [10].
The pharmacokinetics and pharmacodynamics of dofetilide have been evaluated in healthy subjects and in patients with ischaemic heart disease. After oral administration of a single dose of dofetilide in healthy subjects, dofetilide was shown to be well absorbed with a systemic bioavailability greater than 90%, to reach mean maximal plasma concentrations in 2.6 h, and to have a terminal elimination half-life of about 8 h [11, 12]. The metabolism of dofetilide produces no active metabolites [13] and the drug is > 70% eliminated unchanged in the urine. The pharmacokinetic parameters observed in patients with coronary artery disease were similar to those seen in healthy volunteers [14].
The QT and QTc intervals were the only electrocardiographic parameters affected by either a single oral or intravenous dose of dofetilide in both healthy volunteers and patients with coronary artery disease [11, 15]. Increases in the QTc interval after oral administration have a direct linear dependence on plasma dofetilide concentration [11]. The purpose of the present study was to determine the pharmacokinetic and pharmacodynamic relationships during multiple doses for 5 days at 1000–2500 µg daily, given as both two and three divided doses.
Methods
Study design
In this double-blind, randomized, crossover study, subjects were divided into three treatment groups with the following dofetilide doses and treatment regimens: group 1 (n = 8) received 1000 µg day−1, given as 330 µg three times daily and 500 µg twice daily; group 2 (n = 8) received 1500 µg day−1, given as 500 µg three times daily and 750 µg twice daily; and group 3 (n = 9) received 2500 µg day−1, given as 1250 µg twice daily and 830 µg three times daily The order of the two treatment regimens within each group was assigned randomly, and the washout period was 6 days between each 5 day dosing period. Dofetilide was administered as capsules containing 250 µg, 330 µg, or 500 µg along with the appropriate number of placebo capsules. Subjects were dosed in an upright position with two or three capsules taken four times a day according to the schedule presented in Table 1.
Table 1.
Dofetilide administration schedule.
| Times of doses | ||||
|---|---|---|---|---|
| Dosage and regimen | 08.00 h | 15.30 h | 20.00 h | 23.00 h |
| Group 1: 1000 µg day−1 | ||||
| three times daily (µg) | 330 + P | 330 + P | P + P | 330 + P |
| twice daily (µg) | 250 + 250 | P + P | 250 + 250 | P + P |
| Group 2: 1500 µg day−1 | ||||
| three times daily (µg) | 250 + 250 | 250 + 250 | P + P | 250 + 250 |
| twice daily (µg) | 500 + 250 | P + P | 500 + 250 | P + P |
| Group 3: 2500 µg day−1 | ||||
| three times daily (µg) | 330 + 500 + P | 330 + 500 + P | P + P + P | 330 + 500 + P |
| twice daily (µg) | 500 + 500 + 250 | P + P + P | 500 + 500 + 250 | P + P |
P = placebo.
Subjects were required to report to the study unit 36 h before dosing and to remain there until 48 h after receiving the final dose. Alcohol, tobacco, caffeine-containing drinks, strenuous exercise, and all medications other than the study drug were prohibited from 48 h before admission to the study unit until 48 h after the final dose. On days 1 and 5 of each treatment period, 24 h Holter monitor recordings were obtained. Any clinically significant dysrhythmia detected on a 12-lead ECG or Holter monitor was recorded as an adverse event and the subject was withdrawn from the study. Dosing and meals were scheduled so that subjects fasted for 2 h before receiving each dofetilide dose.
This protocol was approved by a local ethics committee, was monitored according to Good Clinical Laboratory Procedures, and was conducted in accordance with the revised declaration of Helsinki/Venice, 1983, and Hong Kong, 1989. All subjects provided written informed consent.
Subjects
Included were 25 healthy male subjects, ranging in age from 18 to 45 years (mean: 25 years) and in weight from 55 to 90 kg (mean: 68 kg).
Subjects were excluded if they had any evidence of a clinically significant disease according to clinical history, physical examination and routine haematology and clinical chemistry tests. They were specifically excluded if they had an abnormal ECGs, a history of cardiac dysrhythmias or fainting or if they had family history of sudden death before the age of 40 years.
Measurements
Pharmacokinetic
Blood samples (5 ml) for measurement of dofetilide plasma concentrations were collected from an indwelling catheter or by venepuncture into heparinized tubes immediately before dosing at 08 00 h on days 1, 3, 4, and 5, and at the following hours after the first morning dose on days 1 and 5: 1, 2, 3, 4, 6, 7, 8 (immediately before the second dose), 8.5, 9.5, 10.5, 12 (immediately before the third dose at 20.00 h), 13, 14, 15 (immediately before the fourth dose at 23.00 h), 16, 20, and 24 (immediately before the next morning dose at 08 00 h). Blood samples were also taken 15, 24, 33, and 48 h after the final dose of study medication (at 23.00 h on day 5). The blood was centrifuged within 30 min of sampling and the plasma stored at −20 °C until analysed by validated, specific radioimmunoassay with a dynamic range extending from 0.04 to 0.8 ng ml−1 and a coefficient of variation of 1.2% to 3.3% [16].
Pharmacodynamic
Recordings of 12-lead ECGs were made during the 24 h preceding the first dose at the following times: 08.00, 09.00, 10.00, 11.00, 12.00, 14.00, 15.30, 16.30, 17.30, 18.30, 20.00, 21.00, 22.00, 23.00, 24.00, and 04.00 h. Recordings were also taken subsequently at times corresponding to those of blood sampling for pharmacokinetics. In addition, recordings were made at 2 h intervals from 08.00 h until 14.00 h, and at 15.00, 16.00, 18.00, and 20.00 h on days 2–4 inclusively for each treatment period.
PR, QT, and QTc intervals and QRS duration were assessed, although only the QTc interval was used to indicate pharmacodynamic activity in this study. Accurate determinations of QT intervals were made by using the same ECG lead throughout the study. ECGs were collected electronically from the analogue output of the ECG machine with use of a validated computer program and intervals were measured also by computer but with operator oversight and confirmation or correction of the cursors.
Holter recordings were made for 24 h periods during the prestudy screen and on days 1 and 5 for every treatment group. Subjects receiving 2500 µg dofetilide/day had Holter recordings made on days 2, 3, and 4 as well.
Data analysis
Pharmacokinetics
The maximum observed plasma dofetilide concentration (Cmax) and the time of its occurrence (tmax) were determined directly from the reported data. The area under the plasma concentration vs time curves over the dosing interval AUC(0,τ) after the first treatments on days 1 and 5 was derived with use of the linear trapezoidal method. After the final dose on day 5, the terminal phase rate constant (Kel) was calculated by log-linear regression analysis of those data points that were visually on the terminal log-linear elimination phase.
Pharmacokinetic-pharmacodynamic evaluation
QTc values were derived with Bazett's formula: QTc = QT (1000/RR)1/2 = QT (HR/60)1/2, where HR is heart rate in beats min−1 and RR is the time in ms between two successive R waves. Inconsistent diurnal variation in QTc (as assessed from pretreatment measurements) was demonstrated and therefore the mean of the pretreatment QTc values taken for 24 h before the first dofetilide dose was used as the baseline QTc. Changes from baseline were derived for each subject by subtraction of the baseline QTc value from QTc intervals obtained after dofetilide administration. Plots of the plasma dofetilide concentration vs the change in the QTc interval were constructed for each subject and with each treatment regimen after the first dose on days 1 and 5. Linear regression, constrained to the origin, was performed on each data set that was evaluable. The slopes of the lines for each subject were examined and analysis of variance was used to detect significant differences in the slope between days 1 and 5 of treatment.
Results
Pharmacokinetics
Plasma concentrations of dofetilide were measured for each dosing regimen. The predose concentration of dofetilide (Ctrough) was measured on days 1, 3, 4, and 5 (Table 2). Each dosing regimen reached a steady state by day 3; there was no increase in Ctrough between days 3 and 5. The relationship of Ctrough to dose showed that Ctrough was consistently higher with the three times daily regimen than with the twice daily regimen.
Table 2.
Summary of pharmacokinetic parameters.
| Group 1 | Group 2 | Group 3 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 330 µg t.d.s. | 500 µg b.d. | 500 µg t.d.s. | 750 µg b.d. | 830 µg t.d.s. | 1250 µg b.d. | ||||||||
| Dofetilide dose measurement | Day 1 | Day 5 | Day 1 | Day 5 | Day 1 | Day 5 | Day 1 | Day 5 | Day 1 | Day 5 | Day 1 | Day 5 | |
| Ctrough (ng ml−1) | Mean | < 0.04 | 1.61 | < 0.04 | 1.24 | < 0.04 | 2.24 | < 0.04 | 2.11 | < 0.04 | 3.65 | < 0.04 | 2.56 |
| s.e.mean | 0.1 | 0.13 | 0.29 | 0.43 | 0.17 | 0.11 | |||||||
| n | 7 | 7 | 7 | 7 | 8 | 8 | 8 | 8 | 7 | 7 | 7 | 7 | |
| Cmax (ng ml−1) | Mean | 1.70 | 3.04 | 2.64 | 3.80 | 2.26 | 4.78 | 3.54 | 5.23 | 3.55 | 7.71 | 5.47 | 10.07 |
| s.e.mean | 0.07 | 0.19 | 0.11 | 0.19 | 0.09 | 0.25 | 0.21 | 0.28 | 0.14 | 0.60 | 0.28 | 0.70 | |
| n | 7 | 7 | 7 | 7 | 8 | 8 | 8 | 8 | 7 | 7 | 7 | 7 | |
| tmax (h) | Mean | 2.29 | 2.29 | 2.00 | 2.00 | 2.13 | 2.00 | 2.13 | 1.88 | 2.29 | 2.14 | 1.57 | 1.57 |
| s.e.mean | 0.18 | 0.18 | 0.31 | 0.22 | 0.13 | 0.00 | 0.13 | 0.40 | 0.18 | 0.14 | 0.20 | 0.30 | |
| n | 7 | 7 | 7 | 7 | 8 | 8 | 8 | 8 | 7 | 7 | 7 | 7 | |
| AUC(0,τ) | Mean | 8.61 | 16.75 | 17.03 | 25.39 | 10.81 | 25.54 | 23.55 | 37.50 | 18.89 | 40.10 | 37.50 | 62.07 |
| (ng ml−1h) | s.e.mean. | 0.43 | 0.86 | 0.82 | 1.48 | 0.34 | 1.74 | 1.32 | 2.62 | 0.75 | 2.05 | 27 | 2.92 |
| n | 7 | 7 | 7 | 7 | 8 | 8 | 8 | 8 | 7 | 7 | 7 | 7 | |
| Kel (h−1) | Mean | 0.700 | 0.068 | 0.073 | 0.069 | 0.073 | 0.069 | ||||||
| s.e.mean | 0.002 | 0.0029 | 0.0024 | 0.0022 | 0.0023 | 0.0026 | |||||||
| n | 7 | 7 | 8 | 8 | 7 | 7 | |||||||
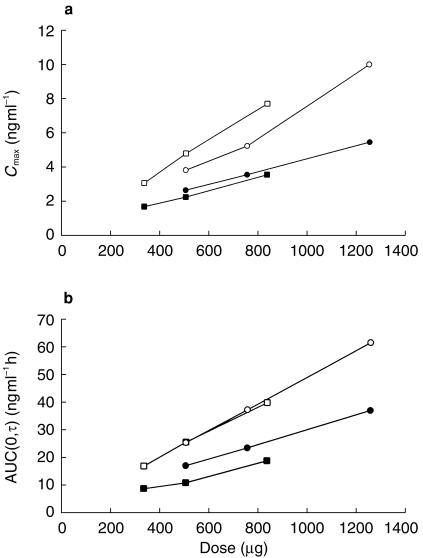
Basic pharmacokinetic parameters were determined for all subjects in all groups and the means of these values are presented in Table 2. Cmax increased in a linear, dose-dependent manner on each of days 1 and 5 for the respective twice daily and three times daily dosing regimens (Figure 1a). Cmax on day 5 was 1.4–2.2 times greater than on day 1. The time at which Cmax occurred, tmax, ranged from 1.57 to 2.29 h.
Figure 1.
a) Cmax (ng ml−1) vs dofetilide dose (µg) on study days 1 (three times daily ▪; twice daily •) and 5 (three times daily □; twice daily ○). b) AUC(0,τ) (ng ml−1 h) vs dofetilide dose on study days 1 (three times daily ▪; twice daily •) and 5 (three times daily □; twice daily ○).
Similarly, AUC(0,τ) increased in a linear, dose-dependent manner on day 1 (initial dose) and on day 5 (after steady state was attained) for the respective twice daily and three times daily dosing regimens (Figure 1b). The steady-state AUC(0,τ) was 1.5–2.1 times higher than the non–steady state AUC(0,τ) of day 1 for each dosing regimen. In either case, the slope of the relationship of AUC(0,τ) to dose remained relatively constant for both regimens; this was most clearly seen in the linearity of all the responses during steady state (day 5). Kel ranged from 0.068 to 0.073 h−1 and was similar for all dosing regimens (Table 2).
The ratio of Cmax to Ctrough was calculated for each subject and for each dosing regimen. At 1000 µg day−1 the mean(s.d.) ratio was 3.20(0.71) for twice daily and 1.93(0.43) for three times daily dosing. For 1500 µg day−1 the mean ratios were 2.86(0.91) and 2.21(0.42), for twice daily and three times daily, respectively, and for 2500 µg day−1 they were 3.95(0.42) and 2.12(0.36), for twice daily and three times daily, respectively.
Pharmacodynamics
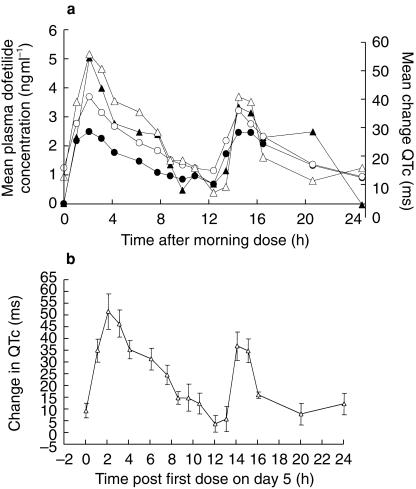
The only consistent effect of dofetilide on any ECG parameter was the prolongation of the QT and QTc intervals. The mean changes in plasma dofetilide concentrations and the corresponding changes in the QTc interval showed a direct relationship, with no indication that changes in QTc lagged behind changes in plasma concentration. An example of this is shown in Figure 2a for the 500 µg twice daily dosing in group 1. The times of peak dofetilide concentration and peak change in the QTc interval were both 2 h post dosing. Therefore, QTcmax did not lag behind Cmax. Although there was a substantial difference in the Cmax occurring during the first dose of dofetilide on day 1 and the first dose on day 5, there was little difference in the respective incremental prolongation of QTc. Figure 2b depicts the steady state mean QTc prolongation at 500 µg twice daily, as in Figure 2a, but with standard error bars to indicate the variability and precision of the estimate of the mean.
Figure 2.
a) Mean plasma dofetilide concentration (ng ml−1) and changes in the QTc interval (ms) during the 24 h after the morning 500 µg dose on days 1 and 5 with a twice daily regimen. The circles represent the mean plasma concentration on days 1 (•) and 5 (○). The triangles represent the QTc interval on days 1 (filled triangles) and 5 (open triangles). b) Mean (▵) and s.e.mean QTc changes (ms) vs time (h) following the first dose at steady state (day 5).
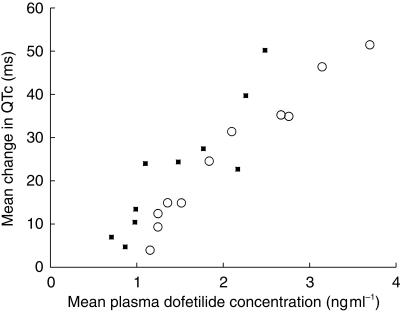
The first 12 h of these same pharmacokinetic and pharmacodynamic data from 500 µg twice daily dosing are plotted as dofetilide plasma concentration vs mean change in the QTc interval in Figure 3. On neither day did the time of maximum QTc occur differently than tmax. This indicated rapid distribution of dofetilide into the effect compartment. However, it did appear that for the same mean plasma dofetilide concentration, there was a greater increase of QTc on day 1 than on day 5, suggesting that the dependence of QTc on plasma dofetilide concentration was decreased during the time steady-state dofetilide plasma concentrations were attained.
Figure 3.
Influence of mean plasma dofetilide concentration (ng ml−1) on changes in the QTc interval (ms) on days 1 (▪) and 5 (○) after the morning 500 µg dose on days 1 and 5. Only the response during the 12 h after the dose is shown.
The slope of the change in the QTc interval as a function of plasma dofetilide concentration was analysed in two different ways. All concentration-QTc measurements for all subjects in each dosing regimen were analysed for that regimen and a slope was calculated from the collective data with use of linear regression. The results of this analysis are presented in Table 3. These slopes ranged from 12.9 to 17.2 ms/ng ml−1 on day 1 and from 9.9 to 12.8 ms/ng ml−1 on day 5. Paired analysis of the slopes on day 1 compared with day 5 showed that across all dosing regimens, slopes were significantly lower on day 5 than on day 1 (P < 0.03).
Table 3.
Slope of change in the QTc interval vs mean dofetilide plasma concentration.
| Day | Group | Regimen (µg) | Mean slope from all data points (ms/ng ml−1) | Mean slope (s.d.) based on analysis of each subject (ms/ng ml−1) |
|---|---|---|---|---|
| Day 1 | Group 1 | 330 three times daily | 14.2 | 20.7 (4.5) |
| 500 twice daily | 14.1 | 15.6 (2.4) | ||
| Group 2 | 500 three times daily | 12.9 | 18.7 (4.5) | |
| 750 twice daily | 13.3 | 14.5 (4.1) | ||
| Group 3 | 830 three times daily | 14.1 | 15.1 (3.3) | |
| 1250 twice daily | 17.2 | 16.2 (2.7) | ||
| Day 5 | Group 1 | 330 three times daily | 12.8 | 16.0 (4.1) |
| 500 twice daily | 11.2 | 13.2 (2.3) | ||
| Group 2 | 500 three times daily | 11.1 | 13.0 (2.9) | |
| 750 twice daily | 12.5 | 13.3 (2.2) | ||
| Group 3 | 830 three times daily | 11.1 | 11.2 (1.6) | |
| 1250 twice daily | 9.9 | 11.1 (2.1) |
An alternative analysis was to derive the same pharmacokinetic-pharmacodynamic relationship for each subject in each regimen and to then calculate the mean slope of these curves (Table 3). These slopes ranged from 14.5 to 20.7 ms/ng ml−1 on day 1 and from 11.1 to 16.0 ms/ng ml−1 on day 5. Paired analysis of the mean slopes on day 1 compared with day 5 showed that across all dosing regimens, slopes were significantly lower on day 5 than on day 1 (P < 0.003). Therefore, an equivalent elevation of dofetilide plasma concentration on day 5 caused significantly less change in the QTc interval than did the same incremental increase of dofetilide concentration on day 1.
Adverse effects
There were no proarrhythmic events during this study and dofetilide was generally well tolerated. While no subjects were discontinued because QTc exceeded 570 ms, at the intermediate dose of 1500 µg day−1, three subjects given 500 µg dofetilide three times daily and one subject given 750 µg dofetilide twice daily had occasional QTc intervals exceeding 500 ms. At the highest dose, 2500 µg day−1, three subjects receiving 830 µg dofetilide three times daily and seven subjects receiving 1250 µg dofetilide twice daily had occasional QTc intervals exceeding 500 ms that were more frequent and more prolonged than those in the subjects receiving 1500 µg day−1.
Treatment was discontinued prematurely in three subjects. One subject (receiving 2500 µg day−1) showed an abnormal ECG (ventricular bigeminy) and another (receiving 2500 µg day−1) experienced several adverse effects, including headache, syncope, and vasodilation. These events were considered by the investigator to be related to the drug treatment. A third subject (receiving 2500 µg day−1) asked to withdraw from the study for reasons unrelated to the study drug.
Discussion
This report is the first to describe the dose response of plasma dofetilide concentrations, and consequent changes in the QTc interval, to multiple oral doses of dofetilide given as capsules in a dosage range from 1000 to 2500 µg day−1. It is also the first report to compare the pharmacokinetics and pharmacodynamics of dofetilide with twice daily and three times daily dosing.
The pharmacokinetic measures obtained in this study are consistent with those reported previously [11, 12] showing dofetilide to be rapidly absorbed after oral administration with predictable increases in plasma dofetilide concentration to a steady state level. In the current study, maximum plasma concentrations were consistently achieved by 2 h postdosing, regardless of the dose or dosing regimen. Additionally, increases in plasma dofetilide concentration to steady state, as indicated by the elevation of Ctrough, occurred during multiple daily dosing regimens and appeared to reach steady state by day 3. Ctrough was linear for twice daily and three times daily dosing regimens across the entire dose range; the slope of the relationship was similar for each regimen. Similarly, Cmax showed a linear relationship to dose with each dose and within each regimen; similar slopes of this relationship existed between regimens as well. tmax was essentially unchanged across each regimen. AUC(0,τ) was measured on day 1 after the first dose and showed a linear relationship to dose within each regimen. The AUC(0,τ) measured at steady state was larger than the day 1 AUC(0,τ) and the magnitude of this difference is consistent with the observed terminal elimination rate and predicted accumulation. The steady state AUC(0,τ) on day 5 demonstrated a linear relationship to dose within each regimen; similar slopes existed between regimens as well. Therefore, the pharmacokinetics of dofetilide appear to be consistent and predictable across doses and between dosing regimens in healthy subjects.
The prolongation of the QTc interval, a well-documented effect of dofetilide [4, 12, 14], showed a linear relationship to plasma dofetilide concentration (Figure 3). Oral administration, unlike intravenous administration of dofetilide [11], did not produce a hysteresis of the concentration-QTc relationship. This suggests that penetration into the effect compartment is rapid and occurs prior to the first sampling point for drug levels and QT.
The response of QTc to the dofetilide plasma concentration varied after the first dose, which was similar to the response of QTc to a single dose [11, 12], and after plasma concentrations had reached steady state. There was no hysteresis in either case; however, the slope of the linear response changed slightly (Table 3; Figure 3). The result was that a dose of dofetilide on day 5 (the first dose given on day 5) raised plasma concentrations to higher values than did the equivalent dose on day 1, but this was not associated with a greater QTc prolongation. The response to both the acute dose and the steady-state dose was linear, but the slope of the response was attenuated during steady state. These data are similar to those of another dofetilide study in which the QTc interval increased from day 1 to day 2. Following this, there was an attenuation of the QTc response until day 5 with no further progression thereafter (Pfizer Inc., Data on file). This finding of attenuation is thus consistent but the mechanism is unexplained.
The use of intravenous dofetilide to determine the pharmacokinetic-pharmacodynamic properties of dofetilide [11] suggested that an approximately 30 ms prolongation of the QTc interval would result from each 1 ng ml−1 increase in the plasma dofetilide concentration. In this study, the responsiveness after a single oral dose was in the range of 14.5–20.7 ms/ng ml−1 and after steady state was achieved, only a 10 to 13 ms prolongation of the QTc interval resulted from each 1 ng ml−1 increase in the plasma dofetilide concentration.
Finally, the pharmacokinetic results of the present study suggest that a three times daily dosing regimen is not vastly superior to a twice daily dosing regimen. The steady-state measurable electrocardiographic effect, prolongation of the QTc interval, responded to plasma dofetilide concentration with no different sensitivity (slope) to a twice daily or three times daily regimen (Table 3). A comparison of the ratios of Cmax to Ctrough for the twice daily and three times daily dosing regimens for the same daily dose showed, as expected, a lower peak-to-trough ratio with the three times daily than with the twice daily dosing regimen. For example, for the currently recommended 1000 µg total daily dose the peak-trough ratio was 3.20 for twice daily dosing compared with 1.93 for three times daily dosing. This difference in ratio between the two regimens was not great enough to consider three times daily dosing a preferred option especially as this study was conducted with 7.5–9 h intervals which is often not achieved in clinical practice with three times daily dosing. Therefore, a twice daily regimen would be preferable to a three times daily regimen because compliance is better with a once or twice a day dosing frequency compared with a three times a day dosing frequency [17].
It is important to note that this study was performed early in the clinical evaluation of dofetilide before the optimal dose was determined. Doses in excess of 500 µg twice daily, the currently recommended maximum dose, were not subsequently developed by Pfizer as the degree of QT prolongation and subsequent risk of proarrhythmia was considered to be too high, especially when administered to a less well controlled and variable patient population.
In conclusion, dofetilide, a novel class III antiarrhythmic drug, shows predictable linear pharmacokinetics and pharmacodynamics after a single dose or after steady state is reached with multiple dosing. Responsiveness to dofetilide, as measured by QTc response to plasma drug, is greater after the first dose than at steady state. The pharmacokinetic and pharmacodynamic profiles after three times daily dosing are not considered to provide a significant advantage compared with twice daily dosing, with the better compliance likely to be achieved with twice daily dosing.
References
- 1.Carmeliet E. Voltage- and time-dependent block of the delayed K+ current in cardiac myocytes by dofetilide. J Pharmacol Exp Ther. 1992;262:809–817. [PubMed] [Google Scholar]
- 2.Gwilt M, Arrowsmith JE, Blackburn KJ, et al. UK−68,798: a novel, potent and highly selective class III antiarrhythmic agent which blocks potassium channels in cardiac cells. J Pharmacol Exp Ther. 1991;256:318–324. [PubMed] [Google Scholar]
- 3.Sedgwick ML, Dalrymple I, Rae AP, Cobbe SM. Effects of the new class III antiarrhythmic drug dofetilide on the atrial and ventricular intracardiac monophasic action potential in patients with angina pectoris. Eur Heart J. 1995;16:1641–1646. doi: 10.1093/oxfordjournals.eurheartj.a060790. [DOI] [PubMed] [Google Scholar]
- 4.Bashir Y, Thomsen P-EB, Kingma JH, et al. Electrophysiologic profile and efficacy of intravenous dofetilide (UK-68,798), a new class III antiarrhythmic drug, in patients with sustained monomorphic ventricular tachycardia. Am J Cardiol. 1995;76:1040–1044. doi: 10.1016/s0002-9149(99)80293-8. [DOI] [PubMed] [Google Scholar]
- 5.Suttorp MJ, Polak PE, van't Hof A, et al. Efficacy and safety of a new selective class III antiarrhythmic agent dofetilide in paroxysmal atrial fibrillation or atrial flutter. Am J Cardiol. 1992;69:417–419. doi: 10.1016/0002-9149(92)90247-v. [DOI] [PubMed] [Google Scholar]
- 6.Frost L, Mortensen PE, Tingleff J, et al. Efficacy and safety of dofetilide, a new class III antiarrhythmic agent, in acute termination of atrial fibrillation or flutter after coronary artery bypass surgery. Int J Cardiol. 1997;58:135–140. doi: 10.1016/s0167-5273(96)02856-2. [DOI] [PubMed] [Google Scholar]
- 7.Crijns HJGM, Van Gelder IC, Kingma JH, et al. Atrial flutter can be terminated by a class III antiarrhythmic drug but not by a class IC drug. Eur Heart J. 1994;15:1403–1408. doi: 10.1093/oxfordjournals.eurheartj.a060402. [DOI] [PubMed] [Google Scholar]
- 8.Falk RH, Pollak A, Singh SN, Friedrich T. Intravenous dofetilide, a class III antiarrhythmic agent, for termination of sustained atrial fibrillation or flutter. J Am Coll Cardiol. 1997;29:385–390. doi: 10.1016/s0735-1097(96)00506-2. [DOI] [PubMed] [Google Scholar]
- 9.Singh SN, Berk MR, Yellen YG, et al. Efficacy and safety of dofetilide in maintaining sinus rhythm in patients with atrial fibrillation/flutter: a multicenter study. Circulation. 1997;96(Suppl):I–383. Abstract. [Google Scholar]
- 10.Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation: analysis and implications. Arch Intern Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 11.LeCoz F, Funck-Brentano C, Morell T, Ghadanfar MM, Jaillon P. Pharmacokinetic and pharmacodynamic modeling of the effects of oral and intravenous administrations of dofetilide on ventricular repolarization. Clin Pharmacol Ther. 1995;57:533–542. doi: 10.1016/0009-9236(95)90038-1. [DOI] [PubMed] [Google Scholar]
- 12.Tham TCK, MacLennan BA, Burke MT, Harron DWG. Pharmacodynamics and pharmacokinetics of the class III antiarrhythmic agent dofetilide (UK-68,798) in humans. J Cardiovasc Pharmacol. 1993;21:507–512. doi: 10.1097/00005344-199303000-00024. [DOI] [PubMed] [Google Scholar]
- 13.Walker DK, Alabaster CT, Congrave GS, et al. Significance of metabolism in the disposition and action of the antidysrhythmic drug, dofetilide: In vitro studies and correlation with in vivo data. Drug Metab Dispos. 1996;24:447–455. [PubMed] [Google Scholar]
- 14.Sedgwick M, Rasmussen HS, Walker D, Cobbe SM. Pharmacokinetic and pharmacodynamic effects of UK-68,798, a new potential class III antiarrhythmic drug. Br J Clin Pharmacol. 1991;31:515–519. doi: 10.1111/j.1365-2125.1991.tb05572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sedgwick ML, Rasmussen HS, Cobbe SM. Clinical and electrophysiologic effects of intravenous dofetilide (UK-68,798), a new class III antiarrhythmic drug, in patients with angina pectoris. Am J Cardiol. 1992;69:513–517. doi: 10.1016/0002-9149(92)90996-c. [DOI] [PubMed] [Google Scholar]
- 16.Walker DK, Aherne GW, Arrowsmith JE, et al. Measurement of the class III antiarrhythmic drug, UK-68,798, in plasma by radioimmunoassay. J Pharm Biomed Anal. 1991;9:141–149. doi: 10.1016/0731-7085(91)80137-x. [DOI] [PubMed] [Google Scholar]
- 17.Eisen SA, Miller DK, Woodward RS, Spitznagel E, Przybeck TR. The effect of prescribed daily dose frequency on patient medication compliance. Arch Intern Med. 1990;150:1881–1884. [PubMed] [Google Scholar]