Abstract
Aims
The treatment of bipolar disorder often includes use of multiple drug therapies. Lithium is one of the most commonly used treatments, but has a narrow therapeutic window. Lamotrigine, an established antiepileptic drug, is emerging as a potentially important new therapy in the treatment of bipolar disorder. The objective of this two-treatment crossover study was to determine whether lamotrigine affects lithium pharmacokinetics.
Methods
Twenty healthy adult men completed the study. Subjects took 2 g lithium gluconate anhydrous every 12 h in the morning and evening for 5 days and in the morning of day 6, with or without 100 mg lamotrigine once daily in the morning for 6 days. Blood and urine samples were collected on day 6 of both treatments to characterize the pharmacokinetics of lithium using noncompartmental methods.
Results
The geometric least-square mean ratio for renal clearance of lithium between the combination treatment and lithium alone treatment was 0.93 (95% confidence interval 0.85–1.02). Both treatments were well tolerated.
Condusions
Lamotrigine does not cause significant change in the pharmacokinetics of lithium.
Keywords: interaction, lamotrigine, lithium, pharmacokinetics
Introduction
The efficacy and safety of lamotrigine, an established antiepileptic drug, in treating bipolar (manic-depressive) disorder are currently under evaluation, with emerging evidence for its effectiveness in both mania and depression [1, 2]. Lamotrigine is eliminated predominantly by glucuronic acid conjugation and the resulting conjugates are removed through the kidneys.
Lithium is a standard treatment for bipolar disorder. It has a low therapeutic index. Optimal serum concentrations appear to be in the range of0.8–1.5 mm for the acute treatment of a manic episode and 0.4–1.2 mm for prophylaxis [3]. Higher concentrations are more liable to cause side-effects such as tremor, diarrhoea, polyuria and weight gain [4]. Permanent cerebellar damage is one of the most serious forms of lithium toxicity upon overdose, although this may also appear in the so-called therapeutic range [5]. Lithium is eliminated almost entirely by renal excretion [6].
Polypharmacy is often required for the management of bipolar disorder [7]. Since the metabolites of lamotrigine are eliminated through the kidneys, lamotrigine may potentially alter the serum concentrations of lithium. The object of this study was to study the potential effect of lamotrigine on multiple-dose pharmacokinetics of lithium.
Methods
Clinical protocol
This was an open, randomized, two-period crossover study in 21 adult white men in good health, as determined by medical history, physical examination and clinical laboratory tests. Institutional review board approval and informed consent from each subject were obtained before the study. The median age and weight of the subjects were 24 (range 20–38) years and 68 (range 57–85) kg. Subjects took 2 g lithium gluconate anhydrous (containing 9.8 mmol of lithium) every 12 h in the morning and evening for 5 days and in the morning of day 6, with and without 100 mg lamotrigine once a day in the morning for 6 days. Neurolithium® 10 ml ampoules and Lamictal® 100 mg chewable/dispersible tablets were used. Test drugs were administered with 200 ml water during meals. The two treatments were separated by a 2 week washout period.
Blood and urine samples were collected on day 6 of both treatments. Blood samples (5 ml) were obtained before and 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 7, 8, 10 and 12 h after the dose of lithium. Urine was collected for 12 h after the dose. Additional blood samples were drawn daily in the morning (lithium alone treatment) or both morning and evening (combination treatment) to monitor trough concentration of lithium. During combination treatment, blood samples (10 ml) were collected daily to assess predose lamotrigine plasma concentrations.
Adverse events were monitored throughout the study. Physical and neurological examinations, 12-lead electrocardiography, vital sign measurements, thyroid function tests and routine clinical laboratory tests were conducted on various occasions.
Drug assays
Blood samples for lithium concentrations were allowed to clot and serum was separated by centrifugation at 0 °C. Blood samples for lamotrigine concentrations were centrifuged at 0 °C to obtain plasma. Serum, urine and plasma samples were stored at or below −20 °C until analysis.
Serum and urine concentrations of lithium were analysed using a Perkin Elmer 3100 atomic absorption spectrophotometer operating with a wavelength of 671 nm and a split of 0.7 nm. A 15mA cathode lamp was used. The temperature of the flame, constituted with a mixture of air and acetylene, was 2400 °C. Concentrations were quantifiable up to 1 mm in serum and 10 mm in urine. Assay bias and precision were less than 10% for both matrices.
Plasma concentrations of lamotrigine were analysed using a previously described time-resolved immunofluorometric assay [8]. Assay bias and interday variability were both less than 10%.
Pharmacokinetic analysis
Serum and urine concentrations of lithium on day 6 were subject to noncompartmental pharmacokinetic analysis using WINNONLIN [9]. The maximum serum concentration (Cmax) and the time of Cmax (tmax) were read directly from the concentration-time curve. The area under the serum concentration-time profile for the 12 h dosing interval (AUC(0,12h)) was obtained by linear trapezoidal summation. Renal clearance (CLR) was calculated as the ratio of the lithium recovered in urine to AUC over the 12 h dosing interval (Ae(0,12)) to AUC(0,12h).
Statistical analysis
Analysis of variance (anova) was performed on all parameters (except tmax) after log-transformation, taking into account variation due to subject, sequence, subject within sequence, period and treatment. For all log-transformed parameters, the treatment difference in least squares mean and the corresponding 95% confidence interval were obtained and exponentiated, and the results were presented in the form of the ratio of geometric least squares means. A nonparametric method [10] was used to compute the median difference and its confidence interval for treatment comparison of tmax.
Results
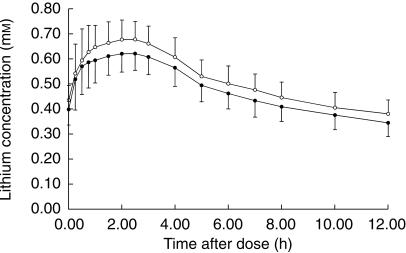
Twenty subjects completed both treatments. One subject withdrew from the study owing to an adverse event (see below). Serum lithium concentrations on day 6 were generally comparable between the two treatments (Figure 1). Although the concentrations during the combination treatment were slightly lower, the differences were unlikely to be of clinical importance (Figure 1). Cmax, tmax, AUC(0,12h) and CLR were all similar between the two treatments (Table 1). The amount of lithium recovered from urine over the 12 h dosing interval on day 6 of both treatments approximated the dose (9.8 mmol). The ratios of geometric least-square means of all parameters (except tmax) were close to 1 (null hypothesis value) and had narrow 95% confidence intervals (between 0.79 and 1.02). The 95% confidence interval for the median difference in tmax was −0.75–0.75 and included the null hypothesis value of 0.
Figure 1.
Mean serum concentrations of lithium after the morning dose on day 6 (○, lithium alone; •, lithium + lamotrigine). Error bars represent one s.d. n = 20.
Table 1.
Pharmacokinetic parameters of lithium.
| Treatment | Comparison | ||
|---|---|---|---|
| LI | LI + LTG | (LI + LTG)/LI | |
| Cmax (mm) | 0.71 (0.08) | 0.65 (0.07) | 0.92 (0.88, 0.97) |
| tmax (h) | 1.75 (0.79) | 1.69 (0.97) | 0.00 (−0.75, 0.75) |
| AUC(0,12h) (mmh) | 6.23 (0.78) | 5.75 (0.73) | 0.92 (0.90, 0.95) |
| Ae(0,12h) (mmol) | 11.2 (1.6) | 9.58 (1.34) | 0.86 (0.79, 0.93) |
| CLR,(l h−1) | 1.83 (0.37) | 1.70 (0.30) | 0.93 (0.85, 1.02) |
LI: lithium. LTG: lamotrigine. For parameters except tmax, values are presented as mean (s.d.) for each treatment and geometric least squares mean ratio (95% confidence interval) for treatment comparison. For tmax, values are presented as mean (s.d.) for each treatment and median difference (95% confidence interval) for treatment comparison. n = 20, except for Ae(0,12h) and CLR during combination treatment, where n = 19 since urine collection by one subject was incomplete.
The trough concentrations of both lithium and lamotrigine approached steady state on day 6 (data not shown). Consistent with the observations on the day 6 serum lithium concentration-time profiles, the trough concentrations of lithium showed only small differences between treatments and the differences were unlikely to be of clinical relevance.
One adverse event was reported. A 28-year-old subject experienced moderate pruritis on the first day of the combination treatment (period 1 for this subject) and subsequently withdrew from the study. The event was attributed to parasitosis by the investigator. One subject's eosinophil count increased from 252/mm3 at baseline to 642/mm3 poststudy (normal range 50–300/mm3). Another subject's aspartate aminotransferase increased from 16 mU ml−1 at baseline to 55 mU ml−1 poststudy (normal range 0–25 mU ml−1). The causes for these clinical laboratory abnormalities were unknown.
Discussion
We have studied the pharmacokinetics of lithium with and without concurrent lamotrigine in this study. All the pharmacokinetic parameters of lithium evaluated (Cmax, tmax, AUC(0,12h), Ae(0,12h) and CLR) were similar during the two treatments. Since lithium is cleared almost exclusively by renal elimination [6] and renal clearance of lithium was not altered by lamotrigine, serum concentrations of lithium are unlikely to be significantly affected by lamotrigine. The mean values of CLR (1.70–1.83 l h−1) were comparable with previously reported values in psychiatric patients (0.6–2.4 l h−1) [6].
In order to reduce the risk of toxicity associated with any potential increase in lithium concentration caused by lamotrigine, we used relatively low dosages (0.98 mmol every 12 h). Consequently, the steady-state trough concentrations (about 0.4 mm) were at the lower end of the established therapeutic window of 0.4–1.5 mm measured 10–12 h after dose [3]. However, the lack of effect by lamotrigine on lithium pharmacokinetics is expected to be applicable to higher lithium concentrations as well, since lithium pharmacokinetics are linear. Nevertheless, the interaction at higher lithium dosages and during long-term coadministration remains to be studied.
We did not study the potential for lithium to alter the systemic concentrations of lamotrigine. Lamotrigine is inactivated predominantly by glucuronic acid conjugation, which is not involved in lithium elimination. We would not expect lithium to affect the pharmacokinetics of lamotrigine.
In conclusion, lamotrigine does not cause significant change in the pharmacokinetics of lithium.
Acknowledgments
We thank Dr Jean-Philippe Decourt and the staff at CEMAF Clinique de l'ADPC for conducting the study and analysing the lithium samples. We also thank Dr Richard Peck for contributing to the study design and Ms Elizabeth Culverhouse for analysing the lamotrigine samples.
References
- 1.Berk M. Lamotrigine and the treatment of mania in bipolar disorder. Eur Neuropsychopharmacol. 1999 doi: 10.1016/s0924-977x(99)00025-5. in press. [DOI] [PubMed] [Google Scholar]
- 2.Calabrese J, Bowden C, Sachs G, Ascher J, Monaghan E, Rudd G,A. double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with Bipolar I depression. J Clin Psychiatry. 1999;60:79–88. doi: 10.4088/jcp.v60n0203. [DOI] [PubMed] [Google Scholar]
- 3.Finley PR, Warner MD, Peabody CA. Clinical Relevance of Drug Interactions with Lithium. Clin Pharmacokin. 1995;29:172–191. doi: 10.2165/00003088-199529030-00004. [DOI] [PubMed] [Google Scholar]
- 4.Gelenberg A, Kane J, Keller M, et al. Comparison of standard and low serum levels of lithium for maintenance treatment of bipolar disorder. N Engl J Med. 1989;321:1489–1493. doi: 10.1056/NEJM198911303212201. [DOI] [PubMed] [Google Scholar]
- 5.Cookson J. Lithium. Balancing risks and benefits. Br J Psychiatry. 1997;171:120–124. doi: 10.1192/bjp.171.2.120. [DOI] [PubMed] [Google Scholar]
- 6.Ward E, Musa MM, Bailey L. Clinical pharmacokinetics of lithium. J Clin Pharmacol. 1994;34:280–285. doi: 10.1002/j.1552-4604.1994.tb01994.x. [DOI] [PubMed] [Google Scholar]
- 7.Frye MA, Kimbrell TA, Dunn RT, et al. Gabapentin does not alter single-dose lithium pharmacokinetics. J Clin Psychopharmacol. 1998;18:461–464. doi: 10.1097/00004714-199812000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Sailstad JM, Findlay JWA. Immunofluorometric assay for lamotrigine (Lamictal) in human plasma. Ther Drug Monit. 1991;13:433–442. doi: 10.1097/00007691-199109000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Scientific Consulting Inc. North Carolina, USA: Cary; 1995. WINNONLIN User's Guide. [Google Scholar]
- 10.Koch GC. The use of non-parametric methods in the statistical analysis of the two-period change-over design. Biometrics. 1972;28:577-588. [PubMed] [Google Scholar]