Abstract
Aims
The purpose of the study was to investigate the effects of asimadoline, a new κ-opioid agonist, on renal function and on hormones related to body fluid balance as well as its tolerability in healthy subjects.
Methods
In a placebo-controlled, randomised, double-blind crossover design we studied the effects of single oral doses of 1, 5, and 10 mg of asimadoline, in 24 healthy volunteers. Two hour control urine collections were followed by 2 h postdose urine collections and subsequently 2.5% saline was given i.v. at a rate of 0.3 ml min−1 kg−1 during another 2 h urine collection. Blood was obtained hourly. Arginine‐vasopressin (AVP), atrial natriuretic peptide (α-hANP), endothelin (ET-1) and cAMP were determined by r.i.a. or ELISA.
Results
GC-MS measurements revealed Cmax values of asimadoline in plasma ranging from 18 ng ml−1 at the 1 mg dose, 91 ng ml−1 at the 5 mg dose, to 214 ng ml−1 at the 10 mg dose after an average of 1.1–1.4 h. Without effects on blood pressure, heart rate, GFR or urine electrolyte excretion, urine volume increased after 1–2 h after administration of 5 and 10 mg asimadoline from 3.3 ± 1.3 to 5.6 ± 1.4 (P < 0.05) and from 3.2 ± 1.6 to 5.5 ± 2.2 ml min−1 (P < 0.01), respectively. CH2O rose from 0.2 ± 1.5 to 2.0 ± 1.6 (P < 0.05) and from 0.6 ± 1.6 to 3.0 ± 1.6 ml min−1 (P < 0.01). Urinary excretion of AVP was suppressed only with the 10 mg dose from 46 ± 23 to 25 ± 15 fmol min−1 (P < 0.05) without and from 410 ± 206 to 181 ± 125 fmol min−1 (P < 0.05) with stimulation by 2.5% saline. Plasma AVP was suppressed only by the 10 mg dose of asimadoline in six of eight subjects during the 2.5% saline infusion. Changes in the α-hANP or ET-1 systems were not affected by asimadoline.
Conclusions
Asimadoline is diuretic in man after single doses of 5 or 10 mg probably through a direct effect at the renal tubular level. Suppression of AVP secretion was observed only at the highest dose level of 10 mg of asimadoline.
Keywords: asimadoline, free-water clearance, κ-opioid agonist, renal function, atrial natriuretic peptide, endothelin, vasopressin
Introduction
Narcotic analgesics of the µ-opioid antagonist type, which also possess κ-opioid receptor agonistic properties, are known to have diuretic properties [1, 2]. Pure κ-opioid receptor agonists dose-dependently induce a diuresis in animals [3–5] and in humans [6–8]. The diuresis results from a rise in free-water clearance with a decrease in urine osmolarity [6]. Although the diuresis was found to be mainly related to suppression of arginine‐vasopressin (AVP) secretion [9], a direct effect of these drugs at the renal tubular level was also suggested [10].
Asimadoline is a new peripherally acting, potent selective κ-opiate receptor agonist from the group of hydroxypyrrolidine ethyldiphenylacetamides developed for the treatment of chronic pain of various origins. In the present study we investigated the pharmacokinetics and pharmacodynamics (renal and hormonal effects), and tolerability of asimadoline in healthy subjects after single oral doses of 1, 5, and 10 mg as compared with placebo. Also, since equivocal data were obtained regarding the mechanisms of the diuresis observed [4, 10, 11], its effects on free-water clearance and on AVP release were studied before and after stimulation of AVP secretion by hypertonic saline infusion. Since atrial natriuretic peptide (ANP) [12] and endothelin (ET) [13, 14] are thought to be involved in the renal regulation of body fluid balance, the effects of asimadoline on these hormones were also studied.
Methods
Healthy volunteers
Twenty-four male Caucasian volunteers age 21–40 years participated in this double-blind single centre study with three parallel groups each of eight subjects. The study was performed in accordance with the declaration of Helsinki and all subjects had given their written informed consent. Study design and protocol were approved by the local Ethics Committee, Paris-Boucicaut. Fasting subjects received tap water, 20 ml kg−1 body weight, at 06.00 h. They were in the supine position during the whole study period except for voiding urine in standing position immediately after collection of venous blood. Each group underwent a randomised, placebo-controlled, two period cross-over investigation.
Subjects received one dose of asimadoline of 1, 5, or 10 mg and placebo. The two administrations were separated by a 14 day wash-out period. Each dose of asimadoline was preceeded by a 2 h control urine collection period and was followed by a 2 h postdose collection period. A third urine collection period of 2 h followed during which 2.5% saline was infused i.v. from 2 to 3 h after administration of asimadoline at a rate of 0.3 ml min−1 kg−1 body weight through a catheter in the left cubital vein. Blood for serum or plasma was obtained from the right cubital vein.
Urine samples were collected over six periods of 1 h each followed by one period of 8 h. Values of each two 1 h collection periods were combined, i.e. results are given for a control period from −2–0 h before and for 0–2 h and 2–4 h after oral administration of asimadoline or placebo.
Tolerability studies
For tolerability investigations blood pressure and heart rate in sitting position, ECG, pulmonary function, haematology and clinical chemistry data, urinary analysis and subjective and objective adverse events were documented.
Pharmacokinetic studies
For pharmacokinetic studies blood samples for determination of plasma concentrations of asimadoline were collected immediately before and 0.5, 1, 1.5, 2, 3, 4 h and then 2 hourly up to 12 h with one final blood sample at 24 h after oral administration.
Pharmacodynamic studies
Studies of renal function included urinary flow rate and electrolyte excretion and glomerular filtration rate (GFR) estimated as endogenous creatinine clearance as well as osmolar and free-water clearances with calculations performed according to conventional formulae.
The hormonal effects of asimadoline were assessed by measurements of plasma concentrations of AVP, α-hANP and ET-1 and by urinary excretion of AVP, ET-1 and cAMP.
Determination of asimadoline in plasma
Measurements of plasma concentrations of asimadoline were performed by NICI-GC/MS using a Hewlett Packard MP 5890 and Fisons Trio 2000 MS-system. To each sample of 1 ml plasma 100 ng of internal standard D4-asimadoline dissolved in 50 µl methanol were added after addition of 0.2 ml 1 n NaOH. The samples were extracted with 5 ml toluene/diisopropylether (3 : 1 v/v). After centrifugation, the organic phase was transferred into a conical glass tube and evaporated to dryness. The dry residue was dissolved in a solution of triethylamine and pentafluorobenzoylchloride, incubated for 30 min and extracted with toluene/isopropylether. It was then evaporated to dryness. The residue was dissolved in 150 µl ethylacetate. 1 to 2 µl of this solution were injected. The GC/MS analysis was performed on a Fisons Trio 2000 and a Finnigan 4515 GC/MS system. A fused silica tubular capillary column of cross-linked methyl-phenyl siloxane type (DB-5, J & W Scientific, Folsom, CA, USA) was used for GC separation. The MS system is operated in the negative ion chemical ionization mode. Selected ion monitoring was performed at m/z 608 for the molecular ion of asimadoline and at m/z 612 for the molecular ion of internal standard D4-asimadoline. Data acquisition and integration of the peak areas were achieved using standard ion recording software (LabBase software for the Fisons Trio 2000; INCOS data system for the Finnigan 4515). The lower limit of quantification in plasma was 1 ng ml−1.
Concentrations of creatinine, sodium, potassium and chloride in plasma and urine were determined in venous blood samples by standard laboratory methods (Technion) and serum osmolarity was determined by microosmometer.
Plasma concentrations of AVP and of α-hANP were determined by radioimmuno-assay (r.i.a.) and of ET-1 by enzyme-linked immunosorbent assay (ELISA), respectively (Amersham, Braunschweig, Germany) after extraction with C18-Sep-Pak cartriges. Urinary concentrations of AVP and cAMP were determined by r.i.a. and of ET-1 by ELISA (Amersham) in unextracted urine samples. The lower limits of detection were ≈1 fmol ml−1 (1.25 pg ml−1) for AVP, 1 fmol ml−1 for α-hANP, 1 fmol ml−1 for ET-1 and 0.25 pmol ml−1 for cAMP, respectively.
Statistics
The pharmacokinetic and statistical analysis was carried out by calculation of pharmacokinetic parameters according to standard methods [15, 16] using the PHARMR NCA program release 1.3e (SIMED S.A., 94008 Creteil, France). The statistical analysis was performed with SASR package release 6.0 (SAS Institute Inc.) according to [17]. Comparison of the dose-normalized Cmax and dose-normalized AUC was tested by analysis of variance using PROC GLM on logarithmically transformed data. The analysis of tmax was based on the nonparametric Kruskall-Wallis test using PROC NPAR1WAY. The experimental area under the curve, AUC(0,4h) was calculated with the actual measurements and the extrapolated AUC(0,∞) was estimated according to standard formula.
For statistical analysis of pharmacodynamic data a paired t-test and Wilcoxon's rank-signed test were used. In text and tables, results are given as means ± s.d. and, for clarity, in figures they are presented as means ± s.e.mean. A P value of < 0.05 was considered to indicate statistically significant differences. In an additional table the 95% confidence intervals (CI) for the differences of the main comparisons of the effects of asimadoline are summarized.
Results
Tolerability
The tolerability examinations did not reveal any major side-effects related to asimadoline. Most of the adverse events observed, such as thirst, dizziness, or paraesthesia were related most probably to the hypertonic saline infusion, since these occurred between 2 and 3 h post administration, i.e. during 2.5% saline i.v., in the absence and presence of asimadoline. No adverse events related to asimadoline were found with the 1 mg dose. Minor events possibly related to asimadoline, such as headache and tiredness, were observed after doses of 5 mg (in four of eight subjects) and 10 mg (in five of eight subjects), but only in one subject in the absence of 2.5% saline infusion.
Pharmacokinetics
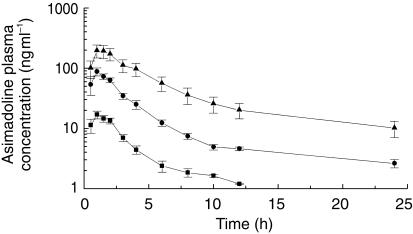
In Figure 1 the time course of plasma concentrations is shown following oral administration of single doses of 1, 5, and 10 mg asimadoline. Values for maximal con-centrations averaged 18.0 ± 5.6, 90.7 ± 32.1, and 213.6 ± 114.5 ng ml−1, respectively. When normalized for dose, no significant differences in maximal plasma concentrations were noted. At all three doses the mean time to reach maximum plasma concentration of asimadoline was approximately 1 h (1.1–1.4 h). Nevertheless, the AUC tended to be higher after administration of 10 mg asimadoline. In addition, the intersubject variability was higher after 10 mg asimadoline than after administration of the lower doses. Terminal plasma half-life of asimadoline was significantly shorter after 1 mg ranging from 1 h to 4 h and averaging 2.55 ± 0.98 h than after administration of higher doses ranging from 6 h to 21 h averaging 15.10 ± 3.50 h after 5 mg and averaging 10.57 ± 3.13 after 10 mg asimadoline. Plasma concentrations declined rapidly and were below the lower limit of detection after 6–12 h. Therefore, the half-life in the 1 mg group could not be calculated in the same way as in the 5 mg and 10 mg groups. AUC(0,∞) for 1, 5, and 10 mg doses were 58.5 ± 21.4, 375.9 ± 92.6, and 1205.2 ± 773.0 ng ml−1 h, respectively.
Figure 1.
Semilogarithmic plot of plasma concentrations of asimadoline during 24 h after oral administration of 1 (▪), 5 (bull;), and 10 mg (▴) asimadoline in each group of eight healthy subjects. Data are given as means ± s.e.mean.
Pharmacodynamics
Plasma sodium concentration in the placebo groups averaged 143 ± 1.3 during the control and the 2 h postadministration period, and 151 ± 1.9 mmol l−1 during the 2 h period after 2.5% saline infusion. In the groups receiving asimadoline plasma sodium concentration amounted to 143 ± 1.3 during the control and the 2 h postadministration periods and rose to 153 ± 1.9 mmol l−1 after 2.5% saline infusion.
Serum osmolarity in the placebo groups averaged 291 ± 5 during the control and the 2 h postadministration period, and 308 ± 5 mosm l−1 during the 2 h period of 2.5% saline infusion. In the groups receiving asimadoline serum osmolarity amounted to 290 ± 5 during the control and the 2 h postadministration periods and rose to 313 ± 5 mosm l−1 during the period of 2.5% saline infusion.
Renal effects
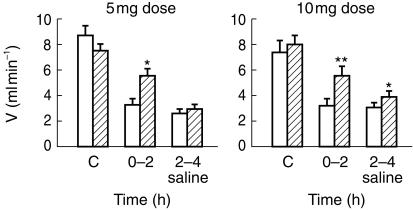
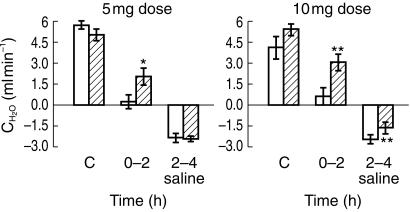
Without effects on blood pressure or heart rate (data not shown) a significant increase in urine volume (Table 1; Figure 2) occurred within 2 h after administration of 5 mg (P < 0.01) and 10 mg (P < 0.01) of asimadoline, respectively, but not in the 1 mg group. This occurred in the absence of changes in GFR (ranging with placebo between 118 ± 8 and 122 ± 15 without and between 133 ± 29 and 140 ± 18 ml min−1 with 2.5% saline infusion; with asimadoline between 105 ± 11 and 126 ± 18 without and between 133 ± 33 and 140 ± 23 ml min−1 with 2.5% saline infusion); (Table 1). Electrolyte excretion was not affected by asimadoline (Table 1). The rise in urine volume was due to a significant rise in free-water clearance (CH2O) (P < 0.05 and P < 0.01 for the 5 and 10 mg doses, respectively) (Table 1; Figure 3). Asimadoline at the 10 mg dose also reduced negative CH2O significantly from −2.5 ± 0.7 to −1.7 ± 1.1 ml min−1 (P < 0.01) during 2.5% saline infusion (Table 1; Figure 3).
Table 1.
Urine volume (V), free-water clearance (CH20), glomerular filtation rate (GFR), and urinary electrolyte excretion (UNaV, UKV,UClV)before (–2–0 h) and after oral administration of 1, 5, or 10 mg asimadoline as compared with placebo in each group of eight healthy subjects in the absence (0–2 h) and presence of 2.5% saline infusion (2–4 h).*P < 0.05;**P < 0.01.
| Parameters | Collection interval | Placebo | 1 mg asimadoline | Placebo | 5 mg asimadoline | Placebo | 10 mg asimadoline |
|---|---|---|---|---|---|---|---|
| V | |||||||
| (ml min−1) | −2–0 h | 7.2 ± 1.6 | 6.2 ± 1.3 | 8.7 ± 2.1 | 7.6 ± 1.0 | 7.4 ± 2.3 | 8.0 ± 2.0 |
| 0–2 h | 2.8 ± 0.8 | 3.3 ± 0.6 | 3.3 ± 1.4 | 5.6 ± 1.4** | 3.2 ± 1.6 | 5.5 ± 2.2** | |
| 2–4 h (2.5% NaCl i.v.) | 2.8 ± 0.8 | 3.0 ± 0.9 | 2.7 ± 0.7 | 3.0 ± 0.8 | 3.1 ± 1.1 | 3.9 ± 1.4 | |
| CH2O | |||||||
| (ml min−1) | −2–0 h | 5.3 ± 1.8 | 4.7 ± 2.2 | 5.7 ± 1.2 | 5.0 ± 0.9 | 4.1 ± 1.9 | 5.4 ± 1.2 |
| 0–2 h | 0.5 ± 1.1 | 1.6 ± 2.2 | −0.2 ± 1.0 | 2.0 ± 1.6** | 0.6 ± 1.6 | 3.0 ± 1.7** | |
| 2–4 h (2.5% NaCl i.v.) | −2.3 ± 0.7 | −2.1 ± 1.2 | −2.4 ± 0.8 | −2.5 ± 0.5 | −2.5 ± 0.7 | −1.7 ± 1.1** | |
| GFR | |||||||
| (ml min−1) | −2–0 h | 113 ± 10 | 109 ± 23 | 122 ± 19 | 105 ± 11 | 123 ± 27 | 116 ± 12 |
| 0–2 h | 118 ± 8 | 126 ± 18 | 122 ± 15 | 124 ± 8 | 120 ± 21 | 117 ± 23 | |
| 2–4 h (2.5% NaCl i.v.) | 140 ± 18 | 140 ± 23 | 136 ± 23 | 138 ± 15 | 133 ± 29 | 133 ± 33 | |
| UNaV | |||||||
| (µmol min−1) | −2–0 h | 146 ± 40 | 131 ± 65 | 156 ± 45 | 143 ± 20 | 135 ± 47 | 117 ± 32 |
| 0–2 h | 193 ± 32 | 171 ± 54 | 195 ± 43 | 213 ± 43 | 178 ± 47 | 138 ± 39 | |
| 2–4 h (2.5% NaCl i.v.) | 581 ± 163 | 642 ± 226 | 619 ± 211 | 625 ± 157 | 647 ± 267 | 623 ± 320 | |
| UKV | |||||||
| (µmol min−1) | −2–0 h | 91 ± 22 | 64 ± 12 | 76 ± 32 | 70 ± 22 | 62 ± 28 | 45 ± 11 |
| 0–2 h | 92 ± 11 | 89 ± 17 | 89 ± 30 | 102 ± 38 | 72 ± 17 | 68 ± 21 | |
| 2–4 h (2.5% NaCl i.v.) | 108 ± 11 | 126 ± 40 | 111 ± 35 | 116 ± 13 | 115 ± 41 | 108 ± 46 | |
| UClV | |||||||
| (µmol min−1) | −2–0 h | 129 ± 54 | 125 ± 55 | 147 ± 45 | 123 ± 34 | 121 ± 48 | 105 ± 31 |
| 0–2 h | 185 ± 47 | 175 ± 54 | 178 ± 48 | 194 ± 45 | 226 ± 121 | 121 ± 44 | |
| 2–4 h (2.5% NaCl i.v.) | 48 ± 245 | 623 ± 278 | 675 ± 228 | 697 ± 178 | 698 ± 270 | 643 ± 331 | |
Figure 2.
Urine volume during the 2 h control period (C; −2–0 h), the 2 h postdose collection period (0–2 h) and during the subsequent 2 h (2–4 h) of 2.5% saline infusion after oral administration of single doses of 5 and 10 mg of asimadoline (hatched column) or placebo (open column) in each group of eight healthy subjects. Data are given as means ± s.e.mean; *P < 0.05, **P < 0.01.
Figure 3.
Free-water clearance during the 2 h control period (C; −2–0), the 2 h postdose collection period (0–2 h) and during the subsequent 2 h (2–4 h) of 2.5% saline infusion after oral administration of single doses of 5 and 10 mg of asimadoline (hatched colum) or placebo (open column) in each group of eight healthy subjects. Data are given as means ± s.e.mean; *P < 0.05, **P < 0.01.
Hormonal effects
Under basal conditions plasma AVP concentration did not change with either dose of asimadoline. It rose significantly after stimulation with 2.5% saline infusion (P < 0.05) and this rise was suppressed at the 10 mg dose of asimadoline in six of eight subjects when compared to placebo (P < 0.05) (Table 2).
Table 2.
Plasma AVP concentration, and urinary AVP and cAMP excretion before (–2–0 h) and after oral administration of 1, 5, or 10 mgasimadoline as compared with placebo in each group of eight healthy subjects in the absence (0–2 h) and presence of 2.5% saline infusion (2–4 h).*P < 0.05.
| Parameter | Collection interval | Placebo | 1 mg asimadoline | Placebo | 5 mg asimadoline | Placebo | 10 mg asimadoline |
|---|---|---|---|---|---|---|---|
| Plasma AVP concentration | |||||||
| (fmol ml−1) | |||||||
| −2–0 h | 2.3 ± 1.4 | 2.0 ± 1.0 | 2.2 ± 0.6 | 2.0 ± 0.3 | 3.6 ± 3.4 | 3.0 ± 1.4 | |
| 0–2 h | 2.9 ± 1.0 | 1.8 ± 0.6 | 2.6 ± 1.9 | 1.9 ± 1.0 | 2.9 ± 2.2 | 3.5 ± 2.3 | |
| 2–4 h (2.5% NaCl i.v.) | 5.4 ± 1.6 | 5.1 ± 1.4 | 4.2 ± 1.4 | 4.1 ± 2.3 | 5.7 ± 2.3 | 4.2 ± 1.9* | |
| Urinary AVP excretion | |||||||
| (fmol min−1) | |||||||
| −2–0 h | 30 ± 17 | 29 ± 22 | 22 ± 13 | 26 ± 9 | 40 ± 27 | 22 ± 13 | |
| 0–2 h | 30 ± 15 | 28 ± 13 | 22 ± 10 | 21 ± 10 | 46 ± 23 | 25 ± 16* | |
| 2–4 h (2.5% NaCl i.v.) | 316 ± 121 | 278 ± 59 | 220 ± 89 | 244 ± 70 | 410 ± 206 | 181 ± 125* | |
| Urinary cAMP excretion | |||||||
| (pmol min−1) | |||||||
| −2–0 h | 2.7 ± 0.8 | 2.4 ± 0.5 | 2.5 ± 0.5 | 2.3 ± 0.3 | 2.8 ± 0.8 | 2.4 ± 0.3 | |
| 0–2 h | 2.8 ± 0.8 | 2.9 ± 0.3 | 2.7 ± 0.3 | 2.8 ± 0.8 | 2.8 ± 0.3 | 2.6 ± 0.3 | |
| 2–4 h (2.5% NaCl i.v.) | 4.7 ± 1.1 | 4.7 ± 0.8 | 4.0 ± 1.1 | 3.9 ± 1.1 | 3.4 ± 0.5 | 3.6 ± 0.5 | |
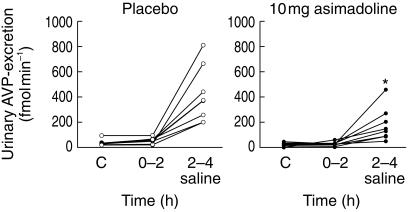
Urinary excretion of AVP was suppressed after 10 mg asimadoline in the absence and presence of 2.5% saline infusion (P < 0.05) (Figure 4; Table 2), but not at the 1 or 5 mg doses.
Figure 4.
Urinary AVP excretion during the 2 h control period (C; −2–0 h), the 2 h postdose collection period (0–2 h) and during the subsequent 2 h (2–4 h) of 2.5% saline infusion after oral administration of a single dose of 10 mg asimadoline or placebo in each group of eight healthy subjects. Data are given as means ± s.e.mean; *P < 0.05 asimadoline vs placebo during 2.5% saline infusion.
Urinary cAMP excretion was unaffected by asimadoline under basal conditions. It rose significantly (P < 0.05) with i.v. 2.5% saline infusion but was not significantly altered by asimadoline (Table 2).
The significant rises in plasma α-hANP concentration (P < 0.01) (Table 3) and in urinary excretion of ET-1 (P < 0.01) with 2.5% saline infusion were not affected by asimadoline. Plasma concentration of ET-1 did not change in any group and was not affected by asimadoline (Table 3).
Table 3.
Plasma ANP and ET concentrations and urinary ET-1 excretion after oral administration of 1, 5, or 10 mg asimadoline as compared with placebo in each group of eight healthy subjects in the absence (0–2 h) and presence of 2.5% saline infusion (2–4 h). #P < 0.01 for effects of 2.5% saline infusion.
| Parameter | Collection interval | Placebo | 1 mg asimadoline | Placebo | 5 mg asimadoline | Placebo | 10 mg asimadoline |
|---|---|---|---|---|---|---|---|
| Plasma ANP concentration | |||||||
| (pmol l−1) | |||||||
| −2–0 h | 9.1 ± 3.7 | 9.1 ± 1.6 | 11.7 ± 3.7 | 10.7 ± 3.7 | 9.4 ± 2.6 | 8.5 ± 1.6 | |
| 0–2 h | 8.8 ± 3.3 | 8.5 ± 1.6 | 10.4 ± 3.7 | 8.8 ± 1.6 | 9.4 ± 1.6 | 7.8 ± 2.6 | |
| 2–4 h (2.5% NaCl i.v.) | 15.9 ± 5.2 # | 15.6 ± 4.2 # | 15.3 ± 2.6 # | 15.9 ± 2.6 # | 15.9 ± 4.2 # | 16.6 ± 1.6 # | |
| Plasma ET-1 concentration | |||||||
| (pmol l−1) | |||||||
| −2–0 h | 4.5 ± 1.1 | 3.9 ± 1.2 | 3.9 ± 1.3 | 3.4 ± 1.2 | 4.0 ± 1.3 | 3.1 ± 1.3 | |
| 0–2 h | 4.0 ± 1.3 | 4.2 ± 1.2 | 4.7 ± 2.4 | 4.1 ± 1.1 | 3.7 ± 1.1 | 3.6 ± 1.3 | |
| 2–4 h (2.5% NaCl i.v.) | 4.5 ± 1.2 | 3.9 ± 1.1 | 4.6 ± 1.4 | 3.8 ± 0.8 | 3.2 ± 1.3 | 3.8 ± 1.4 | |
| Urinary ET-1 excretion | |||||||
| (pmol min−1) | |||||||
| −2–0 h | 28.8 ± 19.2 | 34.8 ± 18.0 | 30.4 ± 16.0 | 26.8 ± 11.6 | 23.2 ± 16.0 | 17.6 ± 7.6 | |
| 0–2 h | 23.2 ± 5.2 | 25.6 ± 6.4 | 27.6 ± 3.2 | 33.2 ± 11.6 | 27.2 ± 5.2 | 21.7 ± 13.3 | |
| 2–4 h (2.5% NaCl i.v.) | 39.2 ± 8.4 # | 42.8 ± 7.6 # | 38.0 ± 9.6 # | 37.6 ± 9.6 # | 39.6 ± 13.6 # | 41.7 ± 17.3 # | |
For statistical significant differences between effects of asimadoline and placebo and between the 5 and 10 mg doses of asimadoline the 95% confidence intervals for differences of the main comparisons are listed in Table 4.
Table 4.
95% confidence intervals (CI) for differences of main comparisons of effects of 5 and 10 mg doses of asimadoline vs placebo and of 5 vs 10 mg doses of asimadoline in the absence or presence of 2.5% saline infusion i.v.(V = urinary flow rate; CH2O = free-water clearance); *P < 0.05, **P < 0.01.
| Parameters | Collection interval 5 mg asimadoline vs placebo mean of difference ± s.d. | 95% CI | 10 mg asimadoline vs placebo mean of difference ± s.d | 95% CI | 10 mg vs 5 mg asimadoline Mean of difference. | 95% CI | |
|---|---|---|---|---|---|---|---|
| V (ml min−1) | 0–2 h | 2.34 ± 1.91 | 0.74,3.93 ** | 2.26 ± 1.40 | 1.09–3.44 ** | −0.13 | −2.24,1.99 |
| 2–4 h (2.5% NaCl) | 0.21 ± 0.71 | −0.38,0.81 | 0.86 ± 1.01 | 0.02–1.71 ** | 0.99 | −0.32,2.30 | |
| CH2O (ml min−1) | |||||||
| 0–2 h | 2.58 ± 2.11 | 0.37,4.79 * | 2.60 ± 1.18 | 1.51–3.69 | 0.53 | −1.22,2.28 | |
| 2–4 h (2.5% NaCl) | −0.04 ± 0.74 | −0.66,0.58 | 0.85 ± 0.61 | 0.34,1.36 | 0.80 | −0.16,1.76 | |
| Plasma AVP concentration | |||||||
| (fmol ml−1) | 0–2 h | −0.68 ± 1.82 | −2.20,0.84 | 0.58 ± 2.66 | −1.65,2.81 | 1.57 | −0.57,3.71 |
| 2–4 h (2.5% NaCl) | −0.15 ± 3.02 | −2.69,2.38 | −1.54 ± 3.36 | −4.35,1.27 * | 0.07 | −2.32,2.46 | |
| Urinary AVP excretion | |||||||
| (fmol min−1) | 0–2 h | −0.88 ± 10.42 | −9.60,7.84 | −20.59 ± 11.94 | −30.58,-10.60* | 3.86 | 10.74,18.46 |
| 2–4 h (2.5% NaCl) | 23.57 ± 100.71 | −60.63,107.8 | 229.82 ± 256.42 | −444.2,-15.45 | −63.12* | −178.49.52.25 | |
| Urinary cAMP excretion | |||||||
| (pmol min−1) | 0–2 h | 0.11 ± 0.89 | −0.64,0.86 | −0.25 ± 0.13 | −0.36,-0.14 | −0.28 | −0.95,0.40 |
| 2–4 h (2.5% NaCl) | −0.14 ± 1.12 | −1.07,0.80 | 0.00 ± 0.83 | −0.77,0.77 | −0.28 | −1.26,0.71 | |
| Plasma ANP concentration | |||||||
| (pmol l−1) | 0–2 h | −1.62 ± 3.13 | −4.23,1.00 | −1.37 ± 3.43 | −4.23,1.50 | −1.01 | −3.24,1.22 |
| 2–4 h (2.5% NaCl) | 0.65 ± 1.42 | −0.54,1.84 | 0.39 ± 3.34 | −2.41,3.18 | 0.52 | −3.34,4.47 | |
| Plasma ET-1 concentration | |||||||
| (pmol l−1) | 0–2 h | −0.64 ± 2.26 | −2.53,1.25 | −0.16 ± 1.28 | −1.13,2.51 | −0.42 | −1.78,0.94 |
| 2–4 h (2.5% NaCl) | −1.85 ± 3.16 | −1.80,0.32 | 0.59 ± 1.24 | −0.45,1.62 | −0.04 | −1.34,1.27 | |
| Urinary ET-1 excretion | |||||||
| (pmol min−1) | 0–2 h | 5.65 ± 13.83 | −5.90,10.31 | −5.29 ± 11.88 | −15.21,4.65 | −11.48 | −25.39,2.42 |
| 2–4 h (2.5% NaCl) | −0.12 ± 12.19 | −10.31,10.08 | 2.17 ± 9.18 | −5.51,9.84 | 3.89 | −11.44,19.20 | |
Discussion
Pre-clinical investigations have indicated that centrally acting selective κ-opiate receptor agonists, such as µ-opiate receptor agonists, can lead to tolerance development [18–20]. In contrast, withdrawal symptoms following chronic administration of κ-opiate receptor agonists differ both qualitatively and quantitatively from those of µ-opiate receptor agonists. Furthermore, selective κ-opiate receptor agonists have no euphoric effect. The addictive potential of selective κ-opiate receptor agonists is apparently low [21].
In preclinical studies the tolerability of asimadoline, a peripherally acting selective κ-opiate receptor agonist, was tested in healthy subjects with single oral doses ranging from 2.5 to 15 mg. Substance-related side-effects of mild intensity occurred only with doses of 10 and 15 mg within 1–4 h after administration. With repeated administration (5 mg or 2.5 mg once or twice daily for 1 week) safety and tolerability again were found to be good and no central side-effects were observed (unpublished data).
In this study, side-effects, such as thirst, dizziness, mild headache, tiredness, palpitations and peribuccal paraesthesia, were reported almost exclusively during the 2.5% saline infusion in the absence or presence of asimadoline. Only one subject in the 10 mg dose group suffered for a short time period from moderate dizziness after administration of asimadoline before infusion of 2.5% saline. No changes in heart rate or blood pressure were found with administration of asimadoline.
Studies on rats, dogs and monkeys, showed that approximately 80 to over 90% of the dose was absorbed by the intestinal tract. The absolute bioavailability is 60% in monkeys and 20% in dogs. This relatively low rate is attributed to first-pass metabolism. The mother-compound and its metabolites are predominantly excreted via the biliary route, i.e. between 35% and 91%, whereas between 29% and approximately 3% are excreted via the kidney within 120 h. Plasma elimination half-life of unchanged asimadoline ranges between approximately 0.4–0.8 h [22; Bender HM, unpublished data].
In the present study the mean dose-normalized maximal plasma concentrations following single oral administration of 1, 5 and 10 mg asimadoline were not significantly different and the time to reach maximal concentrations was not significantly different between the various doses. Nevertheless, it appears that the mean AUC tended to be higher after administration of 10 mg asimadoline. It is concluded, that the kinetics of asimadoline are dose-independent, although a trend for a nonproportional increase of AUC with increasing oral doses has been observed.
During the first 2 h post administration of asimadoline a significant increase in urine volume, free-water clearance and a decrease in urine osmolarity were noted. The effects were more marked in the 10 mg than in the 5 and 1 mg dose groups. GFR and urinary electrolyte excretion remained unaltered. During the second 2 h period post administration, during which 2.5% saline was infused i.v., an increase in serum osmolarity and a decrease in urine volume and free-water clearance were observed. Only the 10 mg dose of asimadoline increased urine volume and decreased negative free-water clearance with a decrease in urine osmolarity when compared with placebo.
During the first 2 h post administration of asimadoline a significant decrease in urinary AVP excretion was observed only in the 10 mg dose group when compared with placebo. Plasma AVP, α-hANP, and ET-1 concentrations, and urinary cAMP and ET-1 excretion remained unaltered. With 2.5% saline infusion plasma AVP concentration and urinary AVP and cAMP excretion rose significantly following this osmotic stimulus. Plasma α-hANP concentration also rose significantly with 2.5% saline infusion as would be expected from extracellular fluid volume expansion with atrial distension [12]. While plasma ET-1 concentration remained unaltered, urinary ET-1 excretion rose significantly in parallel with increased AVP secretion. This may support a short-loop feedback mechanism, in which ET-1 attenuates the effects of AVP by suppression of cAMP synthesis in the inner medullary collecting duct [14].
During 2.5% saline infusion, asimadoline at a dose of 10 mg, decreased plasma AVP concentration and urinary AVP excretion when compared with placebo. This has been observed with other κ-opioid receptor agonists in experimental animals [9, 23, ]. However, since the diuretic effect of asimadoline paralleled suppression of AVP secretion only in the 10 mg group, but not in the 5 mg group, it appears that asimadoline passes the blood–brain barrier only at high plasma concentrations [22] and suppresses AVP secretion [24]. No effects of asimadoline on the rise in urinary cAMP and ET-1 excretion or in plasma α-hANP concentraton during 2.5% saline infusion were observed in contrast to previous observations with niravoline [25]. Urinary cAMP excretion was not suppressed by asimadoline despite the decrease in AVP excretion, since small changes in this parameter, which to a large extent reflects activity of parathyroid hormone in the kidney, may not be detectable.
Controversial results have been reported regarding the mechanisms of action of κ-agonists. Most previous studies showed that κ-opioid agonists act through suppression of AVP secretion [24] via activation of κ-opioid receptors probably located on hypothalamic-neurohypophyseal nerve terminals [24] beyond the blood–brain-barrier rather than of receptors located in the (posterior) neurohypophysis [11]. κ-opioid receptors may be coupled directly to l-type calcium channels and reduce voltage-dependent calcium conductance [25] thereby inhibiting electrically evoked AVP secretion by nerve terminals.
Pure water diuresis induced by κ-opioid agonists may occur with unaltered plasma AVP levels [8, 26, 27]. In support of a direct action of κ-opioid agonists in the kidney is the observation that these agonists are diuretic in Brattleboro rats [28] and antagonize the action of exogenous AVP administered to water-loaded rats [29]. Such κ-opioid binding sites have been demonstrated in the kidney and may allow a direct renal action [30]. Moreover, in rats with bilateral renal demedullation the diuretic response to κ-opioid agonists is significantly attenuated, suggesting a link between the adrenal medulla and κ-opioid-induced diuresis [26, 31, 32]. The diuretic action appears not to be mediated by an increase in afferent renal sympathetic nerve activity [33]. The exact mechanism of the diuretic action of κ-opioid agonists within the kidney requires further study, especially since an inhibition of AVP-induced adenylcyclase activity could not be confirmed [11]. Nevertheless, κ-opiod agonists appear to modulate the peripheral action of AVP in man [8].
In keeping with our results, Ohnishi et al.[6] showed that the dynorphin-A analogue E2078 reduced plasma AVP dose-dependently reaching statistical significance at a 3 mg dose. Although 1 mg and 2 mg of E2078 produced a considerable aquaretic effect, the AVP-lowering effect was not significantly different from the results with placebo. The authors conclude that there is probably a peripheral effect that supplements the aquaretic effect in addition to its central suppression of AVP.
The renal effects observed in this study are consistent with those of previous studies with asimadoline in man (unpublished data) and with data on other κ-opioid agonists, such as CI-977 [34], the dynorphin-A analogue E 2078 [6], niravoline [23], or spiradoline [8]. They are also consistent with the presence of κ-opioid receptors in the kidney [30] to which binding of asimadoline was demonstrated [22].
In conclusion, the tolerability of asimadoline was good and no drug-related adverse events were observed after the 1 mg dose. Only minor reversible adverse events, such as headache and tiredness, were possibly drug-related and were observed at doses of 5 mg and 10 mg asimadoline, respectively; However, these occurred almost exclusively during infusion of hypertonic saline. Major effects at doses of 5 and 10 mg asimadoline were the increased water intake and diuresis, which was due to an increase in free-water clearance. This is caused most probably by a direct effect of asimadoline on water absorption in the medullary collecting duct independent of changes in AVP secretion, although at the high dose of 10 mg asimadoline osmotically induced AVP secretion was found to be suppressed.
Acknowledgments
The authors gratefully acknowledge the support of ASTER study centre, Paris, France (Drs J.J. Thebault and M. Guillaume).
References
- 1.Miller M. Inhibition of ADH release in the rat by narcotic antagonists. Neuroendocrinology. 1975;19:241–251. doi: 10.1159/000122444. [DOI] [PubMed] [Google Scholar]
- 2.Nutt JG, Jasinski DR. Diuretic action of the narcotic antagonist oxilorphan. Clin Pharmacol Ther. 1974;15:361–367. doi: 10.1002/cpt1974154361. [DOI] [PubMed] [Google Scholar]
- 3.Dykstra LA, Gmerek DE, Winger G, Woods JH. k-opioids in rhesus monkeys. I. Diuresis, sedation, analgesia and discriminative stimulus effects. J Pharmacol Exp Ther. 1987;242:413–420. [PubMed] [Google Scholar]
- 4.Slizgi GR, Ludens JH. Studies on the nature and mechanism of the diuretic activity of the opioid analgesic ethylketocycloazocine. J Pharmacol Exp Ther. 1982;220:585–591. [PubMed] [Google Scholar]
- 5.Slizgi GR, Taylor CJ, Ludens JH. Effects of the highly selective k- opioid U-50,488 on renal function in the anesthetized dog. J Pharmacol Exp Ther. 1984;230:641–645. [PubMed] [Google Scholar]
- 6.Ohnishi A, Mihara M, Yasuda S, Tomono Y, Hasegawa J, Tanaka T. Aquaretic effect of the stable dynorphin-A analog E2078 in the human. J Pharmacol Exp Ther. 1994;270:342–347. [PubMed] [Google Scholar]
- 7.Peters GR, Ward NJ, Antal EG, Lai PY, De Maar EW. Diuretic action in man of a selective kappa-opioid agonist. J Clin Pharmacol Exp Ther. 1987;240:128–131. [PubMed] [Google Scholar]
- 8.Rimoy GH, Bhaskar NK, Wright DM, Rubin PC. Mechanism of diuretic action of spiradoline (U-62066E) – a kappa-opioid receptor agonist in the human. Br J Clin Pharmacol. 1991;32:611–615. doi: 10.1111/j.1365-2125.1991.tb03960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wells T, Forsling ML. k-opioid modulation of vasopressin secretion in conscious rats. J Endocrinol. 1991;129:411–419. doi: 10.1677/joe.0.1290411. [DOI] [PubMed] [Google Scholar]
- 10.Ashton N, Balment RJ, Blackburn TP. k-opioid resceptor agonists modulate the renal excretion of water and electrolytes in anaesthetized rats. Br J Pharmacol. 1990;99:181–185. doi: 10.1111/j.1476-5381.1990.tb14674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks DP, Giardina G, Gellai M, et al. Opiate receptors within the blood–brain barrier mediate kappa agonist-induced water diuresis. J Pharmacol Exp Ther. 1993;266:164–171. [PubMed] [Google Scholar]
- 12.Kramer HJ. Atrial natriuretic hormones. Gen Pharmacol. 1988;19:747–753. [PubMed] [Google Scholar]
- 13.Kramer HJ, Bäcker A, Bokemeyer D, Meyer-Lehnert H. Atrial natriuretic peptide and endothelin: modulators of renal function. Clin Invest. 1994;72:703–705. doi: 10.1007/BF00212995. [DOI] [PubMed] [Google Scholar]
- 14.Migas I, Bäcker A, Meyer-Lehnert H, Michel H, Wulfhekel U, Kramer HJ. Characteristics of endothelin receptors and intracellular signalling in porcine inner medullary collecting duct cells. Am J Hypertens. 1993;6:611–618. doi: 10.1093/ajh/6.7.611. [DOI] [PubMed] [Google Scholar]
- 15.Gibaldi M, Perrier D. Drugs and Pharmaceutical Sciences. 2. New york: Marcel Dekker; 1982. Pharmacokinetics. [Google Scholar]
- 16.Rowland M, Tozer T. Clinical Pharmacokinetics – Concepts and Application. 2. Philadelphia, London: Lea & Febiger; 1989. [Google Scholar]
- 17.Rowland M, Tucker T. Symbols in pharmacokinetics. J Pharm Biopharm. 1980;8:497–507. doi: 10.1007/BF01059548. [DOI] [PubMed] [Google Scholar]
- 18.Cowan A, Zhu XY, Mosberg HI, Omnaas JR, Porreca F. Direct dependence studies in rats with agents selective for different types of opioid receptor. J Pharmacol Exp Ther. 1988;246:950–955. [PubMed] [Google Scholar]
- 19.Gmerek DE, Dykstra LA, Woods JH. Kappa o pioids in rhesus monkeys III. Dependence associated with chronic administration. J Pharmacol Exp Ther. 1987;242:428–436. [PubMed] [Google Scholar]
- 20.von Voigtlander PF, Lahti RA, Ludens JH. U-50, 488: a selective and structurally novel non-mu (kappa) opioid agonist. J Pharmacol Exp Ther. 1983;224:7–12. [PubMed] [Google Scholar]
- 21.Millan MJ. Opioid receptors and analgesia. Trends Pharmacol Sci. 1990;11:70–76. doi: 10.1016/0165-6147(90)90321-x. [DOI] [PubMed] [Google Scholar]
- 22.Barber A, Bartoszyk GD, Bender HM, Gottschlich R, Greiner HE, Harting J, Mauler F, Minck K-O, Murray RD, Simon M, Seyfried CA. A pharmacological profile of the novel, peripherally-selective k-opioid receptor agonist, EMD 61753. Br J Pharmacol. 1994;113:1317–1327. doi: 10.1111/j.1476-5381.1994.tb17142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oiso Y, Iwasaki Y, Kondo K, Takatsuki K, Tomita A. Effect of the opioid k-receptor agonist U-50488H on the secretion of arginine-vasopressin. Neuroendocrinology. 1988;48:658–662. doi: 10.1159/000125078. [DOI] [PubMed] [Google Scholar]
- 24.Hamon G, Jouquey S. Kappa agonists and vasopressin secretion. Horm Res. 1990;34:129–132. doi: 10.1159/000181811. [DOI] [PubMed] [Google Scholar]
- 25.Bellissant E, Denolle T, Sinnassamy P, et al. Systemic and regional hemodynamic and biological effects of a new k-opioid agonist, niravoline, in healthy volunteers. J Pharmacol Exp Ther. 1996;278:232–242. [PubMed] [Google Scholar]
- 26.Ashton N, Balment RJ, Blackburn TP. K-opioid -induced changes in renal water and electrolyte management and endocrine secretion. Br J Pharmacol. 1989;97:769–776. doi: 10.1111/j.1476-5381.1989.tb12015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leander JD, Hart JC, Zerbe RL. Diuresis and suppression of vasopressin by kappa-opioids: comparison with mu- and delta-opioids and clonidine. J Pharmacol Exp Ther. 1985;234:463–469. [PubMed] [Google Scholar]
- 28.Slizgi GR, Ludens JH. Role of ADH in ethyketocyclazocine induced diuresis. Studies in the Brattleboro rat. Life Sci. 1986;38:2437–2440. doi: 10.1016/0024-3205(86)90613-2. [DOI] [PubMed] [Google Scholar]
- 29.Gavend M, Gavend MR, Serre F. Etude experimentale de l'action diuretique d'un antagoniste des morphiniques, la ciclazocine. J Pharmacol (Paris) 1978;9:77–88. [Google Scholar]
- 30.Slizgi GR, Ludens JH. Displacement of 3H-EKC binding by opioids in rat kidney: a correlate to diuretic activity. Life Sci. 1985;36:2189–2193. doi: 10.1016/0024-3205(85)90328-5. [DOI] [PubMed] [Google Scholar]
- 31.Blackburn TP, Borkowski KR, Friend J, Rance MJ. On the mechanism of k-opioid diuresis. Br J Pharmacol. 1986;89:593–598. doi: 10.1111/j.1476-5381.1986.tb11160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borkowski KR. Studies on the adrenomedullary dependence of k-opioid agonist-induced diuresis in conscious rats. Br J Pharmacol. 1989;98:1151–1156. doi: 10.1111/j.1476-5381.1989.tb12659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapusta DR, Jones SY, DiBona GF. Role of renal nerves in excretory responses to administration of k-agonists in conscious spontaneously hypertensive rats. J Pharmacol Exp Ther. 1989;251:230–237. [PubMed] [Google Scholar]
- 34.Reece PA, Sedman AJ, Rose S, Wright D, Dawkins R, Rajagopalan R. Diuretic effects, pharmacokinetics, and safety of a new centrally acting kappa-opioid agonist (DI-977) in humans. J Clin Pharmacol. 1994;34:1126–1131. doi: 10.1002/j.1552-4604.1994.tb01991.x. [DOI] [PubMed] [Google Scholar]