Abstract
Aims
To determine the FMO and P450 isoform selectivity for metabolism of benzydamine and caffeine, two potential in vivo probes for human FMO.
Methods
Metabolic incubations were conducted at physiological pH using substrate concentrations of 0.01–10 mm with either recombinant human FMOs, P450s or human liver microsomes serving as the enzyme source. Products of caffeine and benzydamine metabolism were analysed by reversed-phase h.p.l.c. with u.v. and fluorescence detection.
Results
CYP1A2, but none of the human FMOs, catalysed metabolism of caffeine. In contrast, benzydamine was a substrate for human FMO1, FMO3, FMO4 and FMO5. Apparent Km values for benzydamine N-oxygenation were 60 ± 8 µm, 80 ± 8 µm, > 3 mm and > 2 mm, for FMO1, FMO3, FMO4 and FMO5, respectively. The corresponding Vmax values were 46 ± 2 min−1, 36 ± 2 min−1, < 75 min−1 and < 1 min−1. Small quantities of benzydamine N-oxide were also formed by CYPs 1A1, 1A2, 2C19, 2D6 and 3A4.
Conclusions
FMO1 and FMO3 catalyse benzydamine N-oxygenation with the highest efficiency. However, it is likely that the metabolic capacity of hepatic FMO3 is a much greater contributor to plasma levels of the N-oxide metabolite in vivo than is extrahepatic FMO1. Therefore, benzydamine, but not caffeine, is a potential in vivo probe for human FMO3.
Keywords: benzydamine, caffeine, cytochrome P450, flavin-containing monooxygenase, human liver microsomes, N-oxide
Introduction
The identification and validation of drugs as in vivo probes for human monooxygenase function serves at least two important purposes. Firstly, it enables studies on the pharmacogenetic mechanism(s) underlying population variability in drug response to be conducted, and their clinical consequences evaluated. Our current understanding of the importance of pharmacogenetics in antidepressant therapy would have been greatly hindered without the availability of isoform-specific in vivo probes such as sparteine, debrisoquine and dextromethorphan for CYP2D6 and (S)-mephenytoin and omeprazole for CYP2C19 [1]. Secondly, the regulation of individual human monooxygenases can be studied. For example, drug–drug interactions involving agents known to be P450 isoform-specific have shown that phenytoin and several other common antiepileptic agents induce hepatic CYP3A4 [2], and that fluconazole is a potent inhibitor of CYP2C9 [3]. In vivo metabolic probes for practically all of the other major human liver P450s have now been described [4].
The human flavin-containing monooxygenase (FMO) gene family codes for at least four active proteins, FMO1, FMO3, FMO4 and FMO5 [5]. Although trimethylamine has emerged as the prototypical substrate for human FMO3, the major form of the enzyme present in human liver [5, 6], in vivo studies with this odouriferous compound present some problems. Trimethylamine is an endogenous breakdown product of dietary lecithin, and direct analysis of trimethylamine N-oxide is not straightforward. Therefore, there is a need to identify alternative in vivo xenobiotic probes for human FMO3.
In the present study we examined the human FMO and P450 isoform specificity for metabolism of caffeine and benzydamine. Caffeine was chosen because recent indirect studies suggest that the metabolism of caffeine to theobromine may be mediated by human FMO [7]. Benzydamine was chosen because conversion to its N-oxide metabolite is well established as a sensitive indicator of FMO activity [8], although no data have been presented for the isoform specificity of the N-oxygenation reaction in humans.
Methods
Benzydamine hydrochloride, theophylline and theobromine were obtained from Sigma (St Louis, MO). Caffeine and trimethylamine hydrochloride were purchased from Aldrich (Milwaukee, WI). Paraxanthine was obtained from RBI (Natick, MA). Benzydamine N-oxide and dazidamine were kindly provided by the F. Angelini Research Institute (Rome, Italy).
Human FMO1, FMO3, FMO4, and FMO5 were expressed in the baculovirus expression vector system, and insect cell membranes were prepared and flavin concentrations quantified by h.p.l.c. as reported recently [9]. Microsomal preparations of human liver samples, prepared and characterized as previously described [10], were used. Microsomal preparations of 13 human baculovirus-expressed P450 isoforms, CYPs 1A1, 1A2, 2A6, 2B6, 2C8, 2C9, 2C18, 2C19, 2D6, 2E1, 3A4, 3A5 and 4A11, were purchased from GENTEST Corp. (Woburn, MA). Purified CYP1A2 was isolated from insect cells infected with a recombinant baculovirus containing a cDNA for human CYP1A2, according to standard procedures established in our laboratory [11]. Details of this purification procedure will be published elsewhere.
Reaction mixtures (250 µl) contained 0.1 m phosphate buffer pH 7.4, 10–300 pmol heterologously expressed enzyme, or 200 µg microsomal protein, 1000 units of catalase and a NADPH-generating system consisting of 0.5 mm NADP+, 6 mm glucose-6-phosphate and 2.8 units ml−1 of glucose-6-phosphate dehydrogenase. In some incubations trimethylamine (0.5 mm), an FMO3 substrate, was added at greater than 10 times its Km as an inhibitor. After a 2 min preincubation at 37 °C the reaction was initiated by the addition of substrate (0.01–10 mm), and was allowed to continue at 37 °C for 5–30 min. Incubations with caffeine were terminated by the addition of HClO4 to a final concentration of 0.4%, whereas incubations with benzydamine were quenched with an equal volume of acetonitrile containing dazidamine as internal standard. Protein was precipitated by centrifugation and the supernatants were subjected to h.p.l.c. analysis.
Caffeine and its metabolites were analysed using an Nucleosil 3 µm C18 self-packed (150 mm × 2.1 mm) column with 20 mm phosphate buffer (pH 4.8)/acetonitrile/tetrahydrofuran (96/2.5/1.5) as eluent at a flow rate of 0.4 ml min−1 and u.v. detection at 280 nm. Theobromine, paraxanthine and theophylline eluted at 4.0 min, 5.9 min and 6.6 min, respectively. Rates of formation of benzydamine N-oxide were determined by h.p.l.c. as described previously [9]. Kinetic parameters, Km and Vmax, were obtained using the k.cat program for Macintosh (Biometallics) which fits experimental data directly to the Michaelis-Menten equation using a nonlinear regression method.
Results
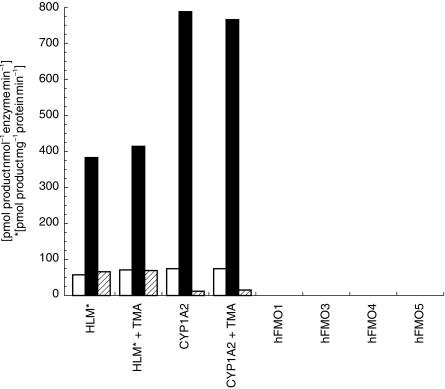
As expected, human liver microsomes and purified CYP1A2 metabolized caffeine principally to paraxanthine, but also formed readily detectable amounts of theobromine and theophylline (Figure 1). However, none of the human FMO isoforms catalysed formation of quantifiable levels of any of the N-demethylated metabolites of caffeine. In addition, the inclusion of 0.5 mm trimethylamine, a specific FMO3 substrate, in metabolic reactions catalysed by either human liver microsomes or CYP1A2 had no significant effect on turnover to the three caffeine metabolites. As a positive control for the use of trimethylamine as a microsomal FMO3 inhibitor, we also determined that 0.5 mm trimethylamine inhibited the formation of benzydamine N-oxide in human liver microsomes by 41% at a substrate concentration of 0.5 mm.
Figure 1.
Formation of theobromine (□, TB), paraxanthine (▪, PX) and theophylline ( , TP) by human liver microsomes, purified human CYP1A2 and recombinant human FMO isoforms at pH 7.4. Caffeine concentration was 10 mm. Data were obtained from a single human liver sample chosen for its representative activity towards trimethylamine N-oxide formation (5 nmol mg−1 min−1). Values are the mean of duplicate determinations which deviated by less than 5%. Recombinant preparations of human FMO1, FMO3 and FMO4 were highly active towards benzydamine as substrate (see Figure 2 and Results). Human FMO5 exhibited a low reaction velocity, but stereoselective formation of (S)-methyl p-tolyl sulfoxide with methyl p-tolyl sulphide as substrate. Therefore, all FMO preparations are catalytically active.
, TP) by human liver microsomes, purified human CYP1A2 and recombinant human FMO isoforms at pH 7.4. Caffeine concentration was 10 mm. Data were obtained from a single human liver sample chosen for its representative activity towards trimethylamine N-oxide formation (5 nmol mg−1 min−1). Values are the mean of duplicate determinations which deviated by less than 5%. Recombinant preparations of human FMO1, FMO3 and FMO4 were highly active towards benzydamine as substrate (see Figure 2 and Results). Human FMO5 exhibited a low reaction velocity, but stereoselective formation of (S)-methyl p-tolyl sulfoxide with methyl p-tolyl sulphide as substrate. Therefore, all FMO preparations are catalytically active.
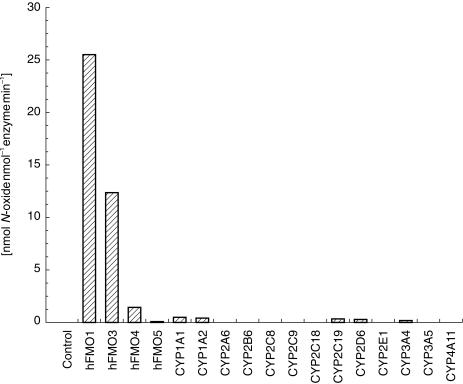
In contrast to the data obtained with caffeine, benzydamine was a substrate for all four FMO isoforms, although only FMO1 and FMO3 displayed high activity at a substrate concentration of 0.1 mm (Figure 2). Human FMO1, FMO3 and FMO4 metabolized benzydamine (0.1 mm) to benzydamine N-oxide at significant rates (Figure 2). Low levels of N-oxide were also formed by human CYP1A1, CYP1A2, CYP2C19, CYP2D6 and CYP3A4.
Figure 2.
Rates of formation of benzydamine N-oxide formation by recombinant human FMO and P450 isoforms at pH 7.4. Benzydamine concentration was 0.1 mm. Values are the mean of duplicate determinations which deviated by less than 5%.
In order to better evaluate the contributions of the human FMOs to benzydamine N-oxide formation under physiological conditions, we determined apparent Km and Vmax values with each enzyme. Km values for benzydamine N-oxygenation were 60 ± 8 µm, 80 ± 8 µm, > 3 mm and > 2 mm, for FMO1, FMO3, FMO4 and FMO5, respectively. The corresponding Vmax values were 46 ± 2 min−1, 36 ± 2 min−1, < 75 min−1 and < 1 min−1. Substrate inhibition with FMO4 and substrate activation with FMO5, at benzydamine concentrations above 3 mm, prevented more accurate determination of the kinetic parameters for these two isoforms. Nonetheless, it is clear that FMO1 and FMO3 are the most efficient catalysts of benzydamine N-oxygenation.
Discussion
Caffeine metabolite ratios have been used widely by many laboratories to investigate not only CYP1A2 phenotype, but also xanthine oxidase and NAT-2 activity in humans [12]. Recently, data have been presented that describe phenotype-genotype correlations for human FMO3 variants in a Korean population using the urinary ratio of theobromine: caffeine [7]. This is intriguing because N-dealkylation reactions are not typical of microsomal FMOs. Therefore, in the present study we evaluated the contribution of microsomal and recombinant human FMOs to formation of the N-dealkylated metabolites of caffeine. The FMO3 substrate trimethylamine did not inhibit NADPH-dependent formation of theobromine, theophylline or paraxanthine by human liver microsomes. Moreover, we were unable to demonstrate N-demethylation of caffeine by recombinant human FMO1, FMO3, FMO4 or FMO5. Therefore, none of these data support the use of caffeine N-demethylation as a probe for FMO3 status in humans.
The present study demonstrates that benzydamine is metabolized to its N-oxide metabolite most efficiently by human FMO1 and FMO3. The recombinant enzyme data match well with previous microsomal experiments [9] which found excellent congruence between benzydamine N-oxygenase activity and trimethylamine N-oxygenase activity in human liver, where human FMO3 is by far the more dominant isoform, and significant levels of benzydamineN-oxygenase activity in human kidney microsomes, where FMO1 predominates [5].
Peak plasma concentrations of benzydamine and its major metabolite benzydamine N-oxide are in the 0.2–0.6 µg/ml range after oral administration of 50 mg of the parent compound to humans [13]. Although, benzydamine N-oxygenation is not an FMO3 isoform-specific reaction, consideration of the tissue distributions of human FMO1 and FMO3 [5] supports the expectation that the metabolic capacity of hepatic FMO3 would be a much greater contributor to plasma levels of the N-oxide in vivo than extrahepatic FMO1.
Therefore, on the basis of benzydamine's ease of administration, facile metabolite analysis and FMO isoform selectivity, this anti-inflammatory agent is a good candidate as an in vivo probe for human FMO. More specifically, we suggest that determination of the plasma benzydamine N-oxide: benzydamine ratio, shortly after oral administration of a test dose of the drug, would be expected to reflect human hepatic FMO3 activity. Further studies are required to put this proposal on a firm pharmacokinetic basis.
Acknowledgments
Funding for this research was provided by the National Institutes of Health Grant GM43511. The authors would like to thank Dr Robert Haining (University of Washington) for preparation of baculovirus-expressed human CYP1A2.
Note added in proof
For further discussion of the use of caffeine as a probe for FMO see: Rettie AE & Lang DH. Can caffeine be used as an in-vivo probe for human flavin-containing monooxygenase activity? Pharmacogenetics 2000; 10: 275–277.
References
- 1.Bertilsson L, Dahl ML, Tybring G. Pharmacogenetics of antidepressants: clinical aspects. Acta Psychiatr Scand Suppl. 1997;391:14–21. doi: 10.1111/j.1600-0447.1997.tb05954.x. [DOI] [PubMed] [Google Scholar]
- 2.Thummel KE, Wilkinson GR. In vitro and in vivo drug interactions involving human CYP3A. Ann Rev Pharmacol Toxicol. 1998;38:389–430. doi: 10.1146/annurev.pharmtox.38.1.389. [DOI] [PubMed] [Google Scholar]
- 3.Black DJ, Kunze KL, Wienkers LC, et al. Warfarin-fluconazole II. A metabolically based drug interaction: In vivo studies. Drug Metab Dispos. 1996;24:422–428. [PubMed] [Google Scholar]
- 4.Kivisto KT, Kroemer HK. Use of probe drugs as predictors of drug metabolism in humans. J Clin Pharmacol. 1997;37:40S–48S. doi: 10.1177/009127009703700121. [DOI] [PubMed] [Google Scholar]
- 5.Rettie AE, Fisher MB. Transformation Enzymes. Oxidative; Non-P450. In: Woolf Tf., editor. Handbook of Drug Metabolism. New York: Marcel Dekker; 1999. pp. 131–151. [Google Scholar]
- 6.Dolphin CT, Janmohamed A, Smith RL, Shephard EA, Phillips IR. Missense mutation in flavin-containing monooxygenase gene FMO3 underlies fish–odour syndrome. Nat Genet. 1997;17:491–494. doi: 10.1038/ng1297-491. [DOI] [PubMed] [Google Scholar]
- 7.Park CS, Chung WG, Kang JH, Ro HK, Lee KH, Cha YN. Phenotyping of flavin-containing monooxygenase using caffeine metabolism and genotyping of FMO3 in a Korean population. Pharmacogenetics. 1999;9:155–164. [PubMed] [Google Scholar]
- 8.Ubeaud G, Schiller CD, Hurbin F, Jaeck D, Coassolo P. Estimation of flavin-containing monooxygenase activity in intact hepatocyte monolayers of rat, hamster, rabbit, dog and human by using N-oxidation of benzydamine. Eur J Pharm Sci. 1999;8:255–260. doi: 10.1016/s0928-0987(99)00016-0. 10.1016/s0928-0987(99)00016-0. [DOI] [PubMed] [Google Scholar]
- 9.Lang DH, Yeung CK, Peter RM, et al. Isoform specificity of trimethylamine N-oxygenation by human flavin-containing monooxygenase (FMO) and P450 isoforms: Selective catalysis by FMO3. Biochem Pharmacol. 1998;56:1005–1012. doi: 10.1016/s0006-2952(98)00218-4. 10.1016/s0006-2952(98)00218-4. [DOI] [PubMed] [Google Scholar]
- 10.Lang DH, Rettie AE, Böcker RH. Identification of enzymes involved in the metabolism of atrazine, terbuthylazine, ametryne and terbutryne in human liver microsomes. Chem Res Tox. 1997;10:1037–1044. doi: 10.1021/tx970081l. [DOI] [PubMed] [Google Scholar]
- 11.Haining RL, Hunter AP, Veronese ME, Trager WF, Rettie AE. Allelic variants of P4502C9: baculovirus-mediated expression, purification, structural characterization, substrate stereoselectivity and prochiral selectivity of the wild-type and I359L mutant forms. Arch Biochem Biophys. 1996;333:447–458. doi: 10.1006/abbi.1996.0414. 10.1006/abbi.1996.0414. [DOI] [PubMed] [Google Scholar]
- 12.Miners JO, Birkett DJ. The use of caffeine as a metabolic probe for human drug-metabolizing enzymes. Gen Pharmacol. 1996;27:245–249. doi: 10.1016/0306-3623(95)02014-4. 10.1016/0306-3623(95)02014-4. [DOI] [PubMed] [Google Scholar]
- 13.Schoenwald RD, Kumakura T, Catanese B. Pharmacokinetics of benzydamine. Int J Tiss Reac. 1987;9:93–97. [PubMed] [Google Scholar]