Abstract
Aims
Nebivolol is a selective, vasodilatory β1-adrenergic receptor antagonist which has been suggested to possess additional antioxidative properties. The aim of the present study was to assess the actions of nebivolol in antihypertensive doses on systemic oxidative stress in healthy volunteers, reflected by 24h urinary excretion of 8-iso-PGF2α.
Methods
In a double-blind, cross-over study, 12 healthy volunteers received 5 mg nebivolol once daily or placebo for a total of 7 days, separated by a wash out period of 2 weeks. After each treatment period 24h urinary excretion of 8-iso-PGF2α was determined by gas chromatography-tandem mass spectrometry.
Results
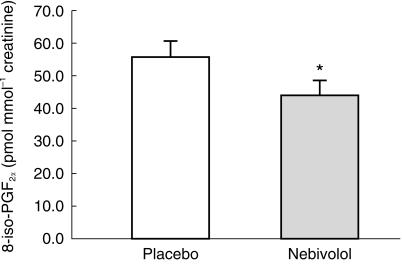
After the 7 day treatment period nebivolol decreased significantly urinary excretion of 8-iso-PGF2α by 24% from 55.3 ± 5.1 pmol mmol−1 creatinine during the placebo period to 42.3 ± 4.7 pmol mmol−1 creatinine (mean ± s.e. mean, P = 0.01), a mean decrease of 13 pmol mmol−1 creatinine (95% CI: −22.8; −3.1).
Conclusions
Our data show for the first time that nebivolol decreases systemic oxidative stress in young healthy volunteers.
Keywords: β-adrenoceptor antagonists, oxidative stress, healthy volunteers
Introduction
Nebivolol, a vasodilating and highly selective β1-adrenoceptor antagonist, has been associated with additional antioxidative effects [1–3]. After hydroxyl radical (OH) induced injury in right ventricular rabbit trabeculae, nebivolol in contrast to propranolol completely abolished blunting of the force-frequency relationship which can normally be observed during the OH injury phase. Hydroxyl radical induced systolic and diastolic dysfunction could be prevented almost completely by nebivolol [3].
Isoprostanes are prostaglandin (PG)-like compounds which are produced by nonenzymatic free radical- catalysed peroxidation of arachidonic acid [4]. Formation of 8-iso-PGF2α, one of the most abundant isoprostanes formed in vivo[5], was found to be increased in animals in which oxidative stress had been experimentally induced as well as in certain cardiovascular disease associated syndromes, e.g. coronary reperfusion with thrombolytic drugs, clamp release in patients undergoing coronary artery bypass grafting [6], hypercholesterolaemia [7] and atherosclerosis [8].
Whether nebivolol decreases oxidative stress after oral doses commonly used in antihypertensive therapy has not been investigated in man. Therefore the present trial was conducted to determine the effect of nebivolol on systemic oxidative stress by measuring urinary excretion of 8-iso-PGF2α in healthy volunteers.
Methods
Subjects
Twelve healthy, non obese volunteers (six male, six female), age (mean ± s.d.) 25.1 ± 2.5 years were included in this study. All had normal clinical history and examination, 12-lead electrocardiogram, haematological and biochemical screen. Only extensive metabolizers of nebivolol were included into the study [9]. None of the volunteers was receiving drugs which might alter free radical status, and dietary habits were constant during the study. Informed consent was obtained to the study protocol previously approved by the local Ethics Committee, and the investigation was conducted in accordance with the Declaration of Helsinki.
Study design
The subjects received in a randomized, double-blind, cross-over design either nebivolol (one tablet, 5 mg daily) or placebo for 7 days. The study periods were separated by a washout phase of 2 weeks. On day 7 of each medication phase, the subjects collected 24h urine for analysis of 8-iso-PGF2α and creatinine.
Quantification of urinary 8-iso-PGF2α
Urinary 8-iso-PGF2α was determined by GC-tandem MS analysis as described previously [10]. Briefly, a 5 ml aliquot of urine was spiked with 5 ng of internal standard [2H 4]-8-iso-PGF2α (Cayman, Ann Arbor, MI, USA), analytes were solid-phase extracted, their pentafluorobenzyl (PFB) derivatives were prepared, separated by thin-layer chromatography, converted to their trimethylsilyl derivatives, and analysed by GC-tandem MS. Quantification was performed by selected reaction monitoring of the product ions at a mass/charge ratio (m/z) of 299 for endogenous 8-iso-PGF2α and m/z of 303 for the internal standard which were generated by collisionally activated dissociation of the parent ions [M – PFB]– at m/z 569 and 573, respectively.
Urinary creatinine levels were determined spectrophotometrically by the alkaline picric acid reaction with an automatic analyser (Beckmann 6641, Galway, Ireland). Urinary excretion rates of 8-iso-PGF2α were corrected for creatinine excretion.
Statistical analyses
All data are given as means ± s.e.mean. Statistical analyses were performed using paired Student's t-test (two-tailed). Statistical significance was assumed for P < 0.05. Data analysis was performed with SPSS (release 9.0.1 for windows).
Results
Following nebivolol administration for a 7 day period urinary excretion of 8-iso-PGF2α decreased significantly from 55.3 ± 5.1 pmol mmol−1 creatinine to 42.3 ± 4.7 pmol mmol−1 creatinine (P < 0.05; Figure 1), a mean decrease of 13 pmol mmol−1 creatinine (95% CI: −22.8; −3.1). Urinary excretion of creatinine was similar in both groups (13.1 ± 1.1 [nebivolol]vs 13.2 ± 1.1 [placebo] mmol/24h; P = 0.82). Urinary 8-iso-PGF2α excretion rates did not correlate with gender or body mass index. Basal levels of urinary 8-iso-PGF2α were in accordance with previously published data [11].
Figure 1.
Creatinine-corrected urinary excretion + s.e. mean of 8-iso-PGF2α in 12 healthy subjects receiving nebivolol 5 mg once daily or placebo. Asterisks indicate significance, i.e. P < 0.05 for nebivolol vs placebo.
Discussion
This study demonstrates that oral administration of nebivolol at standard antihypertensive doses decreases significantly urinary excretion of the isoprostane 8-iso-PGF2α. This finding strongly supports the hypothesis that nebivolol exerts sytemic antioxidative effects. These results are in accordance with a previous in vitro study which showed that nebivolol can effectively prevent a large part of OH induced systolic and diastolic dysfunction in right ventricular rabbit cardiac trabeculae [3].
There is conflicting evidence as to whether other factors such as vitamin E or NSAIDs could possibly effect formation and urinary excretion of 8-iso-PGF2α. Davi et al. found a dose-dependent suppression of enhanced 8-iso-PGF2α formation by vitamin E supplementation in hypercholesterolemic patients [7]. In contrast, supplementation with vitamin E in healthy humans did not affect urinary excretion of 8-iso-PGF2α[12]. In principal the formation of 8-iso-PGF2α in humans seems to be COX-independent [13, 14]. However, in our study supplementation with vitamins or use of NSAIDS was excluded.
Nebivolol has vasodilator activity and there is indirect evidence that this is mediated via the l-arginine/NO system [2]. The finding of the present study that nebivolol decreases systemic oxidative stress supports an interaction between nebivolol and l-arginine/NO system in favour of NO. This interaction could involve inhibition of the production or amplification of the degradation of oxidizing species such as superoxide anion by nebivolol. The consequence of such an interaction would be the availability of NO at higher concentrations despite unchanged NO synthase activity, which would lead to enhanced NO-dependent vasodilation. Such an explanation is attractive since both enantiomers of nebivolol cause vasodilation in human resistance vasculature [2]. This lack of stereospecificity would be consistent with a chemical antioxidant activity of nebivolol [1–3] rather than with mechanisms which involve enzymes or receptors.
Increased oxidative stress, as determined by increased excretion of 8-iso-PGF2α, appears to be an important event in several forms of cardiovascular disease. In human atherosclerosis [8], hypercholesterolemia [7] and coronary reperfusion [6] urinary excretion of 8-iso-PGF2α has been shown to be elevated. Our study shows that oxidative stress expressed in terms of urinary excretion of 8-iso-PGF2α occurs even in healthy humans under basal conditions and can significantly be reduced pharmacologically, for instance by use of nebivolol at therapeutically relevant doses.
The results of the present study provide a rationale for further clinical studies on the antioxidative effects of nebivolol in cardiovascular disease.
Acknowledgments
The excellent laboratory assistance of I. Fuchs is gratefully acknowledged. GC-tandem MS analyses were performed by F.-M. Gutzki. E. Schwedhelm is the recipient of a graduate grant from Hannover Medical School. This study was supported by Berlin Chemie, Berlin, Germany.
References
- 1.Van de Water A, Janssens W, van Neuten J, Xhonneux R, de Cree J. Pharmacological and haemodynamic profile of nebivolol, a chemically novel, potent and selective β1-adrenergic antagonist. J Cardiovasc Pharmacol. 1988;11:552–563. doi: 10.1097/00005344-198805000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Cockcroft JR, Chowienczyk PJ, Brett SE, et al. Nebivolol vasodilates human forearm vasculature: evidence for an l-arginine/NO-dependent mechanism. J Pharmacol Exp Ther. 1995;274:1067–1071. [PubMed] [Google Scholar]
- 3.Janssen PML, Zeitz O, Hasenfuss G. Transient and sustained impacts of hydroxyl radicals on sarcoplasmic reticulum function: protective effects of nebivolol. Eur J Pharmacol. 1999;366:223–232. doi: 10.1016/s0014-2999(98)00907-8. 10.1016/s0014-2999(98)00907-8. [DOI] [PubMed] [Google Scholar]
- 4.Roberts LJ, 2nd, Morrow JD. The generation and actions of isoprostanes. Biochim Biophys Acta. 1997;1345:121–135. doi: 10.1016/s0005-2760(96)00162-2. [DOI] [PubMed] [Google Scholar]
- 5.Morrow JD, Minton TA, Badr KF, Roberts LJ., 2nd Evidence that the F2-isoprostane, 8-epi-prostaglandin F2 alpha, is formed in vivo. Biochim Biophys Acta. 1994;1210:244–248. doi: 10.1016/0005-2760(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 6.Delanty N, Reilly MP, Pratico D, et al. 8-epi PGF2 alpha generation during coronary reperfusion. A potential quantitative marker of oxidant stress in vivo. Circulation. 1997;95:2492–2499. doi: 10.1161/01.cir.95.11.2492. [DOI] [PubMed] [Google Scholar]
- 7.Davi G, Alessandrini P, Mezetti A, et al. In vivo formation of 8-Epi-prostaglandin F2 alpha is increased in hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1997;17:3230–3235. doi: 10.1161/01.atv.17.11.3230. [DOI] [PubMed] [Google Scholar]
- 8.Gniwotta C, Morrow JD, Roberts LJ, 2nd, Kuhn H. Prostaglandin F2-like compounds, F2-isoprostanes, are present in increased amounts in human atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 1997;17:3236–3241. doi: 10.1161/01.atv.17.11.3236. [DOI] [PubMed] [Google Scholar]
- 9.Sachse C, Brockmöller J, Bauer S, Roots I. Cytochrome P450 2D6 variants in a Caucasian population: allele frequencies and phenotypic consequences. Am J Hum Genet. 1997;60:284–295. [PMC free article] [PubMed] [Google Scholar]
- 10.Tsikas D, Schwedhelm E, Fauler J, Gutzki F-M, Mayatepek E. Specific and rapid quantification of 8-iso-PGF2α in urine of healthy humans and patients with Zellweger syndrome by gas chromatography–tandem mass spectrometry. J Chromatogr B. 1998;716:7–17. doi: 10.1016/s0378-4347(98)00275-8. [DOI] [PubMed] [Google Scholar]
- 11.Catella F, Reilly MP, Delanty N, et al. Physiological formation of 8-iso-PGF2αin vivo is not affected by cyclooxygenase inhibition. Adv Prostaglandin Throm Leuk Res. 1995;23:233–236. [PubMed] [Google Scholar]
- 12.Meagher EA, Barry OP, Bensinger S, Rokach J, FitzGerald GA. Effects of chronic therapy with vitamin E on lipid peroxidation in vivo in healthy humans. J Invest Med. 1998;46:204A. [Google Scholar]
- 13.Awad JA, Roberts LJ, 2nd, Burk RF, Morrow JD. Isoprostanes-prostaglandin-like compounds formed in vivo indepenently of cyclooxygenase. Gastroenterol Clinics North Am. 1996;25:409–427. doi: 10.1016/s0889-8553(05)70255-7. [DOI] [PubMed] [Google Scholar]
- 14.Bachi A, Brambilla R, Fanelli R, Bianchi R, Zuccato E, Chiabrando C. Reduction of urinary 8-epi-prostaglandin F2α during cyclooxygenase inhibition in rats but not in man. Br J Pharmacol. 1997;121:1770–1774. doi: 10.1038/sj.bjp.0701321. [DOI] [PMC free article] [PubMed] [Google Scholar]