Abstract
Aims
The aim of this study was to characterize the pharmacodynamics and the pharmacokinetics of S 17092, a new orally active prolyl endopeptidase inhibitor following single and repeated administration in elderly healthy volunteers.
Methods
This was a double-blind, randomized, placebo-controlled, single and multiple dose study in elderly healthy male and female volunteers (n = 36). Four doses were investigated in sequential order: 100, 400, 800 and 1200 mg. Each dose was administered orally once a day in single administration and then, after a 1 week washout period, during 7 days. Pharmacodynamics were assessed by measurement of plasmatic prolyl endopeptidase (PEP) activity, quantitative electroencephalogram (EEG) and psychometric tests. S 17092 concentrations in plasma were quantified by high performance liquid chromatography with tandem mass spectrometric detection.
Results
PEP activity in plasma was dose-dependently inhibited both after administration of a single dose and after repeated doses of S 17092. The mean maximal inhibition was obtained within 0.5–2 h after dosing, while inhibition lasted at least 12 h after dose administration. S 17092 appeared to be a centrally active substance as it induced statistically significant modifications in EEG compared with placebo. S 17092 at 100 mg exerted an acute increase in alpha band following single administration at 4 h and 8 h postdosing. When administered repeatedly over 7 days S 17092 did not appear to induce significant lasting central nervous system (CNS) effects. In psychometric tests, response times in the numeric working memory were significantly reduced compared with placebo, following the 800 mg dose. There were some beneficial residual effects of the 1200 mg dose on day 13: delayed word recall and word recognition sensitivity improved compared with the declines noted under placebo. Maximum measured concentration (Cmax) and area under the curve (AUC) parameters increased in proportion to the dose. The terminal half-life (t½) values ranged between 9 and 31 h on day 1 and between 7 and 18 h on day 14. A high interindividual variability was observed at all dose levels. S 17092 was well tolerated with no clinically significant changes in laboratory or physical parameters observed at any dose.
Conclusions
S 17092 had a potent, dose-dependent inhibitory effect on plasmatic PEP, increased alpha band EEG at the 100 mg dose and improved performance in two verbal memory tests at the 1200 mg dose while there were disruption to the vigilance task. The results obtained in elderly healthy subjects indicated that S 17092 is suitable for once-daily dosing without any serious adverse events.
Keywords: memory enhancer, pharmacodynamics, prolyl endopeptidase inhibitor
Introduction
Various therapeutic strategies to counteract cognitive impairment in Alzheimer's disease have been investi-gated: cholinesterase inhibitors, muscarinic agonists and neuroprotective agents. Factors that could influence the activity or the survival of the cholinergic neurones, as well as those of any other neurotransmitter system that is altered during this disease, are potential therapeutical targets. Among these factors neuromodulators like neuropeptides are of particular interest, as suggested by the following observations. Some neuropeptides such as thyrotropin releasing hormone (TRH) and substance P (SP) have been shown to promote cholinergic function and the release of acetylcholine [1]. It has been demonstrated that several neuropeptides like enkephalin, vasopressin and SP can display neurotrophic actions in vitro and promote processes responsible for the functional recovery following damage to the CNS [2]. Furthermore, a deficiency of SP and vasopressin was reported in postmortem examination of cerebral tissue in dementia associated with neurodegenerative diseases [3–9]. Therefore, any treatment that could positively modulate the brain levels of several neuropeptides together may appear as a promising therapeutic attempt.
PEP is a member of the serine-protease family and it hydrolyses peptide bonds at the l-proline carboxyl terminal. Thus, PEP plays an important role in the catabolism of proline-containing peptides such as SP, arginine-vasopressin, TRH, bradykinin, angiotensin, neurotensin and oxytocin [10].
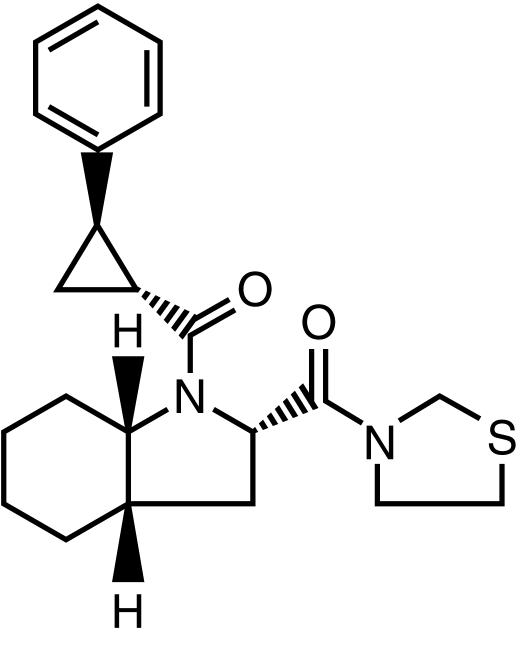
S 17092 (2S, 3aS, 7aS)-1{[(R, R)-2-phenylcyclopropyl] carbonyl}-2-[(thiazolidin-3-yl)carbonyl] octahydro-1H-indole is a recently developed [11] specific prolyl endopeptidase (PEP, EC 3.4.21.26) inhibitor (Figure 1).
Figure 1.
The chemical structure of S 17092.
Preclinical data have indicated that the chronic administration of S 17092 in rodents alleviated scopolamine-induced deficits in passive avoidance and in a spatial discrimination task in a Y maze. Treatment with S 17092 also increased the performance of aged mice in a delayed alternation task and those of normal rats in a social recognition test [12]. Finally, the S 17092 treated old mice performed to a level comparable with the young mice in an age-related relational memory impairment in an eight-arm radial maze [13]. While the tolerability and the pharmacokinetics of S 17092 have been assessed following single dose administration in healthy young volunteers, the present study was designed to characterize the tolerability, pharmacodynamics and pharmacokinetics of S 17092 associated with single and repeated administration to elderly healthy volunteers.
Methods
Subjects
The study population was comprised of 40 (including four replacements) elderly, healthy, nonsmoking men (24) and women (16). The mean age was 60.5 years (s.d. 4.0 years, range 55–71 years) the mean weight was 75.9 kg (s.d. 8.4 kg, range 61.5–99.9 kg), the mean body height was 172.5 cm (s.d. 7.2 cm, range 158–186 cm) and the mean body mass index was 25.5 kg m−2 (s.d. 2.7 kg m−2, range 19.7–30.8 kg m−2). The ratio of males and females in the different groups were 4/4, 3/4, 5/1, 4/3 and 8/4 for the treatment group receiving 100 mg, 400 mg, 800 mg, 1200 mg and placebo, respectively. No differences were observed between the treatment groups with respect to age.
Upon clinical examination, volunteers were found to be free from significant hepatic, gastrointestinal, renal, respiratory, endocrine, haematological, neurological, psychiatric or cardiovascular system abnormalities. The following laboratory determinations were assessed: blood sodium, potassium, calcium, phosphate, chloride, urea, creatinine, albumin, total protein, total bilirubin, triglycerides, cholesterol, gamma-glutamyl transferase, alanine transferase, aspartate transferase, alkaline phosphatase, creatine kinase, blood erythrocytes, haemoglobin, white cell count with differential count, platelets and urine analysis.
None of the subjects had a known or suspected history of alcohol or drug abuse. No medications had been taken within 1 month prior entry into the study.
This study was conducted in accordance with the principles stated in the Declaration of Helsinki. The protocol was approved by the Medical Ethics Commitee of Assen, The Netherlands and all subjects gave written informed consent prior to participation.
Protocol
The study was double-blind, randomized and placebo-controlled. S 17092 was administered in oral doses of 100, 400, 800 and 1200 mg in single administration on day 1 and, after a 1 week washout period, in repeated administration once daily on days 8 through 14. The dose range chosen for the study was based upon results of a single dose study performed on healthy young subjects.
Within each cohort of nine volunteers, three were randomised to receive placebo and six to receive S 17092. Progression to the next higher dose level required the demonstration that the previous dose had been well tolerated.
The drug was taken in the early morning after an overnight fast. The volunteers were required to abstain from smoking, any use of alcohol or physical activity throughout the study period. Consumption of decaffeinated tea or coffee was limited to six cups a day. The subjects were hospitalized 2 days before the single administration for a total of 5 days and were asked to come back for repeated administration on day 7 for a total of 11 days until all the follow-up assessments had been completed.
Sample collection
Venous blood samples (7 ml) for the measurement of S 17092 concentrations in plasma and assessment of plasma PEP activity were collected in heparinized tubes on day 1 and day 14 before and 10, 20, 30, 45, 60 min, 1.5 h, 2 h, 3 h, 4 h, 5 h, 6 h, 8 h, 12 h, 24h, 36 h and 48 h after drug administration. Additional blood samples were collected on day 14 at 60 h, 72 h and 96 h after drug administration. On day 8, day 9, day 10, day 11, day 12 and day 13 a single sample was collected just prior to drug administration. Blood samples were placed on ice immediately after collection. Plasma was then separated by centrifugation and stored at −20 °C and thawed shortly before analysis.
Pharmacokinetic analysis
Plasma concentrations of S 17092 were quantified using a specific and sensitive high-performance liquid chromatography and tandem mass spectrometric (MS/MS) detection method previously validated at the Department for Mass Spectrometry of Pharma Bio-Research Laboratories. The lower limit of quantification was 1 ng ml−1 for S 17092 using 500 µl of plasma. The upper limit of quantification was 200 ng ml−1. The calibration curve was linear over this concentration range.
Due to the irregularity of the profiles, a model-independent analysis of S 17092 individual pharmacokinetic profiles was carried out to determine the following parameters: Cmax (maximum measured concentration), tmax (time associated with the first occurrence of Cmax), AUCt(area under the plasma concentration-curve from time 0 to the time of the last measurable concentration), AUC (area under the curve extrapolated to infinity), t½,z (apparent terminal half-life), Cmin (predose concentration), Rac (accumulation ratio).
Statistical procedure
Regarding the parameters presumed to be dose-dependent, a linear regression was performed between log-transformed parameters (Cmax, AUC on day 1 and AUC(0,24h) on day 14) and the logarithm of the dose. If the slope was significantly different from unity (at 5% level), this indicated a departure from linearity and data at extreme doses were ignored to identify a dose range where linearity of pharmacokinetics was verified. If the slope was not significantly different from unity, a direct proportionality of the dose parameter relationships was concluded. A linear regression forced through the origin was repeated on untransformed data.
Regarding the parameters presumed to be dose-independent (parameters investigated: t½, z and tmax), a Bartlett test was performed to check the homogeneity of variance for parameters t½, z. A two-way anova was then performed to test the possible influence of the factors subject and dose on mean parameters. When a significant dose-effect was found the anova was completed by a Newman-Wallis test for pairwise comparison of the means.
To assess the attainment of steady-state, an evaluation by means of an analysis of variance was performed to determine if Cmin values differ from each other.
Pharmacodynamic assessments
PEP activity
The determination of PEP activity in plasma was performed according to a method previously described [14]. This technique was highly specific for PEP. Briefly, the assay was based on the incubation of plasma with the synthetic substrate Z-glycyl-propyl-4-methylcoumarinyl-7-amide in a potassium phosphate buffer. After a 120 min incubation at 37 °C the reaction was stopped with acetic acid and the release of 7-amino-4-methyl-coumarine (AMC) was measured fluorimetrically and expressed as fluorescence arbitrary units (FAU). A control 0% value was obtained under the same experimental conditions but without initiation of the reaction.
The control FAU was subtracted from the total FAU value. The in vitro linear relationship between FAU and AMC concentration was established separately. The resulting equation was calculated and used to determine the concentration of AMC that was released by PEP activation. PEP activity was expressed in nmol/ml.min. The detection limit, defined as the enzyme activity that could be determined with a coefficient of variation smaller than 10% within a single run was less than 0.1 U l−1. The effect of S 17092 on the PEP activity was assessed on blood samples collected predose and 0.5, 1 h, 2 h, 6 h and 12 h after dosing on day 1 and day 14. The doses 100, 400 and 800 mg were analysed.
Statistical procedure
The minimum PEP activity up to 12 h after dosing and the PEP activity at 12 h after dosing on day 1 and day 14 were analysed by means of two separate one-way anovas with treatment as effect parameter. Differences were considered significant at a 5% level. A significant treatment was further analysed by means of a multiple t-test where each of the active treatments was compared with placebo. The Dunnett's multiplicity correction was applied.
PEP activity and S 17092 plasma concentration
No pharmacokinetic model was attempted. Nevertheless, since concentration measurements were made at the same time points as PD (PEP activity) measurements, a PK/PD analysis was performed. The placebo data per time point were averaged. Subsequently, for each subject, the PD measurements were corrected for placebo. The corrected PD values and concentration data of all subjects were entered into the data base and a population modelling was performed. A direct inhibitory Emax model was applied: E = E0. (1 − Emax.C/C + C50) where Emax is the maximum effect possible, C50 is the concentration producing 50% of the maximum inhibition of effect and E0 is the effect when no drug is present. The parameters of the Emax equation were estimated using the computer program NONMEM version 5.1 (Non Linear Mixed Effect Models) [15].
Electroencephalography (EEG)
During the screening period, subjects were assessed on EEG to ensure that they met the study inclusion criteria for these measures. Subjects had to yield an EEG recording that allowed for a minimum of eight 4 s artifact-free epochs to be analysed, and the resulting spectra from the occipital recordings had to exhibit a minimum of 30% alpha and a dominant peak frequency of 8.5 Hz or more.
As per protocol, the EEG recordings were carried out on day 1 and day 14 in each group prior to the morning dose and again at 4 and 8 h after dosing. All volunteers were exposed to the electrode placement procedure once during the selection (day −21 to day-1) period. Electrical activity was processed with a Grass Neurodata Model 12 amplifier system, digitized with a Burr-Brown PC-2000 AD converter and stored on IBM-PC compatible hard drive.
The EEG was recorded from four amplifiers with tin electrodes positioned on left and right occipital sites and on left and right frontal scalp site regions using linked-earlobe electrodes for monopolar reference recordings. One electrode was placed on the mid-forehead to serve as ground and two additional electrodes were positioned over the supraorbital ridge and external canthous of one eye to monitor vertical and horizontal electro-oculographic artifacts induced by eye movements and blinks. The EEG activity was recorded over a 3 min eyes-closed resting period.
The EEG signals were visually assessed off-line and a minimum of eight artifact-free, 4 s duration epochs were subjected to Fast Fourier Transform analysis for computation of spectral data.
The following spectral EEG parameters were analysed: absolute power for delta, theta, alpha1, alpha2, beta1, beta2 and total band power; relative power for the same spectral bands expressed as a percentage of the entire total band power; alpha/theta ratio; peak frequency in the theta-alpha band limits; and left/right hemisphere asymetry ratios for each of the mentioned parameters.
Absolute and relative EEG amplitude values were log transformed to approximate normal distributions prior to statistical analysis.
Statistical procedure: EEG measures were acquired at a single pretreatment (baseline) time point and at each of the specified postdosing time points following acute administration on day 1 and day 14. Statistical analysis were carried out to assess and compare the central effect of S 17092 vs placebo separately on both day 1 and day 14 (i.e. acute dosing effect), and to assess and compare the central effects of repeated S 17092 vs placebo administration throughout day 8-day 14 (i.e. repeated dosing effect) by comparing baseline EEG measures on day 1 vs day 14.
Single dose effects were assessed by analysing difference score data derived on each of the two test days (day 1 and day 14) by subtracting pretreatment baseline values from post-treatment values at each time point. Separate split-splot analysis of variance procedures involving drug, time and recording site factors were carried out on each EEG measurement on each separate day. Significant (P < 0.05) main effects and interaction effects were followed up using the Student-Newman-Keuls test, the Tukey multiple range test, or t-test.
Repeated dosing effects of S 17092 throughout day 8 – day 14 were assessed by comparing the EEG measurements observed at the pretreatment baseline recordings of day 14 with the recordings observed at the pretreatment baseline recordings of day 1. Effects were assessed by analysing difference score data derived by subtracting the pretreatment baseline values of day 1 from the pretreatment baseline values of day 14. Separate split-splot repeated measures anova procedures involving drug and recording site were carried out on each measurement index. Significant (P < 0.05) main effect and interaction effects were followed up using the Student-Newman-Keuls test, the Tukey multiple range test, or t-test.
Psychometric tests
Cognitive functions were assessed by the CDR computerized cognitive assessment system (Cognitive Drug Research, UK).
Training on the CDR system took place prior to the first day of the trial in order to familiarize the subjects with the procedure and overcome the initial learning variability. Four training sessions were completed by each subject, two being conducted during the screening period and two being conducted on the evening of day −1.
Cognitive assessments were conducted by the subjects on study day 1, day 8 and day 13 at predose, and 2 and 6 h postdose.
A selection of tasks from the CDR system was administered, parallel forms of the tests being presented on each testing session. All tasks were computer-controlled. The tests were administered in the following order: Immediate Word Recall, Picture Presentation, Simple Reaction Task, Digit Vigilance Task, Choice Reaction Task, Spatial Working Memory, Numeric Working Memory, Delayed Word Recall, Delayed Word Recognition, Delayed Picture Recognition.
Statistical procedure
Analysis of performance on day 1
The predosing scores were subtracted from the postdosing scores to obtain the difference from the baseline scores.
Analysis of performance on day 13
The predosing score on day 8 was subtracted from the postdosing score on day 13 to obtain the difference from the baseline scores.
These data were subjected to a split-splot anova, taking the repeated assessments over the day as a within subject factor and the dose of S 17092 as the between subject factor. The SAS procedure GLM was used to conduct the analyses. The 5% level of significance was adopted, and two-tailed testing was employed.
Analysis of carry-over from day 1 to day 8
To identify any carry over from day 1 to day 8, the predosing scores on day 1 and day 8 were subjected to a split-splot anova, taking the repeated assessments over days as a within subject factor and the dose of S 17092 as the between subject factor. The SAS procedure GLM was used to conduct the analyses. The 5% level of significance was adopted, and two-tailed testing was employed.
Analysis of residual effects from day 8 to day 13
to identify any residual effects on day 13, it was compared with the predosing scores on day 8. The data were subjected to a split-splot anova, taking the repeated assessments over days as the within subject factor and the dose of S 17092 as the between subject factor. The SAS procedure GLM was used to conduct the analyses. The 5% level of significance was adopted, and two-tailed testing was employed.
Safety assessments
Enquiries were made twice daily as to whether the subject had experienced any adverse events by asking a general question. Any non critical adverse event was recorded and the following information was obtained for each event: description, date and time of onset and stop, intensity, causal relationship, outcome and possible treatment prescription, and follow-up of the participant.
Blood pressure and pulse rate were recorded after the subject had been in a supine position for 5 min, and after being in a standing position for 2 min. The respiratory rate and oral body temperature were also evaluated. Vital signs measurements were performed on day 1 and before dosing, and 10 min and 0.5, 1, 4 and 12 h after each dose administration as well as 24h after dosing on day 1. Oral body temperature was measured on day −1 and before dosing and 2 and 6 h after dosing on day 1 and day 14 as well as 24h after dosing on day 1.
A 12-lead ECG of at least 5 beats was registered on day −1 and before and 2, and 8 h after each dosing. ECG was also recorded continuously (telemetry) for 8 h after drug administration on day 1, day 11 and day 14.
Laboratory safety tests were performed at the prestudy screening and on the day −1 and day 15.
Descriptive statistics were presented for all subjects who received one or more dose administration of S 17092 or placebo.
Results
Pharmacokinetics
Model-independent pharmacokinetic parameters for S 17092 are summarized in Table 1. Median tmax values ranged from 0.75 to 5 h after administration among the four doses. Cmax, AUC on day 1 and AUC on day 14 increased in proportion to the dose (Cmax= 0.225 x dose, AUC on day 1 = 5.03 x dose, AUC on day 14 = 3.35 x dose).
Table 1.
Mean pharmacokinetic parameters of S 17092 after single (day 1) and repeated (day 8 to day 14) oral administration of S 17092 to healthy elderly volunteers.
| Mean | |||||
|---|---|---|---|---|---|
| Day | Parameters | 100 mg | 400 mg | 800 mg | 1200 mg |
| 1 | Cmax (ng ml−1) | 31.4 | 68.6 | 152 | 292 |
| tmax (h) | 5* | 1* | 1.5* | 2.75* | |
| AUC(0,24h) (ng ml−1 h) | 534 | 862 | 2485 | 4510 | |
| t½,z (h) | 14.2 | 31.1 | 8.97 | 14.6 | |
| AUC (ng ml−1 h) | 939 | 1307 | 3705 | 6424 | |
| 8 | Cmin (ng ml−1) | BLQ | BLQ | BLQ | BLQ |
| 9 | Cmin (ng ml−1) | 17.9 | 30.6 | 96.6 | 159 |
| 10 | Cmin (ng ml−1) | 23.2 | 37.3 | 104 | 150 |
| 11 | Cmin (ng ml−1) | 20.5 | 52.3 | 93.9 | 131 |
| 12 | Cmin (ng ml−1) | 24.9 | 47.5 | 106 | 132 |
| 13 | Cmin (ng ml−1) | 25.9 | 42.5 | 81.6 | 151 |
| 14 | Cmin (ng ml−1) | 26.8 | 45.5 | 71.9 | 114 |
| Cmax (ng ml−1) | 56.2 | 111 | 158 | 286 | |
| tmax (h) | 1* | 0.71* | 0.75* | 0.75* | |
| t½,z (h) | 15.9 | 18.1 | 6.8 | 13 | |
| AUC24 (ng.ml−1 h) | 820 | 1420 | 2534 | 4043 | |
| Rac | 1.36 | 1.8 | 0.98 | 0.94 | |
BLQ: below the limit of quantification (1 ng ml−1)
median.
The values of t½ were dose-independent and ranged between 9 and 31 h on day 1 and between 7 and 18 h on day 14. Stability of Cmin values between day 9 and day 14 showed that steady state was reached. At all dose levels a high interindividual variability was observed.
Pharmacodynamics
PEP activity
Lower mean values for the minimum PEP activity and the PEP activity at 12 h were found after single and repeated administration of S 17092 as compared with placebo. Further, the mean values, particularly at t = 12 h, showed a clear dose dependent reduction of PEP activity. After single dose administration (day 1), the minimum PEP activity values and PEP activity at 12 h after active treatment were significantly lower (P < 0.05) than placebo, except for the 12 h value after a single dose of 100 mg S 17092.
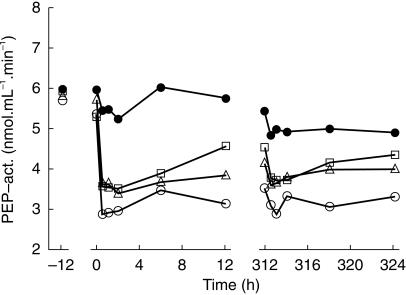
After multiple dose administration no significant differences were found for the 100 mg and 400 mg dose group, whereas for the 800 mg dose group both the minimum PEP activity and the PEP activity at t = 12 h were significantly (P < 0.05) different from placebo (Figure 2). No clear difference was observed for the mean PEP activity on various time points on day 1 and day 14 for the active dose levels. PEP activity in plasma decreased rapidly after oral dose administration of S 17092 showing a mean maximal decrease in activity in 0.5–2 h after dosing both on day 1 and day 14.
Figure 2.
Mean values on PEP activity after single dose administration on day 1 and repeated dose administration on days 8–14 in elderly healthy volunteers. At day 1 the minimum PEP activity values (in nmol ml−1 min−1) were: 4.9 ± 1.1, 3.3 ± 0.9, 3.3 ± 0.6, 2.8 ± 0.6 for placebo (•), 100 mg (□), 400 mg (▵) and 800 mg (○) dose group, respectively. The PEP activity values at 12 h were (in nml ml−1 min−1) were: 5.7 ± 1.1, 4.5 ± 1.6, 3.8 ± 0.5, 3.1 ± 0.9 for placebo, 100 mg, 400 mg and 800 mg dose group, respectively.At day 14 the minimum PEP activity values (in nmol ml−1 min−1) were: 4.5 ± 1.2, 3.5 ± 0.8, 3.5 ± 0.4, 2.8 ± 0.8 for placebo, 100 mg, 400 mg and 800 mg dose group, respectively. The PEP activity values at 12 h (in nmol ml−1 min−1) were: 4.9 ± 1.2, 4.3 ± 1.1, 4.0 ± 0.4, 3.3 ± 0.8 for placebo, 100 mg, 400 mg and 800 mg dose group, respectively.
PK/PD analysis
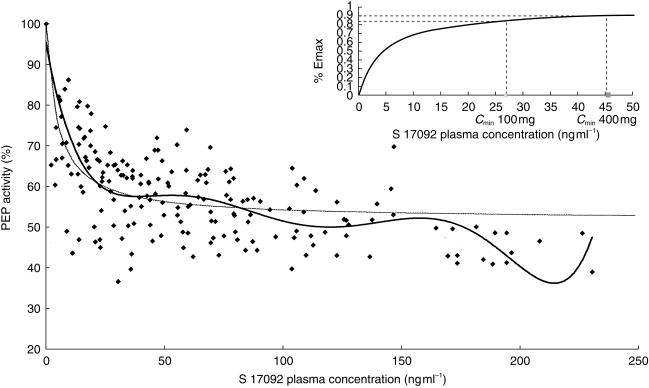
The effect-plasma concentrations profiles for all subjects were well described by the direct inhibitory Emax (maximal PEP inhibition) model, as expected for enzyme activity (Figure 5). Estimates from the Emax model in this population suggested an Emax of 47.8 ± 1.4% with a mean plasma concentration of 4.8 ± 5.0 ng ml−1 required to reduce Emax by 50% (C50).
Figure 5.
PEP activity (%) vs S 17092 plasma concentration, ♦ observed individual data. Thick line: tendency curve of the observed individual data, described by a polynominal relationship of the sixth degree. Thin line: population model described by the inhibitory Emax equation. Insert in right upper corner: % Emax vs S 17092 plasma concentration.
The mean predicted individual values of Emax and C50 for the 100, 400 and 800 mg doses, respectively, equal to 47.8 ± 0.2% and 6.0 ± 4.8 ng ml−1; 47.8 ± 0.4% and 6.7 ± 3.9 ng ml−1; 48.1 ± 0.2% and 5.4 ± 3.6 ng ml−1, are in good agreement with the estimated values of the population PK/PD model.
Electroencephalography
Single dosing day 1
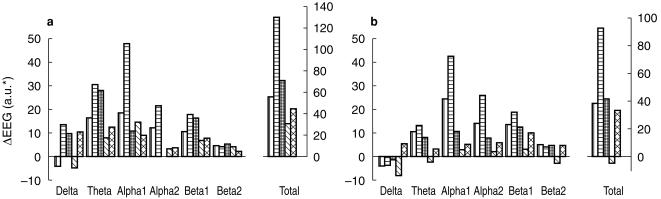
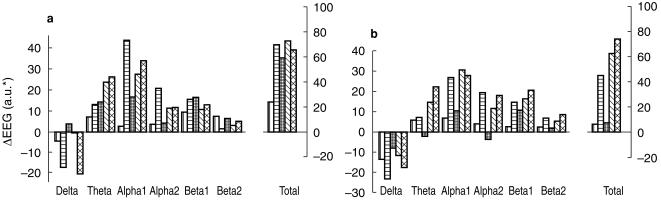
Statistical analysis showed a main drug effect on alpha 1 (F = 2.85, d.f. = 4.31, P < 0.05), alpha 2 (F = 3.44, d.f. = 4.31, P < 0.05), beta 1 (F = 3.25, d.f. = 4.31, P < 0.05) and total (F = 3.15, d.f. = 4.31, P < 0.05) absolute amplitude measures (Figure 3). Follow-up tests revealed that significantly greater amplitude increases were produced by the 100 mg dose compared with placebo and to the 400 mg, 800 mg and 1200 mg doses (P < 0.05) on alpha 1 and that the 100 mg produced significantly greater amplitude increases than the 800 and 1200 mg doses (P < 0.05) on beta 1.
Figure 3.
E; EG absolute power. Single dose effects on day 1. a) 4 h post dosing - pre-dosing, b) 8 h post dosing - pre dosing.  placebo;
placebo;  100 mg,
100 mg,  400 mg;
400 mg;  800 mg;
800 mg;  1200 mg S 17092.o
1200 mg S 17092.o
With total amplitude follow-up tests the 100 mg dose produced significantly greater amplitude increases than the 800 mg dose (P < 0.05). A significant drug effect was also seen with the alpha/theta ratio (F = 3.31, d.f. = 1.26, P < 0.05). Follow-up tests of this effect indicated that the 1200 mg dose produced greater increases in the ratio than placebo (P < 0.05).With respect to relative amplitude measures, only a significant two-way (drug by site) interaction was observed with beta 2, and follow-up tests failed to reveal any specific drug effects. No significant drug effects were observed with peak frequency or hemispheric asymmetry ratios.
Single dosing day 14
Statistical analysis showed a main drug effect on alpha 1 (F = 3.70, d.f. = 4.29, P < 0.05) and alpha 2 (F = 4.05, d.f. = 4.29, P < 0.01) amplitude measures (Figure 4). Follow-up tests revealed that the 100 mg dose produced significantly greater amplitude increases than placebo (P < 0.05) on alpha 1 and that the 100 mg dose produced greater amplitude increases than both placebo and 400 mg (P < 0.05) on alpha 2. A significant drug by site interaction (F = 2.11, d.f. = 12.87, P < 0.05) with alpha 1 suggested that drug effects on this band were dependent upon recording site. Follow-up tests indicated that 100 mg dose resulted in significantly greater alpha 1 amplitude increases compared to placebo at both left and right occipital sites (P < 0.05) but not at left and right frontal sites. No significant drug effects were observed with peak frequency, alpha/theta ratios or hemispheric asymmetry ratios. Analysis of relative EEG differences scores resulted in significant drug effects for alpha 1 (F = 3.07, d.f. = 4.29, P < 0.05) and alpha 2 (F = 2.86, d.f. = 4.29, P < 0.05). Follow-up tests showed that the 100 mg dose resulted in significantly greater relative amplitude increases than placebo (P < 0.05) on alpha 1 and that the 100 mg dose produced greater relative amplitude increases than the 400 mg dose (P < 0.05) on alpha 2.
Figure 4.
E; EG absolute power. Single dose effects on day 14. a) 4 h post dosing - pre-dosing, b) 8 h post dosing - pre dosing.  placebo;
placebo;  100 mg,
100 mg,  400 mg;
400 mg;  800 mg;
800 mg;  1200 mg S 17092.
1200 mg S 17092.
Repeated dosing
No significant drug effects were evident with respect to any of the EEG study measures except for hemispheric absolute theta asymmetry. Follow-up of a significant drug by site interaction (F = 2.99, d.f. = 4.30, P < 0.05) showed that the 1200 mg dose produced a significantly greater decrease (P < 0.05) in the asymmetry index compared with the 400 mg dose due to a reduction of theta in the left hemisphere.
Psychometric tests
Analysis of performance on day 1
There were no significant main effects of dose, nor interactions between dose and the repeated assessment.
Analysis of performance on day 13
There was one significant main effect of dose for the percentage of correct detections in the digit vigilance task. Multiple comparisons using the LSMEANS statement showed this to reflect a lowering of detections with the 1200 mg dose compared with placebo (a drop of 5.3 compared with a rise of 0.03 in placebo; P < 0.01). There was a significant interaction between dose and the repeated assessment for the speed of detections in the vigilance task multiple comparisons using the LSMEANS statement showed the effect for the speed of detections in the vigilance task to reflect slowings of speed in the 100 and 1200 mg doses compared with placebo. For 100 mg, at 2 h, there was a slowing of speed of 42 msec compared to 6 msec with placebo (P < 0.01).
For 1200 mg, there was a significant slowing at 2 h of 29 ms compared with 6 after placebo (P < 0.01), as well as a significant slowing at 6 h of 37 ms compared with no change under placebo at this time (P < 0.01). There were no significant carry-over effects from day 1 to day 8.
There were two interactions between day and dosing condition, indicating that for these measures there were possible evidence of residual effects on day 13. The measures were delayed word recall and word recognition sensitivity. For delayed word recall, the basis for this interaction was an increase in the 1200 mg group from 18% of words recalled correctly on day 8–27% on day 13, whereas under placebo, the score dropped from 24% to 19% over this period. For word recognition sensitivity, there was a similar pattern between placebo and 1200 mg, placebo dropping from 0.52 to 0.50 whereas 1200 mg rose from 0.31 to 0.39. However, there were also drops with 100 mg from 0.53 to 0.29 and with 800 mg from 0.61 to 0.36.
Tolerability
Neither serious nor severe adverse events were observed. Most adverse events were of mild intensity. Five adverse events were of a moderate intensity (4 cases of headache, 1 case of gastro-enteritis), all of these were considered to have a nonobvious or doubtful relation to any of the study treatments. Three adverse events were considered to be possibly related to the study treatments: nausea (1 subject receiving 400 mg and 1 subject receiving 1200 mg) and dizziness (1 subject receiving 1200 mg).
The most frequently reported adverse events, considered to have a nonobvious or doubtful relationship with the study medication, were headache (24 reports), general gastro-intestinal complaints (18 reports), somnolence or fatigue (9 reports), upper respiratory tract infection or irritations (8 reports).
Considering vital signs, respiration rate and oral body temperature, no clinically relevant changes were observed from baseline after any of the study treatments. ECG recordings obtained before and at regular intervals during the study and telemetric heart monitoring did not show clinically relevant abnormalities or changes from baseline that were considered to be related with the study treatments.
There were no changes of laboratory results outside the reference range that were considered to have clinical implications.
Discussion
This study provided evidence for the first time in humans that the pharmacodynamics of a prolyl endopeptidase inhibitor can be assessed through its action on plasmatic PEP activity. Although the percentage of inhibition was not obviously different between doses, the duration of the inhibition showed a clear tendency to be dose-dependent. The concentration-effect data were well approximated by an Emax model, which predicted that for an effect equal to 90% of Emax the drug concentration is 43.9 ng ml−1. This value indicates that the 400 mg dose in repeated administration would be sufficient to obtain a stable E90 (90% of Emax) over the time because the mean minimal concentration of this dose Cmin is 45.5 ng ml−1. However, without integrating the pharmacokinetic parameters the only dose group showing significant difference in PEP activity from placebo at steady state conditions is the 800 mg group.
PEP, which is the target enzyme of S 17092, was long-lastingly inhibited while the administered dose was well-tolerated. Analysis of the concentrations of neuropeptides of which breakdown is thought to be inhibited by the PEP (e.g. substance P, vasopressin, thyrotropin-releasing hormone) will be necessary in a future study to establish whether the inhibition of PEP in plasma as observed in the present study is sufficient to have an effect on the concentrations of these peptides.
The central effectiveness of S 17092 was assessed by means of quantitative EEG as an index of drug penetration into the human brain and to describe time and dose effects of S 17092. Although the EEG data from the rather small group sample size showed marked variability, the exploratory analysis yielded significant drug effects which can be tentatively interpreted as follows: S 17092 appears to be a centrally active substance as it induces statistically significant alterations in EEG compared with placebo. No dose–response relationship was observed as only the 100 mg dose showed EEG effect and the lack of effect in EEG recordings with the 400, 800 and 1200 mg doses is difficult to interpret so far. One possibility is that the 100 mg dose had maximal effect and higher doses produced deleterious effect (inverted U-shape curve). Future studies will be necessary to test the effect of lower dose of S 17092. Another possibility is that a longer duration study is needed to better define a dose-effect relationship (see the alpha 1 band increase tendency with higher doses at day 14 as compared with day 1).
S 17092 at 100 mg induces EEG changes that can be described as ‘vigilance promoting’ since alpha was augmented following single dosing. Although accompanied by amplitude reductions in delta and theta, alpha increases have also been observed with psychostimulant and cognitive-enhancing agents as well as with nicotinic agonists.
No clear dose–response relationship was observed for psychometric measurements. Upon a selection of 10 tasks from the CDR assessment system there were beneficial effects of the 1200 mg dose on two verbal memory tasks while there were disruption to the vigilance task.
The apparent disagreement between the effective dose in EEG recordings and the effective dose in these two verbal tests should be interpreted with caution since it is difficult to show a memory improvement in healthy volunteers especially during very short duration trial. It will be of interest to define an effective dose in memory impaired patients and to perform a more quantitative EEG study in order to better precise the relationship between these two pharmacodynamic parameters. Concerning the relationship between the effective dose in PEP inhibition and the effective dose in psychometric tests an hypothesis could be that the PEP inhibition duration more closely correlates with memory improvement than with the maximal PEP inhibition per se.
This phase I study introduces a new prolyl endopeptidase inhibitor S 17092 and indicates that it is a well-tolerated compound with clear peripheral expression of its mechanism of action after once-daily administration, and with central effects as evidenced by EEG at doses compatible with the PEP inhibition. Furthermore the residual improvements on delayed verbal memory task with the 1200 mg dose may suggest that the compound will have favourable effects on memory. This needs to be confirmed in further studies with memory impaired patients.
Acknowledgments
We gratefully acknowledge Dr J.J. Van Lier, Pharma Bio-Research International, who conducted this clinical trial, Dr K. Wesnes (Cognitive Drug Research, UK), Dr V.J. Knott and Dr B. Gueguen (EEG analysis), J.P. Jeanniot, H. Merdjan, O. Lhote and M. Giraudon (Pharmacokinetic analysis) and P.A. Boyer for helpful comments on the manuscript.
The plasma PEP inhibition was assessed by E. Ruedas (ANSAB laboratory).
References
- 1.Bennett GW, Ballard TM, Watson CD, Fone KCF. Effect of neuropeptides on cognitive function. Exp Gerontol. 1997;32:451–469. doi: 10.1016/s0531-5565(96)00159-3. 10.1016/s0531-5565(96)00159-3. [DOI] [PubMed] [Google Scholar]
- 2.Dekker A, Gispen WH, De Wied D. Axonal regeneration, growth factors and neuropeptides. Life Sci. 1987;41:1667–1678. doi: 10.1016/0024-3205(87)90593-5. [DOI] [PubMed] [Google Scholar]
- 3.Crystal MA, Davies P. Cortical substance P-like immunoreactivity in cases of Alzheimer's disease and senile dementia of Alzheimer type. J Neurochem. 1982;38:1781–1784. doi: 10.1111/j.1471-4159.1982.tb06665.x. [DOI] [PubMed] [Google Scholar]
- 4.Beal FM, Mazurek MF. Substance P-Like immunoreactivity is reduced in Alzheimer's disease cerebral cortex. Neurology. 1987;37:1204–1209. doi: 10.1212/wnl.37.7.1205. [DOI] [PubMed] [Google Scholar]
- 5.Quigley BJ, Kowall NW. Substance P-like immunoreactive neurons are depleted in Alzheimer's disease cerebral cortex. Neurosci. 1991;41:41–60. doi: 10.1016/0306-4522(91)90199-x. [DOI] [PubMed] [Google Scholar]
- 6.Fujiyoshi K, Suga H, Okamoto K, Nakamura S, Kameyama M. Reduction of arginine-vasopressin in the cerebral cortex in Alzheimer type senile dementia. J Neurol Neurosurg Psychiatry. 1987;50:929–932. doi: 10.1136/jnnp.50.7.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazurek MF, Beal FM, Bird ED, Martin JB. Vasopressin in Alzheimer's disease: a study of post-mortem brain concentrations. Ann Neurol. 1986;20:665–670. doi: 10.1002/ana.410200603. [DOI] [PubMed] [Google Scholar]
- 8.Augood SJ, Faull RLM, Loves DR, Emson PC. Reduction in enkephalin and substance P messenger RNA in the striatum of early grade huntington's disease: a detailed cellular in situ hybridization study. Neurosci. 1996;72:1033–1036. doi: 10.1016/0306-4522(95)00595-1. [DOI] [PubMed] [Google Scholar]
- 9.Gottfries CG, Frederiksen SO, Heilig M. Neuropeptides and Alzheimer's disease. Eur Neuropsychopharmacol. 1995;5:491–500. doi: 10.1016/0924-977x(95)00038-q. 10.1016/0924-977x(95)00038-q. [DOI] [PubMed] [Google Scholar]
- 10.Portevin B, Benoist A, Rémond G, Hervé Y, Vincent M, Lepagnol J, De Nanteuil G. New prolyl endopeptidase inhibitors: in vitro and in vivo activities of azabicyclo [2. 2.2.] octane, azabicyclo [2.2.1.] heptane, and perhydroindole derivatives. J Med Chem. 1996;39:2379–2391. doi: 10.1021/jm950858c. [DOI] [PubMed] [Google Scholar]
- 11.Wilk S. Minireview Prolyl endopeptidase. Life Sci. 1983;33:2149–2157. doi: 10.1016/0024-3205(83)90285-0. [DOI] [PubMed] [Google Scholar]
- 12.Lepagnol J, Lebrun C, Morain P, De Nanteuil G, Heidet V. Cognition enhancing effects of S 17092, a potent and long acting inhibitor of post-proline cleaving enzyme. Soc Neurosci Abstract. 1996;22:142. [Google Scholar]
- 13.Marighetto A, Touzani K, Etchamendy N, Jaffard R, De Nanteuil G, Guez D, Robin JL, Morain P. Effect of S 17092 on an age-related cognitive deficit in mice. Soc Neurosci Abstract. 1998;24:686. [Google Scholar]
- 14.Goossens F, De Meester I, Wanhoof G, Scharpé S. A sensitive method for the assay of serum prolyl Endopeptidase. Eur J Clin Chem Clin Biochem. 1992;30:235–238. [PubMed] [Google Scholar]
- 15.Beal SL, Boeckman AJ, Sheiner LB NONMEN. San Francisco Division of Clinical Pharmacology University of California; user's guide parts I-VIII. [Google Scholar]