Abstract
Aims
To estimate the rates of common adverse events in patients treated with the proton pump inhibitors omeprazole, lansoprazole and pantoprazole in general practice in England.
Methods
In prescription-event monitoring cohort studies, data on dispensed prescriptions prescribed by general practitioners in England soon after each drug was launched were linked to subsequent clinical events recorded by the prescriber. 16 205 patients prescribed omeprazole between June 1989 and June 1990, 17 329 patients prescribed lansoprazole between May and November 1994, and 11 541 patients prescribed pantoprazole between December 1996 and June 1997 were studied.
Results
The commonest adverse events in the omeprazole, lansoprazole and pantoprazole cohorts were diarrhoea (incidence: 0.18, 0.39 and 0.23 per 1000 days of exposure, respectively); nausea/vomiting (incidence: 0.16, 0.22 and 0.18 per 1000 days of exposure, respectively); abdominal pain (incidence: 0.17, 0.21 and 0.17 per 1000 days of exposure, respectively); and headache (incidence rates: 0.10, 0.17 and 0.15 per 1000 days of exposure, respectively). The remaining adverse events occurred at rates of less than 0.11 per 1000 days of exposure. There were little absolute differences in the rates of most events between the three proton pump inhibitors. However, diarrhoea was more commonly associated with lansoprazole compared with omeprazole (rate difference: 0.21 per 1000 days of exposure; 95% CI 0.17, 0.25; rate ratio: 2.11; 1.78, 2.51), and there was a clear age-response relationship.
Conclusions
Adverse events occurred relatively infrequently in all three cohorts. There were only small absolute differences in event rates between the three drugs, although these data suggest the hypothesis that lansoprazole is associated with more frequent occurrence of diarrhoea, particularly in the elderly.
Keywords: adverse drug reaction, diarrhoea, general practice, pharmacovigilance, prescription event monitoring, proton pump inhibitors
Introduction
Proton pump inhibitors are highly effective in treating acid-related upper gastrointestinal disease [1, 2]. The first proton pump inhibitor launched in the UK was omeprazole (1989), the second was lansoprazole (1994) and the third was pantoprazole (1997). The use of proton pump inhibitors is increasing rapidly [3, 4], and an estimated 0.8% of the UK population take long-term antisecretory agents [5]. Prescribing doctors are urged to consider a drug's benefits and risks when initiating treatment [6], and when a drug is first launched most of this information will come from premarketing clinical trials. These show no difference in efficacy between proton pump inhibitors [7, 8], and that the commonest adverse events, occurring in 1–4% of patients, are headache, nausea, diarrhoea, abdominal pain, constipation, dizziness, fatigue, rash, and pruritus [2, 7, 8]. However, the adverse event database of newly marketed drugs is limited [9], and it is only after widespread clinical use that the side-effect profile of a drug is ascertained more comprehensively [10]. Although the knowledge gained about adverse effects of drugs used in large cohorts of unselected patients in the community is an important step towards reducing the burden of drug toxicity [11], the importance of adverse drug reactions is often underestimated [11].
Experience of the adverse effects of proton pump inhibitors is greatest for omeprazole, which has been on the market longest [12]. There have been case reports of sexual disturbances [13], gynaecomastia [14], gout [15], lethargy [16], polyarthralgia [17], campylobacter gastroenteritis [18], atrophic gastritis [19], angiooedema [20], subacute myopathy [21], and ocular damage [22]. Case reports are an important method of improving knowledge about drug side-effects but data on the frequency of occurrence of adverse events are not readily available to prescribing doctors, who are therefore unable to put these isolated cases into a population perspective. There is also limited data comparing the side-effect profiles of proton pump inhibitors when used outside the carefully monitored setting of randomised controlled trials. However, relatively weak evidence has recently been published from physician and patient surveys linking lansoprazole with an increased frequency of side-effects, especially diarrhoea, abdominal cramps and headache, when used in the clinical setting [23–25]. Furthermore, a case series reported that microscopic colitis and associated diarrhoea were related to initiation of lansoprazole, and that colonic histology normalized on discontinuation of the drug [26].
Prescription-event monitoring cohort studies on the proton pump inhibitors omeprazole, lansoprazole and pantoprazole have been conducted by the Drug Safety Research Unit. These studies link dispensed prescriptions for drugs prescribed by general practitioners with incident events experienced by the patient after that drug was started [27, 28]. We determined the rates of common adverse events occurring in association with exposure to omeprazole, lansoprazole or pantoprazole. We compared the rates of these common events reported in the lansoprazole and pantoprazole cohorts with the rates reported in the omeprazole cohort, in order to test the null hypothesis that the rates are the same in the three cohorts. We were particularly interested in comparing rates of diarrhoea, abdominal pain and headache, given the a priori evidence for possible differences discussed above [23–25].
Methods
Study design
The methodology of prescription-event monitoring has previously been described [27, 28]. Details of all dispensed prescriptions for selected newly marketed drugs, prescribed by general practitioners in England soon after launch, are provided in confidence by the Prescription Pricing Authority. Enquiries are then sent to the prescribing doctor asking for details and dates of events occurring after the drugs were prescribed. The definition of an event is ‘any new diagnosis, any reason for referral to a consultant or admission to hospital, any unexpected deterioration (or improvement) in a concurrent illness, any suspected adverse drug reaction, or any other complaint which was considered of sufficient importance to enter in the patients’ notes.′ An opinion on whether or not the events were drug related is not required. Other information includes patient demography, indication, and exposure dates. Dose and concomitant medications are not routinely recorded on the questionnaires and all prescriptions for the study drugs issued by general practitioners during the period of surveillance are included in the study regardless of dose, duration of prescription or concomitant prescribing of other drugs. Duration of exposure is accounted for in the analysis by computing person-time of exposure for each patient.
Subjects
Subjects were all patients in England who received, and had dispensed, at least one prescription on an FP10 from their general practitioner for omeprazole between June 1989 and June 1990, lansoprazole between May and November 1994, and pantoprazole between December 1996 and June 1997.
Events
From a list of the commonest 11 events reported in the omeprazole cohort [27], we examined eight clinical events (diarrhoea, nausea/vomiting, abdominal pain, headache, chest pain/tight chest, ischaemic heart disease, malaise/lassitude, depression). The three common events not examined were upper respiratory tract infection, malignancy and dyspepsia because they were thought unlikely to be drug related or that they might be associated with the indication. We also examined the rates of joint pain and myalgia, because concern about these events has previously been raised [17, 21] and they were reported relatively commonly compared with other events in the cohorts [27].
Coding
The events were coded using a specially developed dictionary, designed to deal with terms used by general practitioners, and organized in a system-organ classification. Coding was performed by trained clerks. There was a daily quality assurance procedure supervised by a senior research fellow, and a weekly coding meeting supervised by medical staff. The terms for indications reflect the clinical terms used by reporting general practitioners to describe their reasons for prescribing the drugs. The term ischaemic heart disease included reports of angina and myocardial infarction. Joint pain included reports of joint stiffness, polyarthralgia, and ‘rheumatism.’
Analysis
Analysis was restricted to the first 6 months of drug exposure. Rates were the numbers of each event per 1000 days of exposure during the first 6 months after each drug was started. For individual patients only the first report of each event was included in the calculation of these rates. Person-days of exposure were calculated from the date the drug was first prescribed by the general practitioner, with censoring at the date of the first event during treatment, or the date the subject stopped the drug, or if the drug was not stopped then the end of the 6 month follow-up period. The date of death or the date the subject was no longer registered with the practice was used as the censoring time if these dates occurred before the end of follow-up and the drug had not been discontinued. Rate differences and 95% confidence limits for rate differences were calculated using omeprazole as the baseline, according to standard formulae [29]. Crude and adjusted rate ratios were calculated for the first 6 months of exposure using Cox proportional hazards regression analysis with omeprazole as the reference drug. In this model time is modelled nonparametrically and there is no assumption that rates are constant within each time-band. The adjusted models also included categorical terms for age (9 categories), season of starting the drug (4 categories) and indication for the drug (8 categories). All models were stratified by gender, allowing for different sets of baseline rate parameters for males and females.
Patients from the same general practice may be more alike than patients from different general practices. If such clustering is not accounted for in the analyses confidence intervals and P values may be biased [30]. Therefore all effect estimates were adjusted for potential clustering by general practice.
The proportional hazards assumption was analysed in the crude and adjusted models by inspection of log cumulative hazard curves (Aalen plots), and formally tested by the likelihood ratio test for interaction between exposure and time. Since multiple hypothesis tests were performed, increasing the probability of a ‘significant’ result by chance, the level of statistical significance was set at P < 0.01. Statistical analysis was performed using Stata[31].
Results
The omeprazole cohort comprised 16 205 patients, out of a total of 28 496 (56.9%) patients in England, who received at least one prescription for omeprazole between June 1989 and June 1990; the lansoprazole cohort comprised 17 329 patients, out of a total of 36 722 (47.2%) patients in England, who received at least one prescription between May and November 1994; and the pantoprazole cohort comprised 11 541 patients, out of a total of 28 159 (41.0%) patients in England, who received at least one prescription between December 1996 and June 1997 (Table 1). The age and sex distribution of the cohorts was similar. Approximately two-fifths of each cohort were prescribed the drug for oesophageal reflux/oesophagitis. Omeprazole, the first drug on the UK market, was more frequently prescribed for peptic ulcer, oesophageal reflux and hiatus hernia than lansoprazole or pantoprazole. Lansoprazole (second on the market) was almost twice as frequently prescribed for dyspepsia than omeprazole, and pantoprazole (third on the market) was over two-and-a-half times as frequently prescribed for dyspepsia than omeprazole.
Table 1.
Characteristics of the cohorts.
| Omeprazole | Lansoprazole | Pantoprazole | |
|---|---|---|---|
| Dates of prescriptions | June 1989 to July 1990 | May to November 1994 | December 1996 to June 1997 |
| Total number of green forms posted | 28 496 | 36 722 | 28,159 |
| Total number of green forms returned (% response rates) | 16 205 (56.9) | 17 329 (47.2) | 11 541 (41.0) |
| Total number of patient-months of exposure† | 58268 | 46248 | 28396 |
| Median (IQR¶) number of patient-months of exposure† | 2.6 (1.0–6.0) | 1.7 (0.9–6.0) | 1.9 (0.9–6.0) |
| Males (%) | 7970 (49.2) | 8160 (47.1) | 5350 (46.4) |
| Females (%) | 8073 (49.8) | 8975 (51.8) | 6068 (52.6) |
| Sex not reported (%) | 162 (1.0) | 194 (1.1) | 123 (1.1) |
| Mean age (years) (s.d.*) | 58.1 (16.4) | 55.4 (16.6) | 55.9 (16.5) |
| Indication‡(%Φ) | |||
| Oesophageal reflux/oesophagitis | 5720 (40.8) | 6727 (45.2) | 3276 (42.1) |
| Ulcer peptic/oesophageal | 2411 (17.2) | 1110 (7.5) | 410 (5.3) |
| Hiatus hernia | 1619 (11.5) | 692 (4.6) | 338 (4.3) |
| Dyspepsia | 1352 (9.6) | 2773 (18.6) | 1951 (25.1) |
| Abdominal pain | 712 (5.1) | 1478 (9.9) | 688 (8.8) |
| Other | 2217 (15.8) | 2105 (14.1) | 1122 (14.4) |
| Total with known indication | 14031 | 14885 | 7785 |
In the first 6 months of the study for each patient.
Interquartile range.
Standard deviation.
Ranked by frequency of report in omeprazole cohort.
of total with known indication.
The commonest adverse event in the omeprazole, lansoprazole and pantoprazole cohorts was diarrhoea (incidence rates[95% CI]: 0.18[0.16, 0.20]; 0.39[0.36, 0.42] and 0.23[0.20, 0.27] per 1000 days of exposure, respectively) (Table 2). The next most frequently reported adverse events in all three cohorts were nausea/vomiting, abdominal pain and headache. The remaining events occurred with a frequency of less than or equal to 0.11 per 1000 days of exposure.
Table 2.
Number, rates and rate ratios of common events during the first 6 months of exposure to lansoprazole or pantoprazole compared with omeprazole (reference).
| Number with event | Rate per 1000 days of exposure | Rate difference (95% confidence limits) | Crude rate ratio (95% confidence limits) | Adjusted rate ratio † (95% confidence limits) | |
|---|---|---|---|---|---|
| Diarrhoea | |||||
| Omeprazole | 253 | 0.18 | 0 | 1 | 1 |
| Lansoprazole | 534 | 0.39 | 0.21 (0.17, 0.25) | 2.12 (1.82, 2.47)*** | 2.11 (1.78, 2.51)*** |
| Pantoprazole | 196 | 0.23 | 0.05 (0.01, 0.09) | 1.23 (1.01, 1.50) | 1.26 (1.02, 1.55) |
| Pain abdomen | |||||
| Omeprazole | 240 | 0.17 | 0 | 1 | 1 |
| Lansoprazole | 291 | 0.21 | 0.04 (0.01, 0.07) | 1.22 (1.02, 1.46) | 1.15 (0.95, 1.39) |
| Pantoprazole | 144 | 0.17 | 0 (−0.03, 0.03) | 0.98 (0.79, 1.20) | 0.94 (0.75, 1.17) |
| Nausea/vomiting | |||||
| Omeprazole | 223 | 0.16 | 0 | 1 | 1 |
| Lansoprazole | 304 | 0.22 | 0.06 (0.03, 0.09) | 1.33 (1.12, 1.59)** | 1.33 (1.10, 1.61)* |
| Pantoprazole | 153 | 0.18 | 0.02 (−0.02, 0.06) | 1.08 (0.87, 1.34) | 1.12 (0.90, 1.41) |
| Headache | |||||
| Omeprazole | 147 | 0.10 | 0 | 1 | 1 |
| Lansoprazole | 238 | 0.17 | 0.07 (0.04, 0.10) | 1.63 (1.32, 2.01)*** | 1.58 (1.26, 1.99)*** |
| Pantoprazole | 127 | 0.15 | 0.05 (0.02, 0.08) | 1.37 (1.07, 1.74) | 1.48 (1.14, 1.93)* |
| Pain joint | |||||
| Omeprazole | 119 | 0.08 | 0 | 1 | 1 |
| Lansoprazole | 154 | 0.11 | 0.03 (0.01, 0.05) | 1.35 (1.06, 1.71) | 1.40 (1.08, 1.82) |
| Pantoprazole | 87 | 0.10 | 0.02 (−0.01, 0.05) | 1.22 (0.93, 1.62) | 1.36 (1.01, 1.83) |
| Pain Chest | |||||
| Omeprazole | 97 | 0.07 | 0 | 1 | 1 |
| Lansoprazole | 104 | 0.08 | 0.01 (−0.01, 0.03) | 1.09 (0.82, 1.44) | 1.11 (0.81, 1.52) |
| Pantoprazole | 65 | 0.08 | 0.01 (−0.01, 0.03) | 1.10 (0.81, 1.51) | 1.14 (0.81, 1.60) |
| Ischaemic heart disease | |||||
| Omeprazole | 97 | 0.07 | 0 | 1 | 1 |
| Lansoprazole | 90 | 0.07 | 0 (−0.02, 0.02) | 0.96 (0.71, 1.30) | 1.04 (0.75, 1.44) |
| Pantoprazole | 51 | 0.06 | −0.01 (−0.03, 0.01) | 0.89 (0.63, 1.26) | 0.99 (0.68, 1.45) |
| Malaise | |||||
| Omeprazole | 95 | 0.07 | 0 | 1 | 1 |
| Lansoprazole | 143 | 0.10 | 0.03 (0.01, 0.05) | 1.50 (1.16, 1.93)* | 1.46 (1.11, 1.92)* |
| Pantoprazole | 57 | 0.07 | 0 (−0.02, 0.02) | 0.95 (0.68, 1.33) | 0.93 (0.65, 1.33) |
| Depression | |||||
| Omeprazole | 93 | 0.06 | 0 | 1 | 1 |
| Lansoprazole | 128 | 0.09 | 0.03 (0.01, 0.05) | 1.62 (1.23, 2.12)** | 1.64 (1.24, 2.19)** |
| Pantoprazole | 78 | 0.08 | 0.02 (0.00, 0.04) | 1.42 (1.04, 1.93) | 1.39 (1.01, 1.92) |
| Myalgia | |||||
| Omeprazole | 24 | 0.02 | 0 | 1 | 1 |
| Lansoprazole | 48 | 0.04 | 0.02 (0.01, 0.03) | 2.04 (1.26, 3.29)* | 2.31 (1.36, 3.90)* |
| Pantoprazole | 32 | 0.04 | 0.02 (0.01, 0.03) | 2.20 (1.30, 3.73)* | 2.62 (1.52, 4.50)*** |
All models stratified by gender and with standard errors adjusted for possible clustering effects by general practice surgery.
Adjusted for age, season of starting drug, and indication.
P < 0.01
P < 0.001
P < 0.0001.
The rate difference for diarrhoea when lansoprazole was compared with omeprazole was 0.21 per 1000 days of drug exposure (Table 2). If 1000 patients were treated for one month there would be approximately six extra reports of incident diarrhoea compared with omeprazole (assuming a constant hazard). However, the rate differences for all other events, in both the lansoprazole vs omeprazole comparison and the pantoprazole vs omeprazole comparison were less than 0.07 per 1000 days of drug exposure. If 1000 patients were treated for 1 month there would be fewer than 2.1 extra reports of the event compared with omeprazole (assuming a constant hazard).
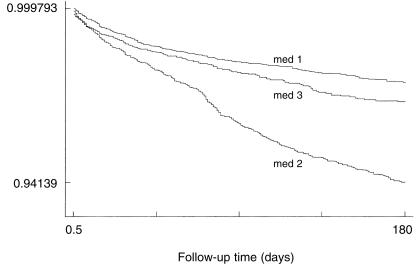
Crude rate ratios for the association between individual proton pump inhibitors and adverse events were similar to the adjusted rate ratios (Table 2). After adjustment for age, sex, indication, season of starting the drug, follow-up time, and clustering at general practice level, lansoprazole was associated with significantly (P < 0.01) higher rates of myalgia (rate ratio: 2.31; 95% CI: 1.36, 3.90); diarrhoea (rate ratio: 2.11; 1.78, 2.51); depression (rate ratio: 1.64; 1.24, 2.19); headache (rate ratio: 1.58; 1.26, 1.99); malaise (rate ratio: 1.46; 1.11, 1.92); and nausea/vomiting (rate ratio: 1.33; 1.10, 1.61) compared with omeprazole. After adjustment for the above factors pantoprazole was associated with significantly (P < 0.01) higher rates of myalgia (2.62; 1.52, 4.50) and headache (1.48; 1.14, 1.93) compared with omeprazole. The differences in proportionate survival from diarrhoea in the three cohorts are illustrated in the Kaplan-Meier survival estimates plotted in Figure 1. There was no evidence of an interaction between drug exposure and indication on outcome. The results were similar when the analysis was restricted to the first month of exposure. Results taking into account clustering at the general practice level were similar to results which did not take this into account.
Figure 1.
Survival curve showing the probability of diarrhoea associated with proton pump inhibitors. Kaplan-Meier survival estimates, med 1 omeprazole; med 2 lansoprazole; med 3 pantoprazole.
Table 3 shows the age-stratified rate ratios of diarrhoea during the first 6 months of exposure to lansoprazole or pantoprazole compared with omeprazole. The adjusted rate ratio of diarrhoea associated with lansoprazole increased in each successive age group from 1.2 (0.6, 2.6) in the 21–30 years age group to 2.9 (2.1, 4.0; P < 0.001) in the 71–80 years age group. There were no age related patterns to the relative differences between omeprazole and lansoprazole for the other events, and there were no age-related patterns to either diarrhoea or other adverse event rates for pantoprazole relative to omeprazole.
Table 3.
Age stratified rate ratio of diarrhoea during the first 6 months of exposure to lansoprazole or pantoprazole compared with omeprazole (reference).
| Drug | 21–30 | 31–40 | 41–50 | Age (years) 51–60 | 61–70 | 71–80 | ≥81 |
|---|---|---|---|---|---|---|---|
| Omeprazole (n) | 12 | 18 | 32 | 36 | 58 | 50 | 25 |
| Lanzoprazole (n) | 17 | 38 | 68 | 95 | 123 | 117 | 43 |
| Pantoprazole (n) | 8 | 17 | 24 | 24 | 40 | 47 | 18 |
| Rate ratio‡(95% CI) | |||||||
| Omeprazole | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Lansoprazole | 1.2 | 1.7 | 1.9 | 2.5 | 2.4 | 2.9 | 2.4 |
| (0.6, 2.6) | (1.0, 3.0) | (1.2, 2.8)* | (1.7, 3.8)** | (1.7, 3.2)** | (2.1, 4.0)** | (1.4, 3.8)** | |
| Pantoprazole | 1.0 | 1.1 | 1.2 | 1.0 | 1.2 | 1.9 | 1.7 |
| (0.4, 2.5) | (0.6, 2.1) | (0.7, 2.0) | (0.6, 1.8) | (0.8, 1.9) | (1.3, 2.8)* | (0.9, 3.1) | |
Adjusted for sex.
P < 0.01
P < 0.001.
Age under 21 years not shown as numbers were very small.
Discussion
The main finding of this analysis was that the commonest adverse events reported during exposure to omeprazole, lansoprazole and pantoprazole occurred relatively infrequently. The large numbers involved in these cohorts provided relatively tight confidence limits around the point estimates, an advantage of this study. The importance of such estimates is that they were derived from ‘real-live’ clinical experience, rather than during the carefully controlled conditions of a clinical trial (which, additionally are often too small to provide precise adverse event rate estimates). These estimates enable the prescriber to assess and rank the frequency of these events within a population perspective. Such an assessment is unavailable from routine prescribing sources used by clinicians such as the British National Formulary (BNF) or the Summary of Product Characteristics (SpC) [32, 33], as these simply provide a raw list of suspected adverse drug reactions.
When choosing between alternative drugs prescribers will need to make some assessment of their comparative efficacy, cost, and side-effect profile [6]. However, the importance of side-effects is often forgotten [11], despite the likelihood that even minor side-effects can impact on patients' acceptability, tolerance and compliance with treatment, as well as have implications for sickness absence from work, costs of treating side-effects, and the costs of additional contact with health services. Our data suggest that there may be differences in the rates of some of the commonly observed side-effects, and the higher rate of diarrhoea associated with lansoprazole is in agreement with previous surveys of physicians experiences and a case-series [23–26]. However, apart from the association between lansoprazole and diarrhoea in comparison with omeprazole, the absolute differences in rates were small and are unlikely to be of clinical importance when deciding on treatment options. We also observed an increasing risk of diarrhoea associated with lansoprazole, but not pantoprazole, with increasing age. This suggests the hypothesis that older age may be a risk factor for diarrhoea in patients prescribed lansoprazole.
The observed event rates are likely to be an underestimate of the adverse events experienced by patients, because they may not report them to their general practitioner or the general practitioner may not have reported them on the green form. Nevertheless, since these events were infrequently reported even after prompted surveillance suggests that these drugs are well tolerated in the general practice setting.
The estimates of effect used in this study (rate difference and rate ratio) may have been subject to selection and response bias, and biases arizing from the different periods of observation of the two studies [34]. Since for any event we found only very small absolute differences between these drugs the main limitation to consider is whether we have missed larger, clinically important differences in event rates. However, we consider that any biases would be likely to increase rather than decrease the chances of observing a noncausal relationship. For example, since the omeprazole cohort was followed up at 1 year, and the lansoprazole and pantoprazole cohorts at 6 months, there may be recall bias in favour of omeprazole. This is because it is usually easier to recall side-effects which have happened more recently.
Response rates were higher in the omeprazole cohort than the lansoprazole cohort, and it is possible that returns in the omeprazole cohort differentially favoured those returns with no or fewer events recorded. Also, the drugs were monitored at different times, separated by up to 8 years, a potential weakness of the study because by the time lansoprazole and pantoprazole were examined most side-effects of proton-pump inhibitors would have been recognized and perhaps be recorded and reported more frequently. It has previously be shown that reporting of events increases when doctors' attention is drawn to specific problems [34, 35]. However, we would have expected a larger difference in rates of diarrhoea when pantoprazole was compared with omeprazole, but this was not observed. The specificity of the association between lansoprazole and diarrhoea, together with the observed age-response relationship, suggest that this association may not be entirely explained by the biases discussed above. It is possible that the duration of prescription for proton-pump inhibitors has changed over time, and that if omeprazole was prescribed for shorter periods, fewer events would be expected. Cox regression analysis controls for the potential confounding effect of follow-up time, which in this study was duration of exposure, and the Aalen plots that we inspected when doing the Cox regression analysis suggested that the rate ratios were of a similar magnitude over the whole follow-up time for each event.
People who had switched from one proton pump inhibitor to another were inevitably included in the study as the methodology did not identify this group of patients. This may have resulted in a higher event rate in lansoprazole and pantoprazole users if switching was related to the side-effects of omeprazole. However, only about 3% of proton pump inhibitors are stopped because of side-effects, making this source of bias an unlikely explanation for our findings [36]. We were unable to examine the effect of different doses on the incidence of side-effects such as diarrhoea.
In conclusion we found that adverse events associated with omeprazole, lansoprazole and pantoprazole were reported infrequently, and that there were only very small differences in the adverse event rates studied between these drugs. The small differences found seem likely to be explained by information or selection bias. However, the data do suggest the hypothesis that diarrhoea occurs more frequently in association with lansoprazole, particularly in older age groups.
Acknowledgments
We are very grateful to general practitioners in England who support prescription-event monitoring studies. We thank the Prescription Pricing Authority, the Family Health Services Authorities of England, and the Office for National Statistics, for their important participation in this program. We thank Gill Pearce who manages the prescription-event monitoring database, Lynda Wilton, senior research fellow, Georgina Spragg for her administrative support and Stella Matthews for secretarial help. There was no funding for this comparative study. The Drug Safety Research Unit has in the past received donations from Wyeth and Astra, amongst other pharmaceutical companies, in support of individual prescription-event monitoring studies.
References
- 1.Blum RA. Lansoprazole and omeprazole in the treatment of acid peptic disorders. Am J Health-Syst Pharm. 1996;53:1401–1415. doi: 10.1093/ajhp/53.12.1401. [DOI] [PubMed] [Google Scholar]
- 2.Shin JM, Besancon M, Prinz C, Simon A, Sachs G. Continuing development of acid pump inhibitors: site of action of pantoprazole. Aliment Pharmacol Ther. 1994;8(Suppl 1):11–23. doi: 10.1111/j.1365-2036.1994.tb00211.x. [DOI] [PubMed] [Google Scholar]
- 3.Martin RM, Lim AG, Kerry SM, Hilton SR. H2-receptor antagonists and proton pump inhibitors in general practice: Prescribing trends in the UK from 1991 to 1996. Gut. 1997;40(Suppl 1):A60. [Google Scholar]
- 4.Department of Health. Statistics of prescriptions dispensed in the Family Health Services Authorities: England 1982–1992. Statistical Bulletin, 1993.
- 5.Moore RA. Helicobacter pylori and peptic ulcer. Oxford: Health Technology Evaluation Association; 1995. [Google Scholar]
- 6.Barber N. What constitutes good prescribing? Br Med J. 1995;310:923–925. doi: 10.1136/bmj.310.6984.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anonymous. Lansoprazole-another proton pump inhibitor. Drug Ther Bull. 1995;33:36–37. doi: 10.1136/dtb.1995.33536. [DOI] [PubMed] [Google Scholar]
- 8.Anonymous. Pantoprazole-a third proton pump inhibitor. Drug Ther Bull. 1997;35:93–94. doi: 10.1136/dtb.1997.351293. [DOI] [PubMed] [Google Scholar]
- 9.Rawlins MD, Jefferys DB. United Kingdom Product Licence applications involving new active substances. 1987–89: their fate after appeals. Br J Clin Pharmacol. 1993;35:599–602. doi: 10.1111/j.1365-2125.1993.tb04188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waller PC, Coulson RA, Wood SM. Regulatory pharmacovigilance in the United Kingdom: current principles and practice. Pharmacoepidemiol Drug Safety. 1996;5:363–375. doi: 10.1002/(SICI)1099-1557(199611)5:6<363::AID-PDS249>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Pirmohamed M, Breckenridge AM, Kitteringham NR, Park BK. Adverse drug reactions. Br Med J. 1998;316:1295–1298. doi: 10.1136/bmj.316.7140.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bateman DN. Proton-pump inhibitors: three of a kind? Lancet. 1997;349:1637–1638. doi: 10.1016/s0140-6736(05)62629-3. [DOI] [PubMed] [Google Scholar]
- 13.Dutertre JP, Soutif D, Jonville AP, Cadenne M, Valat JP, Autret E. Sexual disturbances during omeprazole therapy. Lancet. 1991;338:1022. doi: 10.1016/0140-6736(91)91887-z. [DOI] [PubMed] [Google Scholar]
- 14.Santucci L, Farroni F, Fiorucci S, Morelli A. Gynaecomastia during omeprazole therapy. N Engl J Med. 1991;324:635. doi: 10.1056/NEJM199102283240917. [DOI] [PubMed] [Google Scholar]
- 15.Kraus A, Flores-suarez LF. Acute gout associated with omeprazole. Lancet. 1995;345:461–462. doi: 10.1016/s0140-6736(95)90449-2. [DOI] [PubMed] [Google Scholar]
- 16.Meeuwisse EJM, Groen FC, Dees A, Smit GH, Ottervanger JP. Lethargy with omeprazole. Br Med J. 1997;314:481. doi: 10.1136/bmj.314.7079.481a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beutler M, Hartmann K, Kuhn M, Gartman J. Arthralgia and omeprazole. Br Med J. 1994;309:1620. doi: 10.1136/bmj.309.6969.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neal KR, Scott HM, Slack RCB, Logan RFA. Omeprazole as a risk factor for campylobacter gastroenteritis: case-control study. Br Med J. 1996;312:414–415. doi: 10.1136/bmj.312.7028.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuipers EJ, Lundell RL, Klinkenberg-knol EC, et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux oesophagitis treated with omeprazole or fundoplication. N Engl J Med. 1996;334:1018–1022. doi: 10.1056/NEJM199604183341603. [DOI] [PubMed] [Google Scholar]
- 20.Haeney MR. Angio-oedema and urticaria associated with omeprazole. Br Med J. 1992;305:870. doi: 10.1136/bmj.305.6858.870-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garrote FJ, Lacambra C, Del Ser T, Garcia Diaz B, Obeso G, Solis J. Subacute myopathy during omeprazole therapy. Lancet. 1992;340:672. doi: 10.1016/0140-6736(92)92205-t. [DOI] [PubMed] [Google Scholar]
- 22.Schonhofer P, Werner B, Troger U. Ocular damage associated with proton pump inhibitors. Br Med J. 1997;314:1805. doi: 10.1136/bmj.314.7097.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Creed T, Moran A. Cutting prescribing costs in dyspepsia – effects of changing proton pump inhibitor. Gut. 1999;44(Suppl 1):A4. [Google Scholar]
- 24.Condra LJ, Morreale AP, Stolley SN, Marcus D. Assessment of patient satisfaction with a formulary switch from omeprazole to lansoprazole in gastroesophageal reflux disease maintenance therapy. Am J Managed Care. 1999;5:631–638. [PubMed] [Google Scholar]
- 25.Krinsky ML, Alexis G, Hirano I. Chronic diarrhoea from lansoprazole use in the veteran population. Gastroenterology. 1999;116:A224. [Google Scholar]
- 26.Thomson RD, Bensen SP, Toor A, Maheshwari Y. Lansoprazole associated microscopic colitis. Gastroenterology. 1999;116:A938. doi: 10.1111/j.1572-0241.2002.07066.x. [DOI] [PubMed] [Google Scholar]
- 27.Freemantle SN, Pearce GL, Wilton LV, Mackay FJ, Mann RD. The incidence of the most commonly reported events with 40 newly marketed drugs- a study by Prescription-Event Monitoring. Pharmacoepidemiol Drug Safety. 1997;6(Suppl 1):S1–S8. [Google Scholar]
- 28.Mann RD, Wilton LV, Pearce GL, Mackay FJ, Dunn NR. Prescription-Event Monitoring in 1996-a method of non-interventional observational cohort pharmacovigilance. Pharmacoepidemiol Drug Safety 1997. 6(Suppl 3):S5–S11. doi: 10.1002/(SICI)1099-1557(199710)6:3+<S5::AID-PDS272>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 29.Clayton D, Hills M. Statistical models in epidemiology. OUP: Oxford; 1993. pp. 130–132. [Google Scholar]
- 30.Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGL. Methods for evaluating area wide and organisational based interventions in health and health care: a systematic review. Health Technol Assess. 1999;3 [PubMed] [Google Scholar]
- 31.StataCorp. Stata Statistical Software: Release 5.0. College Station, TX. Stata Corporation, 1997.
- 32.Rawlins MD, Mann RD. Monitoring adverse events and reactions. In: Mann RD, Rawlins MD, Auty RM, editors. A Textbook of Pharmaceutical Medicine. Lancs: Parthenon; 1993. pp. 317–322. [Google Scholar]
- 33.Ferner R, Mann RD. Drug safety and pharmacovigilance. In: Page CP, Curtis MJ, Sutter MC, Walker MJA, Hoffman BB, editors. Integrated Pharmacology. Mosby International: London; 1997. [Google Scholar]
- 34.Rawlins MD. Spontaneous reporting of adverse drug reactions I. Br J Clin Pharmacol. 1988;26:1–5. doi: 10.1111/j.1365-2125.1988.tb03356.x. The data. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones RH. Clinical economics review: gastrointestinal disease in primary care. Aliment Pharmacol Ther. 1996;10:233–239. doi: 10.1111/j.0953-0673.1996.00233.x. [DOI] [PubMed] [Google Scholar]
- 36.Martin RM, Lim AG, Kerry SM, Hilton SR. Trends in prescribing H2-receptor antagonists and proton pump inhibitors in primary care. Alimentary Pharmacol Ther. 1998;12:797–805. doi: 10.1046/j.1365-2036.1998.00374.x. [DOI] [PubMed] [Google Scholar]