Abstract
In earlier studies, the neural cell adhesion molecule, N-CAM, was found to inhibit the proliferation of rat astrocytes both in vitro and in vivo. To identify the gene targets involved, we used subtractive hybridization to examine changes in gene expression that occur after astrocytes are exposed to N-CAM in vitro. While the mRNA levels for N-CAM decreased after such treatment, the levels of mRNAs for glutamine synthetase and calreticulin increased. Since glutamine synthetase and calreticulin are known to be involved in glucocorticoid receptor pathways, we tested a number of steroids for their effects on astrocyte proliferation. Dexamethasone, corticosterone, and aldosterone were all found to inhibit rat cortical astrocyte proliferation in culture in a dose-dependent manner. RU-486, a potent glucocorticoid antagonist, reversed the inhibitory effects of dexamethasone. These observations prompted the hypothesis that the inhibition of proliferation by N-CAM might be mediated through the glucocorticoid receptor pathway. Consistent with this hypothesis, the inhibition of astrocyte proliferation by N-CAM was reversed in part by a number of glucocorticoid antagonists, including RU-486, dehydroepiandrosterone, and progesterone. In transfection experiments with cultured astrocytes, N-CAM treatment increased the expression of a luciferase reporter gene under the control of a minimal promoter linked to a glucocorticoid response element. The enhanced activity of this construct stimulated by N-CAM was abolished in the presence of RU-486. The combined data suggest that astrocyte proliferation is in part regulated by alterations in glucocorticoid receptor pathways.
Keywords: neural cell adhesion molecule, glia, differentiation, nuclear signaling, transcription
Astrocyte proliferation and differentiation are key processes in neural development and play significant roles in the regeneration of neural tissue after a penetrating injury or ischemic trauma. Astrocytes display the neural cell adhesion molecule (N-CAM) on their surface (1–3) and the expression of several cell adhesion molecules is known to change during developmental and regenerative events (4). Our previous studies have shown that addition of soluble N-CAM or certain of its peptides inhibits neonatal astrocyte proliferation in vitro (5) and in vivo following a penetrating injury in adult rat forebrain (6).
These findings raise the question of how signaling is mediated after N-CAM interaction at the astrocyte cell surface. Several recent studies (reviewed in refs. 7 and 8) have addressed the signal pathways activated by the binding of N-CAM to neurons in culture or to neuronal cell lines. N-CAM binding induced changes in intracellular pH, calcium, inositol phosphate levels, and tyrosine phosphorylation (reviewed in ref. 7). Moreover, aggregation of embryonic chicken brain cells leads to down-regulation of N-CAM expression (9). The results of all of these studies suggest that N-CAM binding at the surface of neurons alters intracellular signals that in turn affect gene expression.
In the present experiments, we used subtractive hybridization to identify mRNAs that showed increased or decreased levels of expression after N-CAM treatment of rat astrocytes. Analyses of clones from the subtracted cDNA libraries, and subsequent quantitative analysis of gene expression, revealed that mRNA levels for N-CAM decreased after N-CAM binding, whereas the mRNA levels for glutamine synthetase and calreticulin increased. Transcription of the glutamine synthetase gene is inducible by corticosteroids (10) via a glucocorticoid response element (GRE) in its promoter (11, 12). It is also known that calreticulin can interact with and affect the activity of the glucocorticoid receptor (13, 14).
The finding that mRNA levels for both of these molecules were elevated after N-CAM binding to the cell surface prompted us to examine the effects of corticosteroid agonists and antagonists on astrocyte proliferation in vitro and to compare their effects with those of N-CAM. Glucocorticoids inhibited proliferation and the inhibition by dexamethasone was reversed in the presence of RU-486, a potent glucocorticoid antagonist. We also found that a number of inhibitors in addition to RU-486 partially reversed the inhibition by N-CAM of astrocyte proliferation. Moreover, N-CAM treatment of cells transfected with a construct consisting of a luciferase reporter gene under the control of a minimal promoter linked to two copies of a consensus GRE led to increased reporter gene activity. The results suggest that glucocorticoid receptor pathways are involved in the regulation of astrocyte proliferation.
EXPERIMENTAL PROCEDURES
Reagents.
Aldosterone, dexamethasone, progesterone, 17-β-estradiol, and DHEA (dehydroxyepiandrosterone-3-sulfate) were purchased from Sigma. RU-486 was obtained from Biomol (Plymouth Meeting, PA). N-CAM was purified from early postnatal rat brains by affinity chromatography as described (5). The recombinant third Ig domain of N-CAM was prepared and tested for binding activity as described (15).
Astrocyte Cultures.
Primary cultures of astrocytes were prepared from the forebrains of 3–4-day-old neonatal rats and assayed for proliferation as described (5). More than 95% of the cells were positive for the glial markers GFAP and S100 and for the intermediate filament protein vimentin. The cell types were also characterized using the following marker antibodies (with their specificities indicated in parentheses): A2B5 (type II astrocytes and O2-A progenitors), galactosyl-cerebroside, myelin-associated glycoprotein, myelin-basic protein (oligodendrocytes), neurofilament 160 (neurons), Ox-42 (microglia), fibronectin (fibroblasts). The nonastrocytic cells in the cultures were fibroblasts and occasional oligodendrocytes. The characterization indicated that the cultures were primarily classic in vitro type I astrocytes (16).
Subtractive Hybridization.
Primary astrocytes were seeded into 100-mm tissue culture dishes at a density of 7 × 104 cells per cm2. The day after seeding, the medium was replaced with serum-free media and the cells cultured for an additional 48 hr. Cells were then treated with N-CAM at 10 μg/ml, or with an equivalent volume of PBS. After a 6-hr incubation, the cells were harvested into RNAzol (Tel-Test, Friendswood, TX) and poly(A)+ RNA was isolated using oligo(dT) beads (Qiagen, Chatsworth, CA). In a preliminary time course analysis, a 6-hr treatment with N-CAM was shown to be sufficient to inhibit glial proliferation and was therefore chosen as a suitable time point to identify changes in gene expression. Directional cDNA libraries of the N-CAM-treated and PBS-treated astrocytes were constructed and the differences between these two libraries were enhanced by subtractive hybridization as described (9). After four rounds of subtractive hybridization, the residual cDNA sequences were amplified by PCR and cloned into λZAPII (Stratagene) (9).
Screening was carried out by plating the subtracted cDNA libraries and hybridizing duplicate filters with labeled cDNA probes. To prepare the cDNA probes, RNA was first transcribed using T3 polymerase from λDNA isolated from the two original cDNA libraries. This RNA was then reverse transcribed to yield first-strand cDNA using oligo(dT) as a primer. Twenty nanograms of this DNA was labeled by random oligonucleotide priming (Boehringer Mannheim). Filters were hybridized and washed at high stringency (15 mM NaCl/1.5 mM sodium citrate, pH 7.0/0.1% SDS at 65°C). Plaques that hybridized more strongly with one probe were selected for further analysis. Changes in gene expression were confirmed using slot blots of the cDNA constructs (see below) probed with first-strand cDNA probes made from cells treated for various times with N-CAM. To prepare these probes, 0.5–2 μg of astrocyte poly(A)+ RNA was reverse-transcribed with Superscript reverse transcriptase (GIBCO/Life Technologies) using an oligo(dT) primer. After RNase treatment, the cDNA was ethanol-precipitated and quantitated on ethidium bromide agarose plates. Between 20 and 40 ng of cDNA was radiolabeled with [α-32P]dCTP by random oligonucleotide priming (Boehringer Mannheim), precipitated with spermidine, and hybridized to membranes using Rapid-Hyb (Amersham) buffer at 65°C for 6–8 hr. After washing with 1× SSC/0.1% SDS and then 0.1× SSC/0.1% SDS at 65°C, the blots were exposed to phosphor screens and quantitated by PhosphorImager (Molecular Dynamics) using imagequant software. Hybridization results were normalized to the level of hybridization obtained with glyceraldehyde-3-phosphate dehydrogenase and cyclophilin cDNAs.
Database Searches.
Sequence comparisons were performed using the fasta program (Genetics Computer Group, version no. 7, 1991-UNIX) to scan the GenBank (release no. 95), EMBL (release no. 47), and Swiss-Prot (release no. 33) databases.
DNA Slot Blots.
The cDNA inserts of each clone identified by subtractive hybridization screening were amplified by PCR (see ref. 9) and purified using the Qiaquick PCR purification kit (Qiagen). One microgram of PCR-amplified DNA was denatured by boiling, chilled on ice, and then applied to Hybond-N+ membranes (Amersham) using a slot-blot manifold. DNA was fixed by placing the membrane on Whatman 3MM paper soaked with 0.4 M NaOH for 10 min, followed by a rinse with 300 mM NaCl/30 mM sodium citrate (pH 7.0). Filters were prehybridized with Rapid-Hyb (Amersham) prior to hybridization in the same buffer.
Construction of a Luciferase Reporter Construct Under the Control of the GRE (GRE-luc).
The GRE reporter construct was prepared in the minimal promoter vector pGL3-promoter (Promega). An oligonucleotide (5′-tatataacgcgttgtacaggatgttctctctgcctctgctgtacaggatgttctagatctgccctatagtgagtcgtattac-3′), which contains two copies of a GRE consensus sequence (ref. 17; shown underlined) and which encodes restriction enzyme sites for MluI and BglII (nucleotides in boldface type), was synthesized to construct the GRE-luc plasmid. This oligonucleotide was primed with a short oligonucleotide primer and made double-stranded with Klenow enzyme and dNTPs. After digestion with MluI and BglII, the DNA was cloned upstream of the simian virus 40 promoter in the pGL3-promoter vector to yield the GRE-luc construct. Similar constructs have been used in other studies to measure responsiveness to glucocorticoids (18) and to cell contact (19). Identical methods were used to prepare a construct containing a mutated GRE (17).
Transfection of GRE-luc into Primary Astrocytes by Electroporation.
Primary astrocytes were harvested and resuspended in Opti-MEM I (GIBCO/Life Technologies) at a density of 5 × 106 cells per ml. The resulting astrocyte suspension (0.4 ml) was mixed with 0.5 μg GRE-luc and 0.5 μg CMV-β-gal, which was included as a transfection control to normalize luciferase activity. Cells were electroporated, resuspended in 24 ml of DMEM containing 10% fetal calf serum, and plated in 6-well plates (2 ml per well). After allowing cell recovery for 24 hr, the medium was changed to serum-free DMEM and the cells were incubated for an additional 48 hr before treatment with N-CAM or steroids.
Luciferase and β-Galactosidase (β-gal) Assays.
Cells were washed twice with PBS and solubilized with 150 μl of lysis buffer (100 mM Tris-acetate, pH 7.8/10 mM magnesium acetate/1% Triton-X 100/1 mM EDTA/1 mM DTT). Cell lysates were cleared by centrifugation at 15,000 rpm at 4°C for 10 min. Luciferase assays were performed on 20 μl of cell lysate using an LB 96 P MicroLumat fluorometer (EG & G Berthold/Wallac, Gaithersburg, MD). Lysates were mixed with reaction buffer (66 μM d-luciferin potassium salt/2 mM ATP/100 mM Tris-acetate, pH 7.8/10 mM magnesium acetate/1 mM EDTA) and the fluorescence intensity was measured. To normalize for transfection efficiency, 20-μl aliquots of cell extract were assayed for β-gal activity using the FluoReporter lacZ/Galactosidase kit (Molecular Probes). Levels of activity were quantitated in a CytoFluor 2350 Fluorescence Measurement System (Millipore). The luciferase activity of each sample was normalized to an internal reference standard of β-gal activity.
RESULTS
Preparation of Subtractive Libraries and Identification of Enriched cDNAs.
To identify mRNAs whose levels changed in response to N-CAM treatment of astrocytes, two subtracted cDNA libraries were prepared as described in Experimental Procedures (9). The N-CAM (+) library was enriched for sequences that were more abundant in the astrocytes treated with N-CAM. The N-CAM (−) library was enriched for sequences that were more abundant in the untreated cells, i.e., sequences that are down-regulated after N-CAM treatment. Preliminary analysis of these libraries identified 75 cDNA clones from mRNAs that were differentially expressed. Sequence searches revealed that many of these cDNAs encoded sequences not found in the current databases, but others were identical or homologous to known sequences; examples of these are shown in Table 1. Three such differentially expressed clones were followed up in this study, including N-CAM in the N-CAM (−) library and glutamine synthetase and calreticulin in the N-CAM (+) library.
Table 1.
Changes in mRNAs induced by N-CAM in glial cultures
| mRNAs increased > 2-fold | mRNAs decreased > 2-fold |
|---|---|
| Glutamine synthetase* | N-CAM* |
| Calreticulin* | |
| Hepatoma-derived growth factor | Adenine nucleotide translocator |
| Poly (A) binding protein | SG2NA |
| Elongation factor-1α | |
| Brain identifier transcript | |
| α-tubulin | |
| β-tubulin |
mRNAs that were identified from subtracted cDNA libraries by screening as described in the text and in Experimental Procedures.
The quantitation of these changes is shown in Fig. 1.
To quantitate the levels of the mRNAs corresponding to these clones in untreated and N-CAM-treated astrocytes, mRNA levels in astrocytes were measured by probing slot blots with radiolabeled first-strand cDNA prepared from RNA isolated from either untreated or N-CAM-treated cells (Fig. 1). By comparison to the mRNA levels for cyclophilin and tenascin, which were unchanged after N-CAM treatment (compare stippled and solid bars in Fig. 1), the level of mRNA for N-CAM itself was reduced by 60% after N-CAM treatment of astrocyte cultures. This decrease is comparable to the amount of inhibition of N-CAM mRNA expression in aggregated neurons, as found in our previous studies (9).
Figure 1.
Comparison of levels of gene expression in untreated and N-CAM-treated astrocytes. Duplicate slot blots containing PCR-amplified cDNA inserts for the indicated genes were probed with 32P-labeled first-strand cDNA probes prepared from untreated (stippled bars) or N-CAM-treated (solid bars) astrocyte RNA as described. The hybridization signals represent the pixel values above background that were obtained with a PhosphorImager and analyzed with imagequant software (Molecular Dynamics). These values were normalized against values obtained for glyceraldehyde-3-phosphate dehydrogenase. The hybridization values are the average of at least two independent experiments. CYC, cyclophilin; TN, tenascin; GS, glutamine synthetase; CRT, calreticulin.
In contrast, the mRNA levels for both glutamine synthetase and calreticulin increased 2.5-fold in astrocytes after treatment with N-CAM (Fig. 1). Glutamine synthetase and calreticulin were of particular interest because their genes are linked to glucocorticoid receptor activity. These observations prompted us to investigate the effects of corticosteroid agonists and antagonists on glial proliferation and gene expression and to compare them with the effects of N-CAM.
Effect of Steroids on Astrocyte Proliferation.
The synthetic glucocorticoid dexamethasone, the natural corticosteroid hydrocortisone, the mineralocorticoid aldosterone, and the gonadal steroids progesterone and β-estradiol were examined for their effects on astrocyte proliferation (Fig. 2A). At a concentration of 0.01 μM, dexamethasone reduced glial proliferation to 50% of untreated control cultures and hydrocortisone had a slight effect, whereas the other steroids had no effect at this concentration. At a concentration of 1 μM, inhibition by dexamethasone was similar to its effect at 0.01 μM. At 1 μM, hydrocortisone and aldosterone inhibited proliferation by 40–60%, whereas progesterone and β-estradiol had no significant effect. The effects of dexamethasone on astrocyte proliferation were concentration dependent over the range of 0.1 to 10 nM with the half-maximal inhibition occurring at approximately 0.5 nM (Fig. 2B). Unlike the effects of N-CAM (5), the effects of glucocorticoids were not dependent on cell density (data not shown). At 1 nM dexamethasone, simultaneous addition of 2 μg/ml N-CAM, a concentration that is near the half-maximal for its inhibitory effect (5), was unable to inhibit glial proliferation beyond that induced by dexamethasone alone (data not shown) and the effects of the two reagents did not appear to be additive.
Figure 2.
Effects of steroids on astrocyte proliferation. (A) Astrocytes were treated with 1 μM (stippled bars) or 0.01 μM (solid bars) of dexamethasone, hydrocortisone, aldosterone, β-estradiol, or progesterone, and astrocyte proliferation was measured and compared with that of control untreated cultures as described. The values are expressed as a percentage of the control levels of thymidine incorporation. (B) Concentration-dependent effects of dexamethasone on astrocyte proliferation. Proliferation was measured after treatment with various concentrations of dexamethasone (abscissa). The 0 point on the ordinate represents untreated cultures. Results presented are the average of quadruplicate measurements ± SD.
Glucocorticoid Antagonists Partly Reverse N-CAM Inhibition of Astrocyte Proliferation.
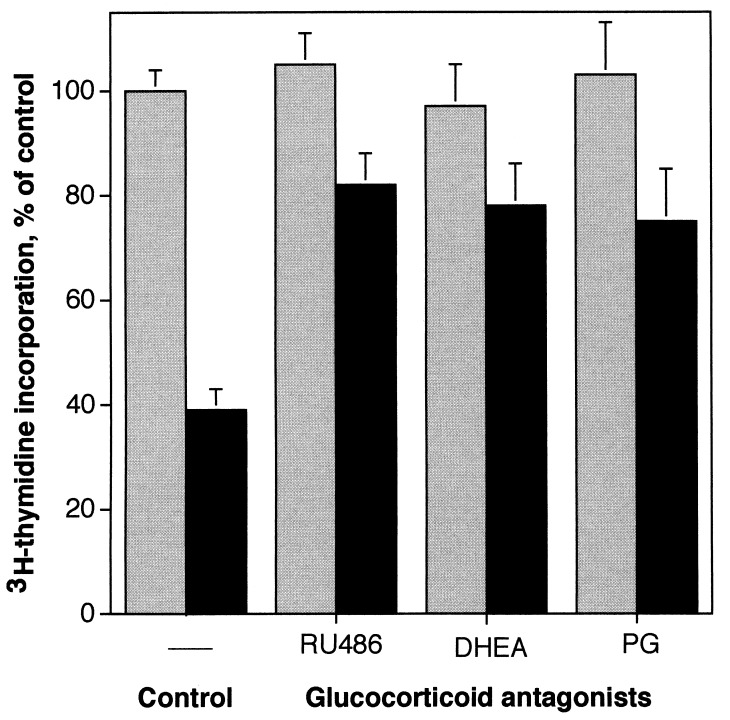
Three different glucocorticoid antagonists, RU-486, DHEA, and progesterone, partially reversed the inhibition of glial proliferation by N-CAM and by the recombinant third Ig domain of N-CAM (Fig. 3; IgIII) and fully reversed the effects of dexamethasone (not shown). The effect of glucocorticoid antagonists was not related to the nature or source of the N-CAM preparation since these agents were able to reverse the inhibition of proliferation both by native N-CAM and the recombinant third Ig domain. Inhibition by IgIII alone was approximately 60% (Fig. 3 Left) and in the presence of each of the three antagonists at 1 μM concentration, inhibition was reduced to approximately 20% (Fig. 3, solid bars). The antagonists alone had no effect on proliferation at this concentration (stippled bars). These observations support the hypothesis that the inhibition of astrocyte proliferation by N-CAM may be mediated in part by glucocorticoid receptor pathways.
Figure 3.
Effects of glucocorticoid receptor antagonists on the inhibition of astrocyte proliferation by the third Ig domain of N-CAM. Glucocorticoid antagonists (1 μM) and the recombinant N-CAM IgIII domain (10 μg/ml) were added simultaneously to cultures in the proliferation assay. Stippled bars, values obtained from cells treated with the antagonists alone; solid bars, those observed with antagonists plus N-CAM IgIII. In control cultures, addition of N-CAM IgIII inhibited proliferation to 40% of control levels. This inhibitory effect was partially reversed in the presence of three different glucocorticoid receptor antagonists, RU-486, DHEA, and progesterone (PG). Results presented are the average of quadruplicate measurements in three separate experiments ± SEM.
Effects of N-CAM on Transcriptional Regulation via the GRE.
Glutamine synthetase mRNA levels were increased in astrocytes treated with N-CAM (see Fig. 1). The gene for this enzyme has a GRE in its promoter (11, 12) and its transcription is elevated by glucocorticoids (10). To determine whether the observed induction of glutamine synthetase by N-CAM treatment of astrocytes involved activation of a GRE, a synthetic GRE promoter–reporter construct (GRE-luc) containing two copies of a consensus GRE upstream of the luciferase gene was prepared. GRE-luc was transfected into primary astrocytes and luciferase activity was tested with or without N-CAM treatment. Baseline activity of the GRE-luc construct in untreated cells was comparable to the activity of the basal promoter control plasmid pGL3 (not shown) suggesting that endogenous steroids, if any, were present at levels too low to activate the GRE-luc construct.
N-CAM treatment of astrocyte cultures for 12 hr resulted in almost a 2-fold increase in the expression of the reporter gene (Fig. 4), an increase similar to that previously shown (12, 19–21) to be induced in retinal Müller glial cells by cell contact. Reporter gene activity returned to baseline levels by 24 hr. In a separate experiment using a 6-hr incubation, N-CAM-stimulated luciferase activity (200 ± 30% of control) was abolished in the presence of the competitive antagonist RU-486 (100 ± 20% of control). Stimulation by N-CAM or IgIII ranged from 1.4- to 2-fold with different cell preparations, and was always inhibited by RU-486. In all cases, the level of stimulation by N-CAM or IgIII was approximately 25% of that stimulated by dexamethasone. Constructs containing a mutated GRE did not respond to dexamethasone or N-CAM.
Figure 4.
Time course of the effects of N-CAM on stimulation of transcription via a GRE. Primary astrocytes were electroporated with the GRE-luc and CMV-β-gal vectors, cultured in serum-free medium for 48 hr, and then treated with 10 μg/ml N-CAM for 1–24 hr. Cell lysates were prepared and luciferase and β-gal activities were measured as described. The data are represented as a percentage of luciferase activity in control cells that were untreated. Each point represents the average of triplicate measurements with error bars showing the SD.
DISCUSSION
Prompted by the observation that N-CAM treatment of astrocytes in culture resulted in increased levels of the mRNAs for glutamine synthetase and calreticulin, we explored the possibility that inhibition of astrocyte proliferation (5, 6) was mediated in part by changes in glucocorticoid receptor pathways. We found that steroids including dexamethasone, corticosterone, and aldosterone inhibited the proliferation of rat cortical astrocytes in culture and that the glucocorticoid antagonist RU-486 prevented the inhibition by dexamethasone. These findings suggested that the effects of N-CAM and N-CAM domains on astrocyte proliferation may be mediated in part through glucocorticoid receptor pathways. Two observations lent support to this notion: (i) the inhibitory effects of N-CAM were reversed in part by RU-486, DHEA, and progesterone and (ii) cells transfected with a luciferase reporter gene linked to a minimal promoter and a GRE showed increased activity after exposure to N-CAM; this effect was also reversed by RU-486.
Although glutamine synthetase and calreticulin message levels were increased after N-CAM treatment, N-CAM mRNA levels decreased upon N-CAM treatment of astrocytes in a manner similar to the decrease of N-CAM observed in confluent fibroblasts (22) and in neurons aggregated via N-CAM (9). N-CAM levels themselves may therefore be controlled by cell–cell contact and may serve as a sensing mechanism to translate extracellular interactions into changes in gene expression and cell proliferation.
The observation that glucocorticoids inhibit astrocyte proliferation has counterparts in a number of other cellular systems (reviewed in ref. 23) and complex effects of steroid agonists and antagonists on glioma cell proliferation have been noted (24–29). In hepatoma cells, treatment with dexamethasone-arrested proliferation in the G1 phase of the cell cycle (30). Studies to determine at what point in the cell cycle N-CAM and steroids arrest progression in astrocytes may provide additional clues to the mechanism of action of each of these antiproliferative agents.
There are striking parallels between the findings on the effect of N-CAM on astrocyte gene expression and previous studies suggesting that cell–cell contact regulates glucocorticoid receptor activity and leads to alterations in glutamine synthetase expression in glia (12, 20, 31, 32). Glutamine synthetase is activated in rat astrocytes both by glucocorticoids (10) and by contact between neuronal and glial cells (31). Moreover, in the absence of contact with neurons, the presence of corticosteroids is insufficient to stimulate glutamine synthetase activity in chicken Müller glial cells (19). Reporter gene constructs containing either the endogenous glutamine synthetase promoter or a synthetic upstream GRE similar to the construct used here have been shown to be induced by neuron–glia contact (12, 19–21), suggesting that this control occurs through activation of the GRE by the glucocorticoid receptor. The results of the present experiments provide a possible molecular basis for the previous observations, although the evidence obtained does not yet prove the hypothesis that N-CAM acts through glucocorticoid pathways.
The likelihood that pathways other than those involving the glucocorticoid receptor may be activated by N-CAM in astrocytes must therefore be addressed. In neurons, a number of second messengers have been shown to be stimulated after N-CAM binding (7), primarily on the basis of assays measuring the ability of N-CAM-expressing cells to support neurite extension in the presence of drugs or other agents that block particular signaling pathways. Analysis of neuronal and glial second messenger pathways activated by N-CAM binding may be facilitated by the parallel use of the previously described assays (8) and the direct assays used here to measure astrocyte gene expression and proliferation.
In this study, baseline activity of the GRE-luc construct in astrocytes was comparable to the activity of the pGL3 minimal promoter vector, suggesting that endogenous levels of steroids in the astrocyte cultures, if any, were insufficient to activate the expression of the GRE-luc construct. Ligand-independent activation of glucocorticoid receptors has been observed in other studies (13, 14, 33–36). This activation can occur through a variety of mechanisms that include enhanced translocation of the glucocorticoid receptor into the nucleus (33–35), alterations in the interactions of the glucocorticoid receptor with any of the numerous proteins with which it interacts in either the cytoplasm or the nucleus (37), and changes in second messengers leading to modulation of glucocorticoid receptor activity (38–40).
While the detailed mechanisms underlying the effects of corticosteroids in vitro remain to be worked out, it would be valuable to examine the effects of these agents on astrocytes in vivo. Injection of N-CAM or its peptides into sites of cortical lesions has been shown (6) to reduce the proliferation of reactive astrocytes. A similar experiment using corticosteroids might yield further support for their use in the treatment of central nervous system injuries (41). Furthermore, the combined use of N-CAM peptides (6) together with steroids may provide an opportunity to reduce the systemic effects of steroids used in the treatment of central nervous system injuries.
Acknowledgments
We thank Mr. Nguyen Tran, Ms. Stacey C. Olson, Mr. Laurent Gousset, Mr. Andrew Benedict, and Ms. Catherine Cowley for excellent technical assistance; Mr. Brian Cox for help with graphics; Drs. Bruce Cunningham, Joseph Gally, Frederick Jones, George Miklos, and Lars Terenius for helpful discussions; and Dr. Bruce Cunningham for providing the recombinant N-CAM proteins. This work was supported by U.S. Public Health Service Grant HD-09635 to G.M.E., a Shannon Award (NS-34874) to K.L.C., National Science Foundation Grant IBN-9422111 to K.L.C., and a grant from the G. Harold and Leila Y. Mathers Foundation to G.M.E. G.M.E. and K.L.C. are consultants to Becton Dickinson and Company.
ABBREVIATIONS
- N-CAM
neural cell adhesion molecule
- GRE
glucocorticoid response element
- GRE-luc
construct with GRE linked to a luciferase reporter gene
- DHEA
dehydroepiandrosterone-3-sulfate
- β-gal
β-galactosidase
References
- 1.Bartsch U, Kirchhoff F, Schachner M. J Comp Neurol. 1989;284:451–462. doi: 10.1002/cne.902840310. [DOI] [PubMed] [Google Scholar]
- 2.Noble M, Albrechtsen M, Moller C, Lyles J, Bock E, Goridis C, Watanabe M, Rutishauser U. Nature (London) 1985;316:725–728. doi: 10.1038/316725a0. [DOI] [PubMed] [Google Scholar]
- 3.Nybroe O, Albrechtsen M, Dahlin J, Linneman D, Lyles J M, Moller C J, Bock E. J Cell Biol. 1985;101:2310–2315. doi: 10.1083/jcb.101.6.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edelman G M, Crossin K L. Annu Rev Biochem. 1991;60:155–190. doi: 10.1146/annurev.bi.60.070191.001103. [DOI] [PubMed] [Google Scholar]
- 5.Sporns O, Edelman G M, Crossin K L. Proc Natl Acad Sci USA. 1995;92:542–546. doi: 10.1073/pnas.92.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krushel L A, Sporns O, Cunningham B A, Crossin K L, Edelman G M. Proc Natl Acad Sci USA. 1995;92:4323–4327. doi: 10.1073/pnas.92.10.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doherty P, Walsh F S. Curr Opin Neurobiol. 1994;4:49–55. doi: 10.1016/0959-4388(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 8.Doherty P, Fazeli M S, Walsh F S. J Neurobiol. 1995;26:437–446. doi: 10.1002/neu.480260315. [DOI] [PubMed] [Google Scholar]
- 9.Mauro V P, Wood I C, Krushel L, Crossin K L, Edelman G M. Proc Natl Acad Sci USA. 1994;91:2868–2872. doi: 10.1073/pnas.91.7.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Banion M K, Young D A, Bohn M C. Mol Brain Res. 1994;22:57–68. doi: 10.1016/0169-328x(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 11.Pu H, Young A P. Gene. 1990;89:259–263. doi: 10.1016/0378-1119(90)90014-i. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Young A P. J Biol Chem. 1991;266:24332–24338. [PubMed] [Google Scholar]
- 13.Burns K, Duggan B, Atkinson E A, Famulski K S, Nemer M, Bleackley R C, Michalak M. Nature (London) 1994;367:476–480. doi: 10.1038/367476a0. [DOI] [PubMed] [Google Scholar]
- 14.Dedhar S, Rennie P S, Shago M, Hagesteijn C-Y, Yang H, Filmus J, Hawley R G, Bruchovsky N, Cheng H, Matusik R J, Giguere V. Nature (London) 1994;367:480–483. doi: 10.1038/367480a0. [DOI] [PubMed] [Google Scholar]
- 15.Ranheim T S, Edelman G M, Cunningham B A. Proc Natl Acad Sci USA. 1996;93:4071–4075. doi: 10.1073/pnas.93.9.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yudkoff M, Daikhin Y, Nissim I, Pleasure D, Stern J, Nissim I. J Neurochem. 1994;63:1508–1515. doi: 10.1046/j.1471-4159.1994.63041508.x. [DOI] [PubMed] [Google Scholar]
- 17.Strähle U, Klock G, Schütz G. Proc Natl Acad Sci USA. 1987;84:7871–7875. doi: 10.1073/pnas.84.22.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strähle U, Schmid W, Schütz G. EMBO J. 1988;7:3389–3395. doi: 10.1002/j.1460-2075.1988.tb03212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reisfeld S, Vardimon L. Mol Endocrinol. 1994;94:1224–1233. doi: 10.1210/mend.8.9.7838155. [DOI] [PubMed] [Google Scholar]
- 20.Vardimon L, Fox L, Degenstein L, Moscona A A. Proc Natl Acad Sci USA. 1988;85:5981–5985. doi: 10.1073/pnas.85.16.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ben-Dror I, Havazelet N, Vardimon L. Proc Natl Acad Sci USA. 1993;90:1117–1121. doi: 10.1073/pnas.90.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aoki J, Umeda M, Takio K, Titani K, Utsumi H, Sasaki M, Inoue K. J Cell Biol. 1991;115:1751–1761. doi: 10.1083/jcb.115.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jusko W J. Toxicology. 1995;102:189–196. doi: 10.1016/0300-483x(95)03047-j. [DOI] [PubMed] [Google Scholar]
- 24.Grasso R J, Johnson C E. Proc Soc Exp Biol Med. 1977;154:238–241. doi: 10.3181/00379727-154-39645. [DOI] [PubMed] [Google Scholar]
- 25.Freshney R I, Sherry A, Hassanzadah M, Freshney M, Crilly P, Morgan D. Br J Cancer. 1980;41:857–866. doi: 10.1038/bjc.1980.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langeveld C H, van Waas M P, Stoof J C, Sutanto W, deKloet E R, Wolbers J G, Heimans J J. J Neurosci Res. 1992;31:524–531. doi: 10.1002/jnr.490310316. [DOI] [PubMed] [Google Scholar]
- 27.Pinski J, Halmos G, Shirahige Y, Wittliff J L, Schally A V. J Clin Endocrinol Metab. 1993;77:1388–1391. doi: 10.1210/jcem.77.5.8077338. [DOI] [PubMed] [Google Scholar]
- 28.Valentini S R, Oliveira M L S, Sasahara R M, Armelin M C S. Braz J Med Biol Res. 1994;27:541–546. [PubMed] [Google Scholar]
- 29.Neuberger T J, Kalimi O, Regelson W, Kalimi M, deVries G H. J Neurosci Res. 1994;38:300–313. doi: 10.1002/jnr.490380308. [DOI] [PubMed] [Google Scholar]
- 30.Sànchez I, Goya L, Vallerga A K, Firestone G L. Cell Growth Differ. 1993;4:215–225. [PubMed] [Google Scholar]
- 31.Wu D K, Scully S, deVellis J. J Neurochem. 1988;50:929–935. doi: 10.1111/j.1471-4159.1988.tb03001.x. [DOI] [PubMed] [Google Scholar]
- 32.Linser P, Moscona A A. Dev Biol. 1983;96:529–534. doi: 10.1016/0012-1606(83)90190-2. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez E R. J Biol Chem. 1992;267:17–20. [PubMed] [Google Scholar]
- 34.Guiochon-Mantel A, Milgrom E. Trends Endocrinol Metab. 1993;4:322–328. doi: 10.1016/1043-2760(93)90074-o. [DOI] [PubMed] [Google Scholar]
- 35.Ning Y-M, Sanchez E R. J Biol Chem. 1993;268:6073–6076. [PubMed] [Google Scholar]
- 36.Tanaka H, Makino Y, Miura T, Hirano F, Okamoto K, Komura K, Sato Y, Makino I. J Immunol. 1996;156:1601–1608. [PubMed] [Google Scholar]
- 37.Bamberger C M, Schulte H M, Chrousos G P. Endocr Rev. 1996;17:245–261. doi: 10.1210/edrv-17-3-245. [DOI] [PubMed] [Google Scholar]
- 38.Rangarajan P N, Umesono K, Evans R M. Mol Endocrinol. 1992;6:1451–1457. doi: 10.1210/mend.6.9.1435789. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H, Li Y-C, Young A P. Proc Natl Acad Sci USA. 1993;90:3880–3884. doi: 10.1073/pnas.90.9.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Espinas M L, Roux J, Pictet R, Grange T. Mol Cell Biol. 1995;15:5346–5354. doi: 10.1128/mcb.15.10.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bracken M B, Shepard M J, Collins W F, Holford T R, Young W, Baskin D S, Eisenberg H E, Flamm E, Leo-Summers L, Maroon J, Marshall L F, Perot P L, Jr, Piepmeier J, Sonntag V K H, Wagner F C, Wilberger J E, Winn H R. N Engl J Med. 1990;322:1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]