Introduction
The oxidative phase of the biotransformation of drugs is a function of hepatic or intestinal cytochromes P-450, also called the mixed-function oxidase system. Three families of cytochromes – CYP1, CYP2, and CYP3 – perform the oxidative metabolism of drugs in humans. Within these families, five isoforms – CYP 1A2, CYP 2C9, CYP 2C19, CYP 2D6, and CYP 3A4 – account for nearly all the side-effects related to drug interactions [1, 2].
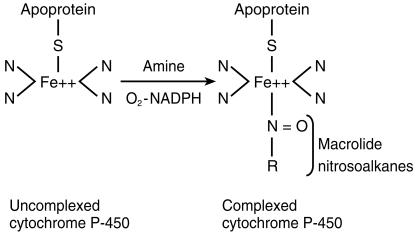
The ability of macrolide antibiotics to interact with the biotransformation of some other drugs has been widely recognized, mostly with erythromycin and troleandomycin because these compounds have been marketed decades ago. Macrolides can induce their own hepatic biotransformation into nitrosoalkanes. These metabolites are derived from the metabolic oxidation of the –N(CH3)2 group of the antibiotic to the corresponding –NO group. Nitrosoalkanes subsequently form inactive CYP 3A4-iron-metabolite complexes (Figure 1) resulting in inhibition of the CYP 3A4-mediated catalytic activity [3]. This mechanism accounts for most of the drug interactions produced by macrolides [4].
Figure 1.
Metabolism of macrolides.
Macrolides differ in their abilities to bind to and inhibit the cytochrome P-450 isoform CYP 3A4 [3, 5–7]. These differences prompted von Rosenstiel & Adam [8] to classify macrolide antibiotics into three groups on the basis of data provided by in vitro experiments:
Group 1 agents include erythromycin and troleandomycin. Both drugs bind strongly to and inhibit markedly CYP 3A4. Since troleandomycin was withdrawn from the market many years ago, this drug will not be discussed further in the present paper;
Clarithromycin belongs to Group 2 agents. This drug exhibits lower affinity for CYP 3A4 as compared with erythromycin, and form complexes to a lesser extent;
Group 3 include azithromycin and dirithromycin. These compounds have been shown to interfere poorly with cytochrome P-450 system in vitro.
Clarithromycin has recently appeared to be similar to erythromycin in some drug interactions (e.g. with psychotropic agents), on the basis of the results of some clinical studies. Furthermore, a number of recent clinical case reports demonstrate that azithromycin and dirithromycin still exhibit some potential for drug interactions, although to a much lesser extent than that with erythromycin.
The discrepancy between what is expected from in vitro data and what may be observed in clinical practice underscores the well-known interindividual variability in the extent of cytochrome P-450 catalytic activity (as much as 10- to 20-fold). This pattern provides some explanation for why some patients appear to be more susceptible than others to a given drug–drug interaction.
It is now clear that the heterogeneity among patients in the ability to perform P-450 metabolism of some drugs is largely the result of genetic factors [1]. Aside from the drug-induced induction/inhibition enzymatic processes, some other nongenetic factors probably contribute to interpatient differences in the liver content or activity of individual P-450:
for example, dietary differences may contribute significantly [9, 10].
CYP 3A4 activity may be non specifically depressed by debilitation or disease, e.g. liver cirrhosis [11] or celiac disease [12].
importantly the infectious process by itsef can affect the activity of CYP; viral or bacterial pulmonary infections have been shown to depress CYP activity. In a sequential study conducted in 14 patients with fever and clinical pneumonia, antipyrine clearance was on average impaired by 36% [13]. Antipyrine elimination proceeds almost exclusively through hepatic biotransformation, and its metabolites are formed by at least 6 hepatic cytochrome P-450 isoforms, namely CYP 1A2, CYP 2B6, CYP 2C8, CYP 2C9, CYP 2C18 and CYP 3A4 [14]. Hence, the infection is likely to induce global inhibition of cytochrome P-450 catalytic activities. This stems probably from the release of endotoxin or interferon during the infection [15, 16]. Conceivably, the combination of interferon- or endotoxin-related CYP depression and the macrolide-induced inhibition of the CYP 3A4 isoform might result in an enhanced metabolic interaction.
An additional factor that may contribute to individual variability in the extent of drug interactions of pharmacokinetic type is the involvement of P-glycoprotein (P-gp). Drug-induced changes in expression of this protein may overlap changes at the level of CYP catalytic activities.
P-gp is an ATP-dependent efflux drug transporter that is constitutively expressed in normal tisues including the gastrointestinal epithelium, the canalicular membrane of the liver, the kidney and capillary endothelial cells in the central nervous system [17, 18]. P-gp appears to play a key role in absorption, distribution and elimination of many anticancer agents as well as other drugs such as digoxin or cyclosporin [19, 20]. Many P-gp inhibitors are also inhibitors of CYP 3A [21]. However, Wandel et al.[22] have demonstrated recently that there is no significant correlation between the ability of the P-gp inhibitors to inhibit P-gp and their ability to inhibit CYP 3A. In the case of macrolide antibiotics, erythromycin has been shown to reverse significantly multidrug resistance in cell-lines, which retain a higher expression of P-gp than the drug-sensitive parental cells [23], suggesting an inhibitory effect of the macrolide on P-gp expression. Also, clarithromycin appears capable of reducing the renal clearance of digoxin through inhibition of P-gp in the kidney epithelial cell [24].
While the involvement of P-gp in some pharmacokinetic drug interactions is an emerging issue, there is no further information at present on the role of this protein in macrolide–associated drug interactions. However, the interaction of some macrolides with cyclosporin might be partially explained by inhibition of P-gp (seebelow).
The present review addresses those macrolide–induced drug interactions (at the CYP level) that have been reported recently and shown to result in potentially serious side-effects in patients. Emphasis is placed on drug interactions involving the relatively recent and widely used macrolides clarithromycin, dirithromycin, and the azalide azithromycin because the last comprehensive reviews available on drug interactions of macrolides were published in 1995 [8, 25]. Following these reports, important drug interactions have emerged (e.g. with cisapride or pimozide) and some others, initially thought to be rare or dubious, have been substantiated or clarified (e.g. with benzodiazepines or HMG-CoA reductase inhibitors).
Recently emerged or substantiated drug interactions
Psychotropic agents
1 a Benzodiazepines
Alprazolam, midazolam, temazepam, and triazolam are among the known substrates of CYP 3A4 [26].
Triazolam:
Using in vitro preparations of human liver microsomes, Greenblatt et al.[27] showed that erythromycin and clarithromycin are potent inhibitors, with similar IC50 values, of triazolam biotransformation. In contrast, azithromycin is a weak inhibitor. Consistent with these findings are data from a controlled clinical study conducted by the same authors in healthy volunteers [27]: both erythromycin and clarithromycin significantly increased triazolam peak plasma concentrations and area under the serum concentration-time curve (AUC), prolonged elimination half-life, and decreased markedly the apparent oral clearance of this benzodiazepine to 33% and 22% of the control value, respectively (characteristics of macrolide regimen are indicated in Table 1). The inhibitory effect of clarithromycin was greater than that of erythromycin, especially on triazolam half-life and AUC. All of the pharmacodynamic effects of triazolam were enhanced by coadministration of erythromycin and clarithromycin. The dynamic interactions are consistent with an increase in triazolam plasma concentrations. The greatest impairment, as assessed by an electro-encephalogram and the digit symbol substitution test, was associated with the triazolam-clarithromycin combination. In contrast, azithromycin produced no effect on the kinetics or dynamics of triazolam.
Table 1.
Summary of recently recognized drug interactions or noninteractions associated with the macrolide antibiotics erythromycin, clarithromycin, azithromycin, or dirithromycin.
| Substrate | Interacting macrolide [ref] | Subjects Type of study | Macrolide regimen | Results | Action |
|---|---|---|---|---|---|
| Alprazolam | Erythromycin [31] | healthy volunteers randomized crossover | 400 mg tid ×10 days | 62% increase in AUC no change in psychomotor function variables (alprazolam was given as a single oral dose) | monitor effects |
| Midazolam | Erythromycin [28] | healthy volunteers randomized crossover | 500 mg tid ×6 days | fourfold increase in AUC significantly altered psychomotor performances | avoid combination with erythromycin or clarithromycin, or reduce midazolam dosage by 75% |
| Clarithromycin [29] | healthy volunteers sequential | 500 mg bid ×7 days | 2.4-fold increase in oral midazolam availability | ||
| Clarithromycin [30] | healthy volunteers randomized | 250 mg bid ×5 days | 3.5-fold increase in AUC, enhanced pharmacodynamics no significant pharmacokinetic or dynamic change | ||
| Azithromycin [30] | 500 mg od ×3 days | ||||
| Triazolam | Erythromycin [27] | healthy volunteers randomized | 500 mg bid ×2 days | threefold increase in AUC, enhanced monitor dynamic effects | monitor effects |
| Clarithomycin [27] | crossover | 500 mg bid ×2 days | fivefold increase in AUC, enhanced effects | ||
| Azithromycin [27] | 500 mg day1, 250 mg day2 | no kinetic or dynamic change | |||
| Clozapine | Erythromycin [32] | case-report | 333 mg tid ×3 days | increased serum clozapine conc. leukocytosis, somnolence, disorientation | avoid combination or halve clozapine dosage and monitor for side-effects |
| Erythromycin [33] | case-report | 250 mg qid ×7 days | twofold increase in serum clozapine conc. tonic-clonic seizure | ||
| Pimozide | Clarithromycin [35] | healthy volunteers randomized crossover | 500 mg bid ×5 days | 113% increase in AUC significant increase in QT interval | combination contraindicated |
| Carbamazepine | Clarithromycin [25] | healthy volunteers randomized crossover | 500 mg bid ×5 days | significant decrease in AUC and Cmax of carbamazepine epoxide metabolite | reduce carbamazepine dosage by 25%-50% |
| Azithromycin [25] | healthy volunteers | 500 mg od ×3 days | no kinetic change | ||
| Disopyramide | Clarithromycin [52] | case report | 250 mg bid ×6 days | ventricular fibrillation marked QT prolongation (625 ms) | monitor closely ECG and plasma drug conc |
| Quinidine | Erythromycin [49] | healthy volunteers sequential | 250 qid ×7 days | 34% decrease in total clearance Cmax increased by 39% | monitor ECG, serum drug conc. and factors predisposing to torsade de pointes |
| Cisapride | Clarithromycin [40] | case-report | 500 mg bid ×3 days | polymorphic ventricular tachycardia QT interval increased to 640 ms | combination contraindicated |
| Clarithromycin [41] | healthy volunteers randomized crossover | 500 mg bid ×5 days | threefold increase in AUC 25 ms increase in QT interval | ||
| Simvastatin | Erythromycin [45] | healthy volunteers randomized crossover | 500 mg tid ×2 days | 6.2 fold increase in AUC | avoid combination, or monitor serum creatine kinase and occurence of muscle aches |
| Lovastatin | Clarithromycin [46] | case report | 500 mg bid ×10 days | acute rhabdomyolysis | same as simvastatin |
| Azithromycin [46] | case report | 250 mg oid ×5 days | acute rhabdomyolysis | ||
| Ergotamine | Clarithromycin [88] | case report | 500 mg bid ×5 days | clinical ergotism, lingual ischaemia | combination contraindicated |
| Loratadine | Erythromycin [84] | healthy volunteers randomized crossover | 500 mg tid ×10 days | 40% and 46% increase in the AUC of loratadine and its active metabolite respectively, no significant change in QT interval | no dosage adjustment required |
| Clarithromycin [85] | healthy volunteers randomized crossover | 500 mg bid ×10 days | 36% and 76% increase in steady- state Cmax and AUC respectively, no significant change in QT interval | no side-effects, no dosage adjustment required | |
| Theophylline po | Clarithromycin [25] | healthy volunteers sequential | 500 mg bid ×4 days | 20% increase in steady-state values of Cmax, trough conc. and AUC | monitor drug conc in patients with baseline values in the upper therapeutic range |
| Dirithromycin [79] | patients with COPD sequential | 500 mg od ×10 days | no significant kinetic change | safe utilization in patients with COPD | |
| Azithromycin [25] | healthy volunteers sequential | 250 mg od ×5 days | no significant kinetic change | ||
| Warfarin | Clarithromycin [60] | case report | 500 mg tid ×3 days | INR increased to 7.3 | monitor INR carefully |
| Clarithromycin [59] | case report | 500 mg bid ×14 days | INR increased to 16.8 | ||
| Azithromycin [62] | case report | 250 mg/d ×3 days | INR increased to 4.9 | ||
| Dirithromycin [42] | healthy volunteers sequential | 500 mg/d ×10 days | no significant kinetic or dynamic (INR) change | ||
| Cyclosporine po | Clarithromycin [70] | case-report | 500 mg tid ×21 days | serum conc. increased 10 times the baseline values, twofold increase of serum creatinine level | monitor trough steady-state conc. |
| Dirithromycin [42] | kidney transplant patients, sequential | 500 mg/d ×14 days | 17% decrease in mean apparent clearance, 13% increase in trough steady-state conc. | ||
| Tacrolimus | Erythromycin [73] | case report | 1g qid ×2 days | trough conc. increased by sixfold | monitor drug conc. |
Abbreviations: AUC = area under the serum concentration-time curve; conc.= concentration; Cmax= maximal concentration in serum; INR = international normalized ratio; COPD = chronic obstructive pulmonary disease.
This study demonstrates that clarithromycin is at least as potent an inhibitor of CYP 3A4 as erythromycin. This finding is further substantiated by studies on the midazolam–macrolide interaction.
Midazolam:
The interaction between erythromycin and orally administered midazolam was investigated in a randomized, crossover study in healthy volunteers [28]. Pretreatment with erythromycin (500 mg) three times daily for 6 days resulted in an almost threefold increase in Cmax and more than a fourfold increase in AUC of midazolam. This interaction resulted in undesirably severe and excessively long-lasting hypnotic effects and was ascribed to the inhibition of CYP 3A4-mediated metabolism of midazolam by erythromycin.
Clarithromycin reduces the clearance of midazolam [29]. Following pretreatment with clarithromycin (500 mg) twice daily for 7 days, the clearance of midazolam was reduced by 64% following iv administration, and by 86% after oral dosing. Oral bioavailability of midazolam was increased significantly from 0.31 to 0.75 (mean values) after dosing with clarithromycin. Using a stable isotope of the benzodiazepine, the authors showed that besides the liver, the intestine is a major site of interaction between oral midazolam and clarithromycin. Additionally, they concluded that interindividual variability in first-pass extraction of high-affinity CYP 3A substrates, such as midazolam, is primarily a function of intestinal enzyme activity.
This drug interaction was described earlier by Yeates et al.[30] who reported a 3.6-fold increase in the AUC of oral midazolam (given as a single dose), with enhancement of psychomotor effects, after a 5-day twice daily treatment with clarithromycin (250 mg). In contrast, the authors found no interaction of azithromycin (500 mg once a day for 3 days) with the pharmacokinetics or pharmacodynamics of midazolam.
Alprazolam:
Alprazolam is another benzodiazepine where the metabolic clearance is impaired by erythromycin. In healthy volunters, erythromycin (400 mg) three times daily for 10 days produced a 62% increase in the AUC(0,48 h), a 60% decrease in oral clearance and a more than twofold increase in elimination half-life of alprazolam when given orally as a single dose [31].
In summary, coadministration of a macrolide antibiotic (with the exception of azithromycin) with one of the highly metabolized benzodiazepines mentioned above should be avoided, or the dose of the benzodiazepine should be substantially reduced.
1 b Neuroleptics
Clozapine is a new antipsychotic agent used in the management of schizophrenia resistant to other neuroleptic medications. Erythromycin has been reported to interact with this agent, presumably through inhibition of its metabolic clearance; case-reports of serious side-effects – including seizure, somnolence, disorientation, difficulty in coordination and ambulation – associated with the coadministration of erythromycin and clozapine (Table 1) have been published [32, 33]. This drug combination should therefore be avoided.
Pimozide is a neuroleptic agent used in the treatment of delusion, schizophrenia, and other psychiatric disorders. Two fatal cases of ventricular dysrhythmia have been ascribed to the drug association pimozide × clarithromycin, presumably due to excessive prolongation of QT interval [34]. In a controlled pharmacokinetic study, clarithromycin (500 mg) twice daily for 5 days inhibited the metabolic clearance (CYP3A-mediated) of pimozide, resulting in elevation of plasma concentrations, significant prolongation of the QT interval, thereby increasing the risk of pimozide cardiotoxicity [35]. Accordingly, this drug association is contraindicated.
2 Cisapride
Cisapride is a widely used drug for gastro-oesophageal reflux, gastroparesis, and dyspepsia. The drug undergoes extensive first-pass metabolism in both the liver and the intestine [36].
Cisapride caused tachycardia and extrasystoles in a review of records of over 13000 patients receiving the agent [37]. The first report of an arrhythmic drug interaction with cisapride was with erythromycin [38]; the patient developed a QT interval of 550 ms from a normal baseline value with progression to polymorphic nonsustained ventricular tachycardia. Over 50% of the reports of torsade de pointes, prolonged QT intervals, and deaths associated with cisapride are due to interactions with drugs known to inhibit the CYP3A4 and, consequently, the metabolism of cisapride [39]. Risk factors for dysrhythmia were identified as history of coronary disease and dysrhythmia, renal insufficiency, and electrolyte imbalance (including hypokalaemia, hypomagnesaemia, and hypocalcemia) [39].
Clarithromycin seems to exhibit a similar adverse reaction when coadministered with cisapride [40]. Data from a case-report and results from a controlled trial on this drug interaction are summarized in Table 1. Potentiation of the cardiotoxic effect of cisapride resulting from the inhibition of the CYP 3A4 – mediated metabolism of the drug by clarithromycin [40, 41] is probably the mechanism underlying the adverse drug reaction.
No studies have yet been reported regarding the potential of the other macrolides for such an interaction with cisapride [42, 43]. Until additional data are available, however, concomitant use of cisapride with any macrolide antibiotic should be avoided.
3 HMG-CoA reductase inhibitors
These agents are metabolized by CYP 3A4 and have dose-related toxic effects on skeletal muscle that may range from diffuse myalgia and myopathy to severe rhabdomyolysis [44].
In a clinical pharmacokinetic study, erythromycin (500 mg) twice daily for 2 days increased the AUC of simvastatin in serum sixfold [45], due presumably to an inhibitory effect of erythromycin on CYP 3A4. Accordingly, concurrent administration of erythromycin and simvastatin should be avoided.
Rhabdomyolysis is a complication known to be associated with concomitant use of lovastatin and erythromycin. In order to reduce this risk, limiting the daily dose of lovastatin to 20 mg has been advocated [8]. Rhabdomyolysis has also been ascribed to the concomitant administration of lovastatin and clarithromycin or azithromycin (one case-report each,Table 1) [46]. Therefore, possible adverse effects, such as elevated serum creatine kinase and muscle tenderness, should be closely monitored when a combination of simvastatin or lovastatin with erythromycin (and probably other macrolides) is used.
4 Class IA antiarrhythmic drugs
Quinidine is eliminated from the body primarily through hepatic biotransformation, and approximately 50% of its metabolism is catalysed by CYP 3A4 [47]. Spinler et al.[48] reported that addition of intravenous erythromycin lactobionate (1 g) four times daily to chronic quinidine therapy resulted in a 50% decrease of the total clearance of quinidine after 5 days of macrolide therapy. In an open clinical study in healthy volunteers, the pharmacokinetics of a single oral dose of quinidine (200 mg) was evaluated before and during administration of erythromycin (250 mg) 4 times daily for 7 days [49]. Erythromycin reduced the total clearance of quinidine and its partial clearance by 3-hydroxylation and N-oxidation by 34, 50 and 33%, respectively, as the median Cmax increased by 39%, inhibition of both hepatic and intestinal CYP 3A4 activity by erythromycin was considered to account for these observations, while the role of P-gp was thought to be ancillary.
Therefore, quinidine concentrations should be monitored closely if quinidine and erythromycin are coadministered. Additionaly, given the antiarrhythmic activity of both drugs [50], electrocardiograms (ECG) should be performed and other factors that may predispose to a prolonged QT interval and torsades de pointes should be treated.
Two cases of life-threatening ventricular dysrhythmia resulting from the interaction between disopyramide-erythromycin have been reported [51].
One case involving the interaction with clarithromycin (Table 1) has been reported recently [52]. In all of these cases, serious ventricular dysrhythmia was induced by a marked prolongation of QT interval, i.e. ≥ 600 ms. This effect was ascribed to the rise in serum disopyramide concentrations, resulting from the impaired clearance of the drug (primarily metabolized through CYP 3A4) during macrolide treatment.
The aforementioned recommendations regarding quinidine hold true also for disopyramide in the case of concurrent administration of erythromycin or clarithromycin.
5 Warfarin
There are reports describing an increase in the hypoprothrombinaemic effect of warfarin sodium following the administration of erythromycin [8, 25]. Prothrombin times increased up to twofold after 7 days of therapy and were occasionally associated with bleeding complications. However, there is a discrepancy between such data and the changes observed in pharmacokinetic studies [53, 54]. For example in the trial by Bachman et al.[53], erythromycin decreased warfarin clearance by 14% in healthy volunteers. Warfarin is a racemic mixture of R-and S-warfarin, with the S-form two-to five-fold more potent. Both forms are metabolized by cytochrome P-450 with predominant involvement of CYP 1A1, CYP 1A2, CYP 2C9, CYP 2C19, and CYP 3A4 [55]. S-warfarin is metabolized primarily by CYP 2C9, while CYP 1A2 and CYP 3A4 account for most of the metabolism of R-warfarin.
The relatively limited changes in the pharmacokinetics of warfarin in healthy volunteers under treatment with erythromycin is consistent with inhibition of CYP 3A4, and to a lesser extent CYP 1A2 by erythromycin [2, 56]. Hence, this drug interaction is likely to be potentiated by other factors, particularly the disease state [57, 58]. As indicated earlier in this review, the process of infection, especially of pulmonary infection, can reduce substantially overall cytochrome P-450 catalytic activity [13].
Regarding the semisynthetic macrolides, only four cases of an interaction with warfarin have been published involving clarithromycin and azithromycin (2 cases each) [59–62]. Dirithromycin has no effect on prothrombin time in healthy volunteers receiving warfarin [42] (Table 1). Nevertheless, the unpredictability of this drug interaction demands careful monitoring of prothrombin time in patients treated concomitantly with warfarin and a macrolide.
Updated overview of previously well-recognized and clinically relevant drug interactions induced by macrolides
1 Immunosuppressive agents
Cyclosporin is extensively metabolized by CYP 3A pathway so there is considerable potential for interaction with macrolides [63]. This is of particular relevance since cyclosporin has low therapeutic index and its renal toxicity is concentration-related. Numerous clinical reports of significant increases in AUC and decreases in the clearance of cyclosporin following the administration of erythromycin are available [63]. This drug interaction seems to result from erythromycin-induced inhibition of CYP 3A4 in both liver and intestine [1, 64, 65]. It is thought that presystemic metabolism in the gastro-intestinal tract plays a leading role in the total metabolic clearance of cyclosporin and may explain the well-known erratic bioavailability of the drug [66].
In addition to CYP 3A4, it has emerged recently that P-gp is involved in the pharmacokinetic behaviour of cyclosporin. The drug is a substrate of P-gp [20] and some drug interactions with this immunosuppressive agent are mediated by P-gp [67]. Gupta et al.[68] reported that erythromycin increases the absolute bioavailability of orally administered cyclosporin. This effect might be ascribed to impairment of the presystemic metabolism of cyclosporin in the intestine as a result of erythromycin-induced inhibition of the intestinal CYP 3A4. However, given that P-gp is also present in the intestinal epithelial cells [17, 69] and that erythromycin has been shown to inhibit the expression of P-gp in tumoral cell lines in vitro[23], the increased bioavailability of cyclosporin during erythromycin treatment might be accounted for by inhibition of both CYP and P-gp in the intestine.
Cases of interaction between clarithromycin and cyclosporin with subsequent cyclosporin toxicity have been reported [8, 70]. According to Spicer et al.[70] (Table 1) the underlying mechanism is the macrolide-related inhibition of CYP 3A4, leading to decreased clearance and increased blood concentrations of cyclosporin. However, given that clarithromycin can inhibit P-gp [24], involvement of this protein in this drug interaction is conceivable.
The effect of dirithromycin on the steady-state pharmacokinetics of cyclosporin was evaluated in 15 stable kidney transplant patients [71]. Administration of dirithromycin (500 mg) daily for 14 days resulted in a 17% decrease in the apparent clearance of cyclosporin, a 16% increase in the average steady-state cyclo-sporin concentration, and a 13% increase in trough steady-state cyclosporin concentrations. All of these alterations were statistically significant. Although this interaction is limited, it still appears that a 17% decrease in cyclosporin clearance may be clinically significant.
A possible clinical interaction between cyclosporin and azithromycin was reported in a kidney transplant recipient [72].
In summary, when macrolides are used in patients under cyclosporin therapy, trough serum cyclosporin concentrations and serum creatinine concentrations must frequently be monitored to allow appropriate adjustment of the cyclosporin dosage.
Tacrolimus is a new immunosuppressive agent that is extensively metabolized by the CYP 3A4 and like cyclosporin is transported by P-gp [20]. The drug appears interact with erythromycin, as described in several case-reports [73]. For example, Desmond Padhi et al.[73] reported a clinical case where the addition of erythromycin (1 g) twice daily in a patient under chronic therapy with tacrolimus induced a sixfold increase in trough concentrations of tacrolimus after only 2 days of erythromycin therapy. The extent of the interaction seems to be similar to that between erythromycin and cyclosporin.
Accordingly, management of this situation is identical to that advised for the cyclosporin–macrolide interaction.
2 Theophylline
Interactions of macrolides with theophylline are fairly well-documented. In most studies, erythromycin and clarithromycin decreased theophilline clearance by 20–25% after 7 days of concomitant administration [8, 25]. The interaction is most likely to occur in patients receiving relatively high erythromycin dosages (> 1.5 g day−1) and in those receiving prolonged therapy [74].
Theophylline is metabolized in man by N-demethylation and by 8-hydroxylation. A number of studies has been published during the past 10 years directed toward the characterization of the CYP isoforms involved in the metabolism of theophylline. It is generally agreed that CYP 1A2 catalyses the N-demethylation [75–77] and is also involved in 8-hydroxylation [76, 77]. It seems that, in addition to CYP 1A2, CYP 2E1 and CYP 3A4 play a role in 8-hydroxylation [77, 78]. Inhibitors of CYP 3A4 (including troleandomycin) inhibit both N-demethylation [56] and 8-hydroxylation in vitro[77]. However, these experiments showed that CYP 3A4 inhibitors reduced N-demethylation by a maximum of 16%. On this basis, the well–known interaction of macrolide antibiotics with theophylline in vivo could be explained by the inhibition of CYP 1A2 and CYP 3A4. However, given the relatively weak inhibitory effect of macrolides on the CYP 1A2 activity in vitro, the inhibitory effect of macrolides on theophylline metabolism may be enhanced in subjects who exhibit low CYP 1A2 activity and subsequently higher CYP 3A4 activity, the latter isoenzyme standing as a substitute metabolic pathway [1]. This hypothesis has been substantiated recently in an in vitro study [77]. Such a pattern might explain why a number of prospective clinical trials fail to show a statistically significant reduction in theophylline clearance when this drug is coadministrated with erythromycin [4].
In two controlled studies, dirithromycin therapy did not appear to alter significantly the disposition of theophylline in healthy subjects or patients (Table 1) [42, 79]. The kinetics of iv aminophylline or oral theophylline were not altered in healthy volunteers after, respectively, a 3 or 5 day treatment with azithromycin (250 mg day−1). However, a case-report has been published of a moderate interaction of this macrolide with theophylline [80].
From a practical viewpoint, given the relative unpredictability of the extent of the theophylline–macrolide interaction, it remains necessary to monitor serum theophylline concentrations whatever the macrolide being used, especially in those patients with baseline theophylline concentrations in the upper therapeutic range (15–20 mg l−1).
3 Carbamazepine
Numerous case-reports and several pharmacokinetic studies document the well–known interaction between erythromycin and carbamazepine.
Following oral administration of this macrolide, a two-to fourfold increase in carbamazepine serum concentration is observed, with the extent of the interaction related to the dose of erythromycin [8]. In carbamazepine-treated patients, serious manifestations of toxicity occur within the 3 days of the start of erythromycin therapy. A number of case-reports have also been published regarding the interaction with clarithromycin [8]. The mechanism underlying this drug interaction is assumed to be the macrolide-induced inhibition of the CYP 3A4 isoform for which carbamazepine is a substrate [81].
Azithromycin and dirithromycin appear to be free from interaction with carbamazepine [42, 43].
In summary, serum carbamazepine concentrations should be closely monitored and patients observed for signs of toxicity in case of coadministration of erythromycin or clarithromycin.
4 Non sedating antihistamines
Terfenadine is a non sedating antihistamine that undergoes nearly complete first-pass biotransformation through CYP 3A4 pathway to form an acid metabolite. In susceptible individuals, accumulation of the parent compound can cause QT interval prolongation that may result in torsade de pointes [82]. Hazardous drug interactions involving this agent and some macrolides have been described [8]. Given that the drug is essentially withdrawn from the market, it will not be discussed further.
Loratadine, another H1-receptor antagonist, is metabolized primarily by CYP 3A4 and, to a lesser extent, by CYP 2D6 [83]. Erythromycin (500 mg) three times daily for 10 days reduced the metabolic clearance of loratadine [84] in a controlled clinical study. However, no changes in the QT interval and safety profile of loratadine were observed.
Carr et al.[85] evaluated the potential for an interaction between clarithromycin (500 mg) twice daily for 10 days and loratadine in a randomized crossover study in healthy volunteers. Clarithromycin increased the steady-state maximum observed plasma concentration and AUC over a dosing interval for loratadine (+ 36% and + 76%, respectively) and for descarboethoxy-loratadine, the active metabolite of loratadine (+ 69% and + 49%, respectively). No corresponding electrocardiographic pharmacodynamic interaction was observed. The authors concluded that given the wide margin of safety associated with loratadine, the observed pharmacokinetic interaction is probably unimportant.
Astemizole undergoes extensive first-pass metabolism to active metabolites and, like terfenadine, the parent compound is the cardiotoxic entity [82]. In many cases, drug interactions with terfenadine have been extrapolated to astemizole. Warnings about the concomitant use of terfenadine and macrolides apply also for astemizole [86].
5 Ergot alkaloids
Clinical ergotism has resulted from the coadministration of ergots and erythromycin [87]. This adverse effect is ascribed to the macrolide-induced inhibition of CYP 3A4, which is responsible for the metabolism of the ergots. Recently, a clinical case of ergotism with lingual ischaemia induced by a clarithromycin–ergotamine interaction has been described [88]. Accordingly, concomitant use of ergot alkaloids with erythromycin or clarithromycin is contraindicated. Until now, no case of such an interaction has been reported with azithro-mycin or dirithromycin. However, avoiding coadministration of ergot alkaloids with any of the macrolide antibiotics appears sensible.
Conclusions
A limiting factor of most controlled studies reviewed above is that they are conducted in healthy volunteers. Although these studies give initial indication of potential clinically significant drug interactions, the most accurate description of an interaction comes from conducting the study in the patient population that will use the combination and are receiving one of these drugs on a long-term basis. This underscores the importance of postmarketing surveillance of drug safety profiles. In this regard, reporting adverse events encountered in clinical practice appears to be essential, especially for recently available drugs. A knowledge of drug interactions, for example those induced by macrolides, contributes to safer management of patients.
References
- 1.Watkins PB. Drug metabolism by cytochrome P-450 in the liver and small bowel. Gastroenterol Clin N Am. 1992;21:511–526. [PubMed] [Google Scholar]
- 2.Slaughter RL, Edwards DJ. Recent advances: the cytochrome P – 450 enzymes. Ann Pharmacother. 1995;29:619–624. doi: 10.1177/106002809502900612. [DOI] [PubMed] [Google Scholar]
- 3.Pessayre D, Larrey D, Funck-Brentano C, et al. Drug interactions and hepatitis produced by some macrolide antibiotics. J Antimicrob Chemother. 1985;16(Suppl H):181–194. doi: 10.1093/jac/16.suppl_a.181. [DOI] [PubMed] [Google Scholar]
- 4.Periti P, Mazzei T, Mini E, Novelli A. Pharmacokinetic drug interactions of macrolides. Clin Pharmacokin. 1992;23:106–131. doi: 10.2165/00003088-199223020-00004. [DOI] [PubMed] [Google Scholar]
- 5.Gascon PM, Dayer P. Comparative effects of macrolide antibiotics on liver mono-oxygenases. Clin Pharmacol Ther. 1991;49:158. Abstract. [Google Scholar]
- 6.Ohmori S, Ishii I, Kurina SI, et al. Effects of clarithromycin and its metabolites on the mixed function oxydase system in hepatic microsomes of rats. Drug Metab Dispos. 1993;21:358–363. [PubMed] [Google Scholar]
- 7.Lindstrom TD, Hanssen BR, Wrighton SA. Cytochrome P-450 complex formation by dirithromycin and other macrolides in rat and in human liver. Antimicrob Agents Chemother. 1993;37:265–269. doi: 10.1128/aac.37.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Von Rosenstiel NA, Adam D. Macrolide antibacterials. Drug interactions of clinical significance. Drug Safety. 1995;13:105–122. doi: 10.2165/00002018-199513020-00005. [DOI] [PubMed] [Google Scholar]
- 9.Britto MR, McKean HE, Bruckner GG, Wedlung PJ. Polymorphisms in oxidation drug metabolism: relationship to food preference. Br J Clin Pharmacol. 1991;32:235–237. doi: 10.1111/j.1365-2125.1991.tb03887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuhr U, Dummert AL. The fate of naringin in humans: a key to grapefruit juice–drug interactions. Clin Pharmacol Ther. 1995;58:20–28. doi: 10.1016/0009-9236(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 11.Westphal JF, Brogard JM. Clinical pharmacokinetics of newer antibacterial agents in liver disease. Clin Pharmacokin. 1993;24:46–58. doi: 10.2165/00003088-199324010-00004. [DOI] [PubMed] [Google Scholar]
- 12.Lang CC, Brown RM, Kinirons MT, et al. Decreased intestinal CYP3A4 in celiac disease: reversal after successful gluten-free diet: a potential source of interindividual variability in first-pass drug metabolism. Clin Pharmacol Ther. 1996;59:41–46. doi: 10.1016/S0009-9236(96)90022-3. [DOI] [PubMed] [Google Scholar]
- 13.Sonne J, Dossing M, Loft S, Andreassen PB. Antipyrine clearance in pneumonia. Clin Pharmacol Ther. 1985;37:701–703. doi: 10.1038/clpt.1985.117. [DOI] [PubMed] [Google Scholar]
- 14.Engel G, Hofmann U, Heidemann H, et al. Antipyrine as a probe for human oxidative drug metabolism: identification of the cytochrome P 450 enzymes catalizing 4-hydroxy-antipyrine, 3-hydroxymethylantipyrine, and norantipyrine formation. Clin Pharmacol Ther. 1996;59:613–623. doi: 10.1016/S0009-9236(96)90001-6. [DOI] [PubMed] [Google Scholar]
- 15.Williams SJ, Baird-Lambert JA, Farrell GC. Inhibition of theophyllin metabolism by interferon. Lancet. 1987;ii:939–941. doi: 10.1016/s0140-6736(87)91422-x. [DOI] [PubMed] [Google Scholar]
- 16.Kitaichi K, Takagi K, Ixase M, et al. Decreased antipyrine clearance following endotoxin administration: in vivo evidence of the role of nitric oxide. Antimicrob Agents Chemother. 1999;43:2697–2701. doi: 10.1128/aac.43.11.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thiebaut F, Tsuruo T, Hamada H, et al. Cellular localization of the multidrug resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiebaut F, Tsuruo T, Hamada H, et al. Immunohistochemical localization in normal tissues of different epitopes in the multidrug transport protein P170: evidence for localization in brain capillaries and crossreactivity of one antibody with a muscle protein. J Histochem Cytochem. 1989;37:159–164. doi: 10.1177/37.2.2463300. [DOI] [PubMed] [Google Scholar]
- 19.Tanigawara Y, Okamura N, Hirai M, et al. Transport of digoxine by human P-glycoprotein expressed in a porcine kidney epithelial cell line (LLC-PK1) J Pharmacol Exp Ther. 1992;263:840–845. [PubMed] [Google Scholar]
- 20.Saeki T, Ueda K, Tanigawara Y, et al. Human P-glycoprotein transports cyclosporin A and FK 506. J Biol Chem. 1993;268:6077–6080. [PubMed] [Google Scholar]
- 21.Kim RB, Wandel C, Leake B, et al. Interrelationship between substrates and inhibitors of human CYP 3A and P-glycoprotein. Pharm Res. 1999;16:408–413. doi: 10.1023/a:1018877803319. [DOI] [PubMed] [Google Scholar]
- 22.Wandel C, Kim RB, Kajiji S, et al. P-glycoprotein and cytochrome P-450 3A inhibition: dissociation of inhibitory potencies. Cancer Res. 1999;59:3944–3948. [PubMed] [Google Scholar]
- 23.Hofsli E, Nissen-Meyer J. Reversal of drug resistance by erythromycin: erythromycin increases the accumulation of actinomycin D and doxorubicin in multi-drug resistant cells. Int J Cancer. 1989;44:149–154. doi: 10.1002/ijc.2910440126. [DOI] [PubMed] [Google Scholar]
- 24.Wakasugi H, Yano I, Ito T, et al. Effect of clarithromycin on renal excretion of digoxin: interaction with P-glycoprotein. Clin Pharmacol Ther. 1998;64:123–128. doi: 10.1016/S0009-9236(98)90030-3. [DOI] [PubMed] [Google Scholar]
- 25.Amsden GW. Macrolides versus azalides: a drug interaction update. Ann Pharmacother. 1995;29:906–917. doi: 10.1177/106002809502900913. [DOI] [PubMed] [Google Scholar]
- 26.Von Moltke LL, Greenblatt DJ, Schmider J, Harmatz JS, Shader RI. Metabolism of drugs by P450, 3A isoforms. Clin Pharmacokinet. 1995;29(Suppl 1):33–44. doi: 10.2165/00003088-199500291-00007. Implications for drug interactions in psychopharmacology. [DOI] [PubMed] [Google Scholar]
- 27.Greenblatt DJ, Von Moltke LL, Harmatz JS, et al. Inhibition of triazolam clearance by macrolide antimicrobial agents: in vitro correlates and dynamic consequences. Clin Pharmacol Ther. 1998;64:278–285. doi: 10.1016/S0009-9236(98)90176-X. [DOI] [PubMed] [Google Scholar]
- 28.Olkkola KT, Aranko K, Luurila H, et al. A potentially hazardous interaction between erythromycin and midazolam. Clin Pharmacol Ther. 1993;53:298–305. doi: 10.1038/clpt.1993.25. [DOI] [PubMed] [Google Scholar]
- 29.Gorski JC, Jones DR, Haener-Daniels BD, et al. The contribution of intestinal and hepatic CYP 3A to the interaction between midazolam and clarithromycin. Clin Pharmacol Ther. 1998;64:133–143. doi: 10.1016/S0009-9236(98)90146-1. [DOI] [PubMed] [Google Scholar]
- 30.Yeates RA, Lauten H, Zimmerman T. Interaction between midazolam and clarithromycin: comparison with azithromycin. Int J Clin Pharmacol Ther. 1996;34:400–405. [PubMed] [Google Scholar]
- 31.Yasui NY, Otani K, Kaneko S, et al. A kinetic and dynamic study of oral alprazolam with and without erythromycin in humans: in vivo evidence for the involvement of CYP 3A4 in alprazolam metabolism. Clin Pharmacol Ther. 1996;59:514–519. doi: 10.1016/S0009-9236(96)90179-4. [DOI] [PubMed] [Google Scholar]
- 32.Glassner Cohen LG, Chesley S, Eugenio S, et al. Erythromycin-induced clozapin toxic reaction. Arch Intern Med. 1996;156:675–677. [PubMed] [Google Scholar]
- 33.Funderberg LG, Vertrees JE, True JE. Seizure following addition of erythromycin to clozapin treatment. Am J Psychiatry. 1994;151:1840–1841. doi: 10.1176/ajp.151.12.1840b. [DOI] [PubMed] [Google Scholar]
- 34.Flockhart DA, Richard E, Woosley RL, Pearle PL, Drici MDA. metabolic interaction between clarithromycin and pimozide may result in cardiac toxicity (abstract PII-36) Clin Pharmacol Ther. 1996;59:189. [Google Scholar]
- 35.Desta Z, Kerbusch T, Flockhart DA. Effect of clarithromycin on the pharmacokinetics and pharmacodynamics of pimozide in healthy poor and extensive metabolizers of cytochrome P-450 2D6 (CYP 2D6) Clin Pharmacol Ther. 1999;65:10–20. doi: 10.1016/S0009-9236(99)70117-7. [DOI] [PubMed] [Google Scholar]
- 36.Barone JA, Jessen LM, Colaizzi JL, Bierman RH. Cisapride: a gastrointestinal prokinetic drug. Ann Pharmacother. 1994;28:488–500. doi: 10.1177/106002809402800413. [DOI] [PubMed] [Google Scholar]
- 37.Imman W, Kubota K. Tachycardia during cisapride treatment. Br Med J. 1992;305:1019. doi: 10.1136/bmj.305.6860.1019-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bran S, Murray W, Hirsch IB, Plamer JP. Long QT syndrome during high-dose cisapride. Arch Intern Med. 1995;155:765–768. [PubMed] [Google Scholar]
- 39.Wysowski DK, Bacsanyi J. Cisapride and fatal arrhythmia. N Engl J Med. 1996;335:290–291. doi: 10.1056/NEJM199607253350416. [DOI] [PubMed] [Google Scholar]
- 40.Piquette RK. Torsade de pointes induced by cisapride/clarythromycin interaction. Ann Pharmacother. 1999;33:22–26. doi: 10.1345/aph.18107. [DOI] [PubMed] [Google Scholar]
- 41.Van Haarst AD, Van Kloster GA, Van Gerven JM, et al. The influence of cisapride and clarithromycin on QT intervals in healthy volunteers. Clin Pharmacol Ther. 1998;64:542–546. doi: 10.1016/S0009-9236(98)90137-0. [DOI] [PubMed] [Google Scholar]
- 42.Watkins VS, Polk RE, Stotka JL. Drug interactions of macrolides: emphasis on dirithro-mycin. Ann Pharmacother. 1997;31:349–356. doi: 10.1177/106002809703100314. [DOI] [PubMed] [Google Scholar]
- 43.Garey KW, Amsden GW. Intravenous azithromycin. Ann Pharmacother. 1999;33:218–228. doi: 10.1345/aph.18046. [DOI] [PubMed] [Google Scholar]
- 44.Illingworth DR, Tobert JA. A review of clinical trials comparing HMG-CoA inhibitors. Clin Ther. 1994;16:366–385. [PubMed] [Google Scholar]
- 45.Kantola T, Kivisto KT, Neuvonen PJ. Erythromycin and verapamil considerably increase serum simvastatin and simvastatin acid concentrations. Clin Pharmacol Ther. 1998;64:177–182. doi: 10.1016/S0009-9236(98)90151-5. [DOI] [PubMed] [Google Scholar]
- 46.Grunden JW, Fisher KA. Lovastatin-induced rhabdomyolysis possibly associated with clarithromycin and azithromycin. Ann Pharmacother. 1997;31:859–863. doi: 10.1177/106002809703100710. [DOI] [PubMed] [Google Scholar]
- 47.Guengerich FP, Muller Enoch D, Blair IA. Oxidation of quinidine by human liver cytochrome. Mol Pharmacol. 1986;30:287–295. [PubMed] [Google Scholar]
- 48.Spinler SA, Cheng JW, Kindwall KE, Charland SL. Possible inhibition of hepatic metabolism of quinidine by erythromycin. Clin Pharmacol Ther. 1995;57:89–94. doi: 10.1016/0009-9236(95)90270-8. [DOI] [PubMed] [Google Scholar]
- 49.Damkier P, Hansen LL, Brosen K. Effect of diclofenac, disulfiram, itraconazole, grapefruit juice, and erythromycin on the pharmacokinetics of quinidine. Br J Clin Pharmacol. 1999;48:829–838. doi: 10.1046/j.1365-2125.1999.00099.x. 10.1046/j.1365-2125.1999.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oberg K, Baumann JL. QT interval prolongation and torsades de pointes due to erythromycin lactobionate. Chemotherapy. 1995;15:687–692. [PubMed] [Google Scholar]
- 51.Ragosta M, Weihl AC, Rosenfeld LE. Potentially fatal interaction between erythromycin and disopyramide. Am J Med. 1989;86:465–466. doi: 10.1016/0002-9343(89)90346-x. [DOI] [PubMed] [Google Scholar]
- 52.Paar D, Terjung B, Sauerbruch T. Life–threatening interaction between clarithromycin and disopyramide. Lancet. 1997;249:326–327. doi: 10.1016/s0140-6736(05)62825-5. [DOI] [PubMed] [Google Scholar]
- 53.Bachmann K, Schwartz JL, Forney R, Frogameni A, Jauregi LE. The effect of erythromycin on the disposition kinetics of warfarin. Pharmacology. 1984;28:171–176. doi: 10.1159/000137958. [DOI] [PubMed] [Google Scholar]
- 54.Weibert RT, Lorentz SM, Townsend RJ, Cook CE, Klauber MR, Jagger PI. Effects of erythromycin in patients receiving long-term warfarin therapy. Clin Pharm. 1989;8:210–214. [PubMed] [Google Scholar]
- 55.Kaminsky LS, Zhang ZY. Human P450 metabolism of warfarin. Pharmacol Ther. 1997;73:67–74. doi: 10.1016/s0163-7258(96)00140-4. 10.1016/s0163-7258(96)00140-4. [DOI] [PubMed] [Google Scholar]
- 56.Chang TK, Gonzales FJ, Waxman DJ. Evaluation of triacetyloleandomycin, α-naphtho-flavone and diethyldithiocarbamate as selective chemical probes for inhibition of human cytochrome P450. Arch Biochem Biophys. 1994;311:437–442. doi: 10.1006/abbi.1994.1259. 10.1006/abbi.1994.1259. [DOI] [PubMed] [Google Scholar]
- 57.Shepherd AM, Hewick DS, Moreland TA, Stevenson IM. Age as a determinant of sensitivity to warfarin. Br J Clin Pharmacol. 1977;4:315–320. doi: 10.1111/j.1365-2125.1977.tb00719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Westphal JF, Vetter D, Brogard JM. Hepatic side-effects of antibiotics. J Antimicrob Chemother. 1994;33:387–401. doi: 10.1093/jac/33.3.387. [DOI] [PubMed] [Google Scholar]
- 59.Recker MW, Kier KL. Potential interaction between clarithromycin and warfarin. Ann Pharmacother. 1997;31:996–998. doi: 10.1177/106002809703100907. [DOI] [PubMed] [Google Scholar]
- 60.Gooderham MJ, Bolli P, Fernandez PG. Concomitant digoxin toxicity and warfarin interaction in a patient receiving clarithromycin. Ann Pharmacother. 1999;33:796–799. doi: 10.1345/aph.18330. [DOI] [PubMed] [Google Scholar]
- 61.Lane G. Increased hypoprothrombinemic effect of warfarin possibly induced by azithro-mycin (letter) Ann Pharmacother. 1996;30:884–885. doi: 10.1177/106002809603000735. [DOI] [PubMed] [Google Scholar]
- 62.Woldtvedt BR, Cahoon CL, Bradley LA, Miller SJ. Possible increased anticoagulation effect of warfarin induced by azithromycin (letter) Ann Pharmacother. 1998;32:269–270. doi: 10.1345/aph.17165. [DOI] [PubMed] [Google Scholar]
- 63.Yee GC, McGuire TR. Pharmacokinetic drug interactions with cyclosporine (Part I) Clin Pharmacokinet. 1990;19:319–332. doi: 10.2165/00003088-199019040-00004. [DOI] [PubMed] [Google Scholar]
- 64.Webber IR, Peters WH, Back DJ. Cyclosporin metabolism by human gastroinestinal mucosal microsomes. Br J Clin Pharmacol. 1992;33:661–664. doi: 10.1111/j.1365-2125.1992.tb04098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kolars JC, Schmiedlin-Ren P, Schwartz JD, et al. Identification of rifampicin inductible P-450III A4 (CYP3A4) in human small bowel enterocytes. J Clin Invest. 1992;90:1871–1878. doi: 10.1172/JCI116064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hebert MF, Roberts JP, Prucksaritanont T, Benet LZ. Bioavailability of cyclosporine with concomitant rifampin administration is markedly less than predicted by hepatic enzyme induction. Clin Pharmacol Ther. 1992;52:453–457. doi: 10.1038/clpt.1992.171. [DOI] [PubMed] [Google Scholar]
- 67.Okamura N, Hirai M, Tanigawara Y, et al. Digoxin–cyclosporin A interaction: modulation of the multidrug transporter P-glycoprotein in the kidney. J Pharmacol Exp Ther. 1993;266:1614–1619. [PubMed] [Google Scholar]
- 68.Gupta SK, Bakran A, Johnson RW, Rowland M. Erythromycin enhances the absorption of cyclosporin (letter) Br J Clin Pharmacol. 1988;25:401–402. doi: 10.1111/j.1365-2125.1988.tb03320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Greiner B, Eichelbaum R, Fritz P, et al. The role of intestinal P-glycoprotein in the interaction of digoxin and rifampin. J Clin Invest. 1999;104:147–153. doi: 10.1172/JCI6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spicer ST, Liddle C, Chapman JR, et al. The mechanism of cyclosporin toxicity induced by clarythromycin. Br J Clin Pharmacol. 1997;43:194–196. doi: 10.1046/j.1365-2125.1997.54310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bachmann K, Sullivan TJ, Reese JH, et al. The influence of dirithromycin on the pharma-cokinetics of cyclosporine in healthy subjects and in renal transplant patients. Am J Ther. 1995;2:490–498. doi: 10.1097/00045391-199506000-00009. [DOI] [PubMed] [Google Scholar]
- 72.Ljutic D, Rumboldt Z. Possible interaction between azithromycin and cyclosporin: a case report. Nephron. 1995;70:130. doi: 10.1159/000188567. [DOI] [PubMed] [Google Scholar]
- 73.Desmond Padhi ID, Long P, Basha M, Anandan IV. Interaction between tacrolimus and erythromycin. Ther Drug Monitor. 1997;19:120–121. doi: 10.1097/00007691-199702000-00024. [DOI] [PubMed] [Google Scholar]
- 74.Prince RA, Wing DS, Weinberger MM, et al. Effect of erythromycin on theophylline kinetics. J Allergy Clin Immunol. 1981;68:427–431. doi: 10.1016/0091-6749(81)90196-2. [DOI] [PubMed] [Google Scholar]
- 75.Sarkar MA, Hunt C, Guzelian PS, Karnes HT. Characterisation of human liver cytochrome P450 involved in theophylline metabolism. Drug Metab Dispos. 1992;20:31–37. [PubMed] [Google Scholar]
- 76.Fuhr E, Doehmer J, Battula N, et al. Biotransformation of caffeine and theophylline in mammalian cell lines genetically engineered for expression of single cytochrome P450 isoforms. Biochem Pharmacol. 1992;43:225–235. doi: 10.1016/0006-2952(92)90282-n. [DOI] [PubMed] [Google Scholar]
- 77.Tjia JF, Colbert J, Back DJ. Theophylline metabolism in human liver microsomes: inhibitory studies. J Pharmacol Exp Ther. 1996;276:912–917. [PubMed] [Google Scholar]
- 78.Sarkar MA, Jackson B. Theophylline N-demethylations as probes for P450 1A1 and P450 1A2. Drug Metab Dispos. 1994;22:827–834. [PubMed] [Google Scholar]
- 79.Bachmann K, Jauragui L, Sides G, Sullivan TJ. Steady-state pharmacokinetics of theophylline in COPD patients treated with dirithromycin. J Clin Pharmacol. 1993;33:861–865. doi: 10.1002/j.1552-4604.1993.tb01964.x. [DOI] [PubMed] [Google Scholar]
- 80.Pollack PT, Slayter KL. Reduced serum theophylline concentrations after discontinuation of azithromycin: evidence for an unusual interaction. Pharmacotherapy. 1997;17:827–829. [PubMed] [Google Scholar]
- 81.Barzaghi N, Gatti G, Crema F, et al. Inhibition by erythromycin of the conversion of carbamazepine to its active 10,11-epoxide metabolite. Br J Clin Pharmacol. 1987;24:936–938. doi: 10.1111/j.1365-2125.1987.tb03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kivisto KT, Neuvonen PJ, Klotz U. Inhibition of terfenadine metabolism: pharmaco-kinetic and pharmacodynamic consequences. Clin Pharmacokinet. 1994;27:1–5. doi: 10.2165/00003088-199427010-00001. [DOI] [PubMed] [Google Scholar]
- 83.Yumibe N, Huie K, Chen KJ, Clement RP, Caten MN. Identification of human liver cytochrome P450s involved in the microsomal metabolism of the antihistaminic drug loratadine (Abstract) J Allergy Clin Immunol. 1994;93:234. doi: 10.1159/000237063. (:1 Part; 2) [DOI] [PubMed] [Google Scholar]
- 84.Brannan MD, Reidenberg P, Radwanski E, et al. Loratadine administered concomitantly with erythromycin: pharmacokinetic and electrocardiographic evaluation. Clin Pharmacol Ther. 1995;58:269–278. doi: 10.1016/0009-9236(95)90243-0. [DOI] [PubMed] [Google Scholar]
- 85.Carr RA, Edmonds A, Shi H, et al. Steady-state pharmacokinetics and electrocardiographic pharmacodynamics of clarithromycin and loratadine after individual or concomitant administration. Antimicrob Agents Chemother. 1998;42:1176–1180. doi: 10.1128/aac.42.5.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nightingale SL. Warnings issues on non-sedating antihistamines terfenadine and astemizole. JAMA. 1992;268:705. [Google Scholar]
- 87.Ghali R, DeLean J, Douville Y, et al. Erythromycin-associated ergotamine intoxication: arteriographic and electrophysiologic analysis of a rare cause of severe ischemia of the lower extremities and associated ischemic neuropathy. Ann Vasc Surg. 1993;7:291–297. doi: 10.1007/BF02000258. [DOI] [PubMed] [Google Scholar]
- 88.Horowitz RS, Dart RC, Gomez HF. Clinical ergotism with lingual ischemia induced by clarithromycin–ergotamine interaction. Arch Intern Med. 1996;156:456–458. [PubMed] [Google Scholar]